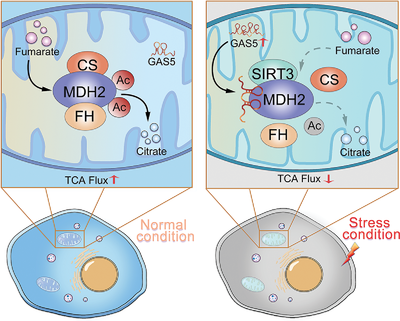
Metabolon: a novel cellular structure that regulates specific metabolic pathways
Graphical Abstract
This manuscript of research highlight focused on one paper recently published in Nature Metabolism entitled “Mitochondrial Long Non-coding RNA GAS5 Tunes TCA Metabolism in Response to Nutrient Stress” from Lin Aifu's group in Zhejiang University. In this manuscript, we discussed the novel findings in Lin's paper and concluded that the metabolon is emerging as a novel cellular structure that regulates specific metabolic pathways.
List of abbreviations
-
- GAS5
-
- growth arrest-specific transcript 5
-
- TCA
-
- tricarboxylic acid
-
- lncRNA
-
- long non-coding RNA
-
- MDH2
-
- malate dehydrogenase
-
- FH
-
- fumarate hydratase
-
- CS
-
- citrate synthase
-
- ΔG0'
-
- inverse standard Gibbs free energy
Tumor cells exhibit several metabolic abnormalities, such as uptake disorders of glucose and amino acids, increased nutrient consumption rates, use of glycolysis/tricarboxylic acid (TCA) cycle intermediates for biosynthesis and NADPH production, and increased demand for nitrogen sources [1]. As an increasing understanding of cancer cell metabolism has been gained in recent years, the metabolic changes and molecular mechanisms associated with tumors in different tumor stages have been gradually revealed. These molecular mechanisms mainly include the following categories: (1) genomic alterations change the nutrient absorption mode of tumor cells [2]; (2) abnormal expression or activity changes of metabolic enzymes reprogram the direction of metabolic flow [3, 4]; and (3) changes in the levels of specific metabolites can affect the biological functions of both tumor cells and tumor-infiltrating immune cells [5, 6]. However, our knowledge on metabolic enzyme interactions in tumor cells remains shallow and what we do know is mostly limited to their physical interactions.
In 1987, Paul Srere defined the term “metabolon” as a supramolecular complex of sequential metabolic enzymes and cellular structural elements [7]. In recent years, scientists have assumed that metabolon is a three-dimensional structure of a loose multi-protein complex formed by certain metabolic enzymes and could promote various biological processes in tumor cells [8]. Metabolic substrates can undergo certain metabolic reactions, such as TCA, by entering metabolon. Therefore, metabolon is considered to be the "key device" for intracellular biosynthesis. In a recent study [9], researchers from Penn State University used a new imaging technique and mass spectrometry to directly observe the functional metabolon involved in purine (the most abundant cellular metabolite) production for the first time. They reported that metabolic enzymes were not randomly distributed in the whole cell but in the form of a metabolon which performs specific metabolic processes. However, the functions and mechanisms of metabolon remained undiscovered.
On January 4, 2021, Lin and colleagues [10] from Zhejiang University (Hangzhou, Zhejiang, China) published a study entitled “Mitochondrial Long Non-coding RNA GAS5 Tunes TCA Metabolism in Response to Nutrient Stress”. In this study, they established a new organelle immune affinity purification system to isolate mitochondria and lysosomes, followed by RNA sequencing. This purification system avoids cross-contamination between organelles and can better retain the integrity of organelles for biochemical characterization because there is no exogenous transfection or chemical treatment of cells in advance. It is suitable for dynamic analysis of organelle components and extraction analysis of organelles in tissues under different stress conditions. Moreover, the organelle lncRNA map was drawn in this study. The data revealed the important regulatory role of GAS5 (growth arrest-specific transcript 5), which is a mitochondrial long non-coding RNA (lncRNA). The authors found that mitochondrial protein malate dehydrogenase (MDH2) could be directly targeted by GAS5. MDH2 is a speed-limiting enzyme in the mitochondrial TCA cycle that maintains the steady cycle of energy metabolism. The present study reported that MDH2 coupled with fumarate hydratase (FH) and citrate synthase (CS) to form a metabolon, which helps MDH2 complete the inverse standard Gibbs free energy (Δ G0') reaction in the cell through the tandem reaction of substrates. Knockdown of GAS5 enhanced the stability of MDH2 metabolon and weaken its sensitivity to glucose. In contrast, overexpression of GAS5 simulated the signal of glucose deficiency, inhibited the formation of FH-MDH2-CS metabolon, consequently caused abnormal levels of citric acid and malic acid, and imbalance of mitochondrial energy metabolism; thereby inhibiting cell growth and proliferation in cancer. Mechanistically, GAS5 can transduce cellular energy status to regulate the SIRT3-MDH2 association, modulate MDH2 deacetylation and evertually control FH-MDH2-CS complex formation. As illustrated in Figure 1, GAS5 could inhibit the interaction between MDH2 and FH/CS to form a metabolon through dynamic mitochondrial translocation, thereby downregulating the metabolic flow of the TCA cycle and inhibiting mitochondrial metabolism. This study indicated that the mitochondrial TCA cycle plays an important role in supporting tumor initiation and progression. Additionally, down-regulation of GAS5 facilitates tumor cells to avoid the inhibitory effect of mitochondrial metabolism and promotes tumor progression in the absence of energy substances
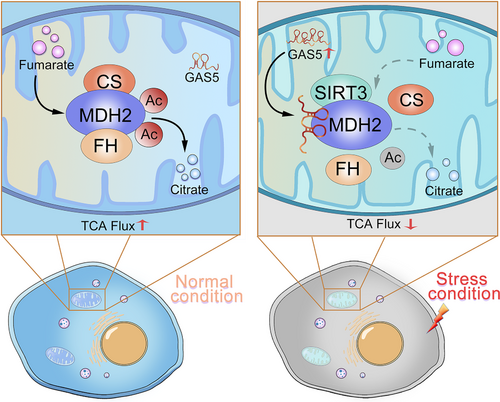
In recent years, the subcellular compartmentalizations of organelles in eukaryotic cells have been demonstrated to have certain biological functions [11, 12]. At the same time, metabolons, the complexes formed between intracellular metabolic enzymes, are also speculated to have biological functions. Lin's study shows that the metabolon is regulated by specific factors in tumor cells, results in the alterations of interactions between metabolic enzymes, which in turn can affect a series of downstream metabolic reactions. Given that many biochemical reactions in cells are completed by a series of enzymes, and the metabolic processes of tumor cells are significantly different from those of normal cells, tumor cells can quickly adapt to the tumor microenvironment and malignant growth, and even metastasize through metabolic reprogramming. In this process, the metabolon formed by metabolic enzymes must also be changed to adapt to the high-intensity metabolic reaction in tumor cells. If researchers can reveal the specific functions and mechanisms of metabolons in tumor cells, and design biological molecules that target metabolons, this could provide new avenues for tumor treatment. Based on current knowledge in this field, metabolons are not relatively stable organelles, but have loose three-dimensional structures. They can undergo various conformational changes along with metabolic processes to complete the corresponding metabolic reactions. Therefore, scientists need to find the key molecules in the metabolons, such as the lncRNA GAS5 which is mentioned above, to clarify the functions and mechanisms of metabolons. MDH2, a target of GAS5, can be used as a breakthrough point to try to regulate the metabolon and further tackle the challenging process of tumor inhibition. Moreover, it has been reported that abnormal signals in tumor cells can induce metabolic enzymes to perform non-metabolic functions, including metabolic enzyme-mediated protein post-translational modifications [13]. However, whether metabolon can perform non-metabolic functions and exert its unique effects in tumor cells remain to be explored.
This latest research on endogenous metabolic enzymes mostly focuses on the enzyme itself, but in fact, in the process of an enzymatic reaction, the spatial position of the enzyme and its substrates can determine the direction of the catalytic reaction. As a special three-dimensional structure of enzyme complex, the metabolon may undertake many key enzymatic reactions and exert multiple functions in the metabolic reprogramming of tumor cells. The molecular mechanisms underlying the metabolic alterations in tumorigenesis and development still warrants further investigation.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported by the National Natural Science Foundation of China (81974430), the Natural Science Foundation of Guangdong Province (2019A1515012037), and the National Heart, Lung, and Blood Institute, NIH, USA (5R00HL136924-03).
AUTHORS' CONTRIBUTIONS
S.E Elf and J. Fan contributed substantially to the conception of this manuscript. T. Tian drafted the manuscript. J. Fan provided critical revision of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.