Neoadjuvant immune checkpoint inhibitor plus chemotherapy in rare tracheal tumors
Abbreviations
-
- CT
-
- computerized tomography
-
- LCNEC
-
- large-cell neuroendocrine carcinoma
-
- LELC
-
- lymphoepithelioma-like carcinoma
-
- PET
-
- Positron emission tomography
Primary malignant tumors of the trachea account for 0.01%-0.4% of all cancer cases [1]. Due to their locally aggressive growth, surgical resection including carinal resection and airway reconstruction was most frequently applied for these diseases [2]. However, tracheal surgery is technically challenging and is associated with high rates of operative morbidity and mortality. Effective neoadjuvant treatment is expected to reduce the tumor load and lessen the extent of resecting primary tracheal tumors. However, evidence regarding neoadjuvant treatment for tracheal tumors remains relatively scarce and platinum-based doublet chemotherapy remains the standard in this setting. Immune checkpoint inhibitors are a promising approach to neoadjuvant treatment. A phase II clinical trial (NADIM[3]) evaluated nivolumab with paclitaxel plus carboplatin as neoadjuvant regiment for resectable stage IIIA non-small cell lung cancer. The major pathological response rate was 83%, and the pathological complete response rate was 63% [3]. The updated results of the phase III trial (Checkmate 816) showed that neoadjuvant nivolumab in combination with platinum-based chemotherapy could significantly improve pathological complete response rate compared to chemotherapy alone (24.0% vs. 2.2%, odds ratio = 13.94, 99% confidence interval [CI] = 3.49-55.75) [4]. Hence, to deepen our understanding on the treatment of primary tracheal tumors, we report the application of neoadjuvant immunochemotherapy in two rare tracheal tumor cases.
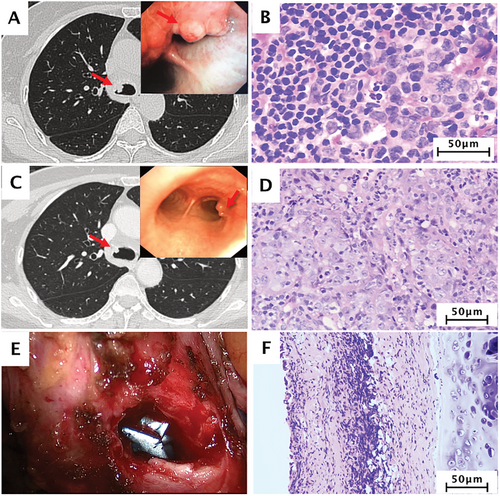
Case 1: A 57-year-old woman presented with a gradually worsening cough for 2 weeks. She had no history of cardiothoracic operation or tuberculosis. Chest computerized tomography (CT) revealed a rounded, solid nodule measuring approximately 1.1 cm in diameter, located at the opening of the right main bronchus, with no clear boundary. 18F-FDG positron emission tomography-computed tomography (PET/CT) showed two foci of intense activity, located at the opening of the right main bronchus and station 7 lymph nodes, respectively (Figure 1A). After a bronchoscopic biopsy, she was diagnosed with primary tracheal lymphoepithelioma-like carcinoma (LELC; Figure 1B, Supplementary Figure S1, and S2). The clinical stage was cT1N1M0 stage IV, defined by Bhattacharyya's criteria [5]. After a multidisciplinary team discussion, she received three cycles of chemotherapy (docetaxel 75mg/m2 + carboplatin, area under the curve [AUC] 5) plus nivolumab (360 mg) every 21 days as neoadjuvant treatment. The patient tolerated the regimen very well, and only experienced grade 2 neutropenia and alopecia which resolved without any treatment. After three cycles of neo-immunochemotherapy, repeated bronchoscopy showed a tiny residual lesion on the right lower tracheal wall, and no residual tumor was observed (Figure 1C and D, Supplementary Figure S3). Subsequently, the patient received carinal-preserving tracheal tumor resection under video-assisted thoracic surgery and recovered without complications. The final pathological diagnosis confirmed a complete pathological response, ypT0N0M0 stage 0 (Figure 1E and F, Supplementary Figure S4). The patient continued to receive treatment with single-agent, nivolumab, and her clinical course is regularly followed up for one year. As of March 2021, she is still disease-free for 18 months.
Case 2: A 48-year-old man presented with a gradually worsening cough and shortness of breath for 2 months. This patient also had no history of cardiothoracic operation or tuberculosis. Chest CT revealed a 1.6-cm rounded, solid lesion in the lower trachea and showed an unclear margin between the lesion and carina (Supplementary Figure S5A). The subtotal lump was laser-resected to relieve airway obstruction, and his condition was diagnosed as primary tracheal large-cell neuroendocrine carcinoma (LCNEC, Supplementary Figures S5B, S6, and S7). We performed a bronchoscopic biopsy on the residual tumor tissue which remained in the carina (Supplementary Figure S5C and D), and determined that the clinical stage was cT1N0M0 stage I defined by Bhattacharyya's category [5]. After a multidisciplinary team discussion, he received three cycles of chemotherapy (paclitaxel 200mg/m2 + carboplatin, AUC 5) plus nivolumab (360 mg) every 21 days as neoadjuvant therapy. No obvious adverse events were observed. After induction treatment, the bronchoscopy showed no evident residual lesion, and no residual tumor was observed after treatment (Supplementary Figure S5E and F). Thus, further surgical resection was recommended due to the possibility of false-negative bronchoscopic biopsy, and lack of sufficient evidence in this clinical setting. However, the patient refused surgery or radiotherapy due to the concern about treatment complications and was therefore recommended frequent follow-up appointments. As of January 2021, he has been still disease-free for eight months.
To our knowledge, tracheal LELC and LCNEC are extremely rare diseases [6]. The cases reported here provide important contributions to the literature on these diseases. On the other hand, tracheal tumor resection and airway reconstruction, especially for tumors with wide extension or involving the carinal, are challenging for thoracic surgeons [2]. This preliminary report demonstrated satisfactory clinical efficacy of neoadjuvant immunochemotherapy for tracheal tumors, which could lessen resection extent.
The biggest limitation of these two cases was the lack of additional exploration of potential biomarkers. Nevertheless, there are several points herein warrant further exploration. First, could definitive chemoradiotherapy following consolidation immunotherapy enable some tracheal tumor patients to avoid surgery? Second, how to implement postoperative adjuvant immunotherapy? Furthermore, as shown in Case 2, if there is no evidence of tumor cells after multiple bronchoscopic biopsies, is surgery necessary? From this case, we have reasons to consider that close observation could be one of the feasible schemes.
In conclusion, this preliminary report showed the potential clinical role of neoadjuvant immunochemotherapy for rare tracheal tumors. We hope that the treatment implemented could be used as reference to improve the treatment outcomes of these patients.
AUTHORSHIP
B.Y.J., W.Z.Z., and Y.L.W. contributed to the conception and design of the study. All authors collected the clinical data and interpreted data for the work. L.X.Y. and X.P.C. contributed to the analysis and interpretation of data. B.Y.J. and J.T.Z. wrote the manuscript. All authors read and approved the final manuscript.
FUNDING
This work was supported by the High-level Hospital Construction Project (DFJH201910 to B.Y.J.) and Guangdong Provincial People's Hospital Young Talent Project (GDPPHYTP201902 to W.Z.Z.). The funding source had no role in the preparation of this manuscript.
ACKNOWLEDGMENTS
We owe thanks to the patients and their families. We thank the multidisciplinary team support at Guangdong Provincial People's Hospital.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Boards at the Guangdong Provincial People's Hospital (No. GDREC2019523H).
CONSENT FOR PUBLICATION
All the patients provided written informed consent.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




