Licochalcone A suppresses Sp1 expression with potential anti-myeloma activity
Abbreviations
-
- ARP1
-
- Arkansas P1
-
- CD138
-
- Cluster of differentiation 138
-
- c-Myc
-
- Myc proto-oncogene protein
-
- DMSO
-
- dimethyl sulfoxide
-
- IRF4
-
- Interferon Regulatory Factor 4
-
- LCA
-
- Licochalcone A
-
- miR-23b
-
- microRNA-23b
-
- MM
-
- Multiple myeloma
-
- NOD-SCID
-
- Non-obese diabetic/severe combined immunodeficiency
-
- OCI-MY5
-
- Ontario Cancer Institute-Myeloma 5
-
- ORR
-
- objective response rate
-
- PBS
-
- phosphate-buffered saline
-
- siRNA
-
- small interfering RNA
-
- Sp1
-
- Specificity protein 1
-
- SS
-
- Side Scatter
-
- STRING
-
- Search Tool for the Retrieval of Interacting Genes
-
- TCM
-
- Traditional Chinese Medicine
-
- UAMS
-
- University of Arkansas for Medical Sciences
Dear editor,
Following up on Fulciniti et al.’s [1] work demonstrating the involvement of the transcription factor, Specificity protein 1 (Sp1), in the biology of multiple myeloma (MM) cell growth and drug resistance, we sought to determine whether inhibition of Sp1 might overcome the relapse of MM. Up to now, inhibition of Sp1 in MM was demonstrated by using microRNA-23b (miR-23b) or small interfering RNA (siRNA), which significantly suppressed cell growth and induced cell apoptosis via induction of caspase-3/7/9 activity [1, 2]. However, the clinical development of miR-23b-targeted or siRNA-treated replacement therapy has been limited by adverse events and narrow therapeutic window in clinical trials [3]. Therefore, natural chemotherapeutic compounds which can suppress tumor growth and reduce adverse events could be of clinical priority for MM treatment.
Traditional Chinese medicine (TCM) and its bioactive components have been widely used alone or as a complementary approach for cancer treatment in East Asia over hundreds of years. Extensive evidence indicated that TCM possesses definite advantages over conventional therapies in terms of fewer adverse events and lesser economic burden [4]. Among the medicinal licorice species (glycyrrhiza glabra, glycyrrhiza uralensis and glycyrrhiza inflata), glycyrrhiza inflata has been described as the king of herbs by TCM doctors and has shown a wide spectrum of pharmacological effects with significant clinical efficacy [5]. Seven retrochalcones, echinatin and licochalcones A, C, D, E, K, and L, were isolated and characterized from the chloroform extracts of glycyrrhiza inflata. Due to the multiple components and complicated clinical application, the biological function of glycyrrhiza inflata and its anti-tumor mechanism are poorly understood. Network pharmacology is based on the theories of integrated system biology, multi-directional pharmacology, and bioinformatics, providing novel strategies to investigate the relation between TCM and targeted genes (the detailed methods are described in the Supplementary Methods) [6]. Before treatment, the target network of glycyrrhiza inflata and bioactive compounds of Sp1 were predicted with SymMap [7] (Figure 1A). Licochalcone A (LCA) was connected with MM and Sp1 in predicted network pharmacology. LCA (3-dimethylallyl-4, 4′-dihydroxy-6-methoxychalcone, formula, C21H22O4, molecular weight, 338.4) is a natural compound derived from the root of glycyrrhiza inflata (Figure 1B), and is known to have anti-cancer, anti-bacterial and anti-inflammation effects [8]. However, no research has been reported on the significance of LCA in treating MM. Following the efficacy of LCA on other cancers and the prediction of SymMap, we hypothesized that LCA might have an anti-cancer effect on MM.
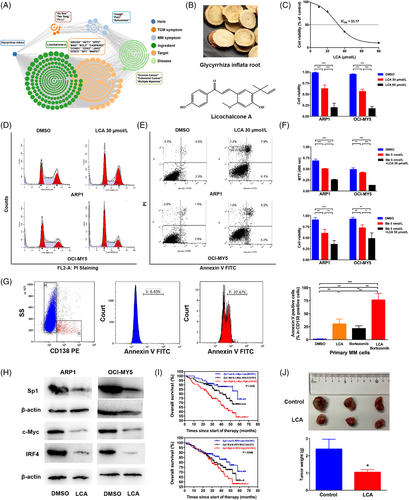
Licochalcone A is an Sp1 antagonist with strong anti-myeloma activity. (A) TCM symptom-Chinese medicine-target network of the Chinese medicine glycyrrhiza inflata. (B) Chinese herb of glycyrrhiza inflata root and chemical structure of licochalcone A. (C) Upper, ARP1 cells were incubated with various concentrations of LCA for 24 h. The cell viability was measured by MTT assay. Lower, the viability of two MM cell lines (ARP1 and OCI-MY5) after LCA (0, 30, 60 μmol/L) treatment for 24 h. The values were measured using Trypan blue staining. (D) and (E) ARP1 and OCI-MY5 cells were treated with DMSO and LCA (30 μmol/L) for 24 h, and the cell cycle (D) and apoptosis distribution (E) were analyzed by flow cytometry. (F) MTT (Upper panel) and Trypan blue (Lower panel) assays of ARP1 and OCI-MY5 cells treated with bortezomib alone or in the presence of LCA (30 μmol/L). G. CD138+ primary MM cells were treated with bortezomib (5 nmol/L) or LCA (30 μmol/L), and cell apoptosis was assessed by Annexin V staining, the two-tailed Student's t-test was utilized to analyze the differences in DMSO vs. LCA; DMSO vs. Bortezomib; DMSO vs. LCA + Bortezomib; LCA + Bortezomib vs. LCA; LCA + Bortezomib vs. Bortezomib, significant comparisons were labeled in this figure. (H) ARP1 and OCI-MY5 cells were treated with DMSO and LCA (30 μmol/L) for 24 h, and the protein levels of Sp1, c-Myc and IRF4 were determined by Western blotting. (I) Kaplan-Meier analyses of overall survival among MM patients with different expressions of Sp1/c-Myc (Upper) and Sp1/IRF4 (Lower). (J) Upper, the NOD-SCID mice injected subcutaneously with RPMI-8226 cells were euthanized, and tumors were isolated. Lower, the mean tumor weight of the PBS-treated group was higher than that of the LCA-treated group. *P < 0.05, **P < 0.01, ***P < 0.001.
Abbreviations: Sp1, Specificity protein 1; MM, Multiple myeloma; LCA, Licochalcone A; TCM, Traditional Chinese Medicine; ARP1, Arkansas P1; OCI-MY5, Ontario Cancer Institute-Myeloma 5; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; CD138, cluster of differentiation 138; DMSO, dimethyl sulfoxide; PBS, phosphate-buffered saline; SS, Side Scatter; IRF4, Interferon Regulatory Factor 4; c-Myc, Myc proto-oncogene protein; NOD-SCID, Non-obese diabetic/severe combined immunodeficiency.
Based on the above findings, we designed pre-clinical studies to assess the anti-MM effect of LCA as monotherapy or combination therapy in vivo and in vitro. Here, we evaluated whether LCA could inhibit cell viability and proliferation. Arkansas P1 (ARP1) and Ontario Cancer Institute-Myeloma 5 (OCI-MY5) cells were treated for 24 h with increasing concentrations of LCA. Of note, the viability of cells treated with 30 μmol/L LCA was obviously decreased compared with the dimethyl sulfoxide (DMSO) group (DMSO vs. LCA: ARP1, 0.98 ± 0.01 vs. 0.62 ± 0.03, P < 0.001; OCI-MY5, 0.95 ± 0.01 vs. 0.56 ± 0.02, P < 0.001; H929, 0.93 ± 0.01 vs. 0.61 ± 0.04, P < 0.01; U266, 0.94 ± 0.01 vs. 0.63 ± 0.03, P < 0.001; Figure 1C, Supplementary Figure S1A). The strong anti-proliferative impact of LCA on MM cells indicated that LCA could induce cell cycle arrest. To this end, we evaluated the effect of LCA on cell cycle via flow cytometry and detected increases in the sub-G1 phase (data presented as the mean ± standard error of the mean [SEM], DMSO vs. LCA: 28.31% ± 0.65% vs. 40.03% ± 0.66%, P < 0.001; 36.23% ± 0.68% vs. 48.12% ± 0.68%, P < 0.001; Figure 1D, Supplementary Figure S1B). From Annexin V staining, as compared with DMSO-treated cells, 30 μmol/L LCA-treated cells showed significantly higher apoptosis rates (DMSO vs. LCA: 5.91% ± 0.42% vs. 17.01% ± 1.52%, P < 0.001; 7.11% ± 0.25% vs. 16.85% ± 1.23%, P = 0.004; Figure 1E, Supplementary Figure S2). Given its anti-myeloma activity as monotherapy in MM cell lines, we hypothesized that the combination therapy of LCA with bortezomib might further enhance anti-myeloma activity. Our results showed that LCA together with bortezomib exhibited strong synergistic effects on cell viability and proliferation in MM cells as revealed by a combination index < 1 (Supplementary Figure S1C). Monotherapy with bortezomib at 5 nmol/L only partially induced MM cell death, whereas LCA at the suboptimal concentration (5 μmol/L) in combination with bortezomib (5 nmol/L) enhanced MM cell death (Figure 1F, Supplementary Figure S1D), indicating a cooperative induction of MM cell death by LCA and bortezomib. We then assessed whether LCA could affect the sensitivity of primary MM cells to chemotherapy. LCA almost reduced the viability of primary MM cells by half, which was equivalent to the extent observed in the control group treated with bortezomib, even though LCA alone did not alter the viability of cluster of differentiation 138negative (CD138−) cells (Quantifications of Annexin V positive cells from CD138+ cells shown in Figure 1G, the data are presented as the mean ± SEM; DMSO vs. LCA: 0.93% ± 0.49% vs. 30.30% ± 5.43%, P = 0.005; DMSO vs. Bortezomib: 0.93% ± 0.49% vs. 21.39% ± 3.12%, P = 0.003; DMSO vs. LCA + Bortezomib: 0.93% ± 0.49% vs. 76.49% ± 6.24%, P < 0.001; LCA + Bortezomib vs. LCA: 76.49% ± 6.24% vs. 30.30% ± 5.43%, P = 0.007; LCA + Bortezomib vs. Bortezomib: 76.49% ± 6.24% vs. 21.39% ± 3.12%, P = 0.002).
Interferon regulatory factor 4 (IRF4) and c-Myc are regarded as “Achilles heels” in MM cells and regulate the expression of various genes related to cell growth and survival, suggesting constitutive activation of c-Myc and IRF4 is critical for the epigenetic nature of MM cell pathogenesis [9]. For this reason, IRF4 and c-Myc are currently recognized as pro-survival mediators to be targeted in anti-MM treatments. A previous study demonstrated that Sp1 acted through c-Myc and IRF4 activation in MM cell lines and that Sp1 degradation by treatment with panobinostat caused an extensive reduction in c-Myc and IRF4 protein levels [10]. Hence, we examined the protein levels by Western blotting in DMSO- or LCA-treated cells and found that LCA prominently suppressed Sp1, IRF4, and c-Myc (Figure 1H). We also investigated how Sp1 activation in MM, specifically through interactions with c-Myc and IRF4, affected the survival of MM patients. As shown on Supplementary Figure S1E, Sp1 expression was significantly correlated with IRF4 expression (r = 0.350, P < 0.001) and c-Myc expression (r = 0.104, P < 0.05) in the GSE2658 dataset. Analysis of Sp1-associated proteins using the Search Tool for the Retrieval of Interacting Genes (STRING) protein interaction database showed a direct interactive link with c-Myc (Supplementary Figure S1F). Furthermore, 351 MM patients (University of Arkansas for Medical Sciences (UAMS) cohort) were divided into 3 subgroups according to the expression of Sp1/c-Myc and Sp1/IRF4, and survival curves showed that the Sp1 high/c-Myc high group and Sp1 high/IRF4 high group have the worst survival outcome (Figure 1I). The above results indicated that LCA effectively reduced the expression of Sp1, c-Myc, and IRF4, which contributed to MM cell death and survival. To further explore the therapeutic utilization of LCA in vivo, RPMI-8226 cells were subcutaneously injected into non-obese diabetic/severe combined immunodeficiency (NOD-SCID) mice treated with phosphate-buffered saline (PBS) or LCA. The mean weight of PBS-treated tumors was significantly higher than that of LCA-treated tumors, confirming the assertion that LCA could suppress tumor growth in vivo (Figure 1J).
Taken together, the synergistic anti-MM effect of LCA in combination with bortezomib indicated that combining LCA with chemotherapy could increase objective response rate (ORR) and should be further investigated. Sp1 inhibition via LCA has the potential to become a novel therapeutic strategy for patients with relapsed or refractory MM and deserves clinical investigations as monotherapy or combination therapy.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research study was approved by the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article or uploaded as supplementary files.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Chunyan Gu (Nanjing University of Chinese Medicine, China) for donating the MM cell lines.
AUTHORSHIP
HB, MZ and HZ contributed equally to this work. HB and BC designed this study; HB, MZ, HZ, QYH, HX and PPX conducted the experiments, performed the statistical analysis, and drafted the manuscript. BC conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors have read and approved the manuscript.




