A phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors
Clinical trial registration number: NCT02857166.
Abstract
Background
Several programmed cell death ligand 1 (PD-L1)/programmed cell death protein 1 (PD-1) antibodies have been approved for cancer treatment worldwide. Their pharmacokinetic and pharmacodynamic characteristics have been reported mainly in western countries, but related data in Chinese patients are limited. This study was conducted to investigate the safety, efficacy, pharmacokinetics, and pharmacodynamics of an anti-PD-1 antibody, toripalimab, in Chinese patients.
Methods
A single-center phase I study was conducted in Sun Yat-sen University Cancer Center. Eligible patients were adults with histologically confirmed, treatment-refractory, advanced, solitary malignant tumors. Toripalimab was intravenously infused every 2 weeks in dose-escalating cohorts at 0.3 mg/kg, 1 mg/kg, 3 mg/kg, 10 mg/kg, and 240 mg. The study followed standard 3 + 3 design.
Results
Between 15th March 2016 and 27th September 2016, 25 patients were enrolled, of whom 3 (12.0%), 7 (28.0%), 6 (24.0%), 6 (24.0%), 3 (12.0%) received 0.3 mg/kg, 1 mg/kg, 3 mg/kg, 10 mg/kg, and 240 mg toripalimab, respectively. After a median follow-up time of 5.0 months (range: 1.5-19.8 months), we observed that the commonest treatment-related adverse events (TRAEs) were fatigue (64.0%) and rash (24.0%). No grade 3 or higher TRAEs were observed. No dose-limiting toxicity, treatment-related serious adverse events (SAEs), or treatment-related death occurred. Objective response rate was 12.5%. The half-life of toripalimab was 150-222 h after a single dose infusion. Most patients, including those from the 0.3 mg/kg group, maintained complete PD-1 receptor occupancy (> 80%) on activated T cells since receiving the first dose of toripalimab.
Conclusions
Toripalimab is a promising anti-PD-1 antibody, which was well tolerated and demonstrated anti-tumor activity in treatment-refractory advanced solitary malignant tumors. Further exploration in various tumors and combination therapies is warranted.
Abbreviations
-
- ADA
-
- anti-drug antibody
-
- ALT
-
- alanine transaminase
-
- AST
-
- aspartate transaminase
-
- AUC
-
- area under the curve
-
- CCP
-
- confirmation cut point
-
- CFDA
-
- China Food and Drug Administration
-
- CI
-
- consistency index
-
- CR
-
- complete remission
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- DCR
-
- disease control rate
-
- DLT
-
- dose-limiting toxicity
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- irAE
-
- immune-related adverse event
-
- LAG-3
-
- lymphocyte-activation gene 3
-
- ORR
-
- objective response rate
-
- PD
-
- progressed disease
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed cell death ligand 1
-
- PR
-
- partial remission
-
- PS
-
- performance status
-
- SAE
-
- serious adverse event
-
- SCP
-
- screening cut point
-
- SD
-
- stable disease
-
- SD
-
- standard deviation
-
- TRAE
-
- treatment-related adverse event
-
- ULN
-
- upper limit of normalik
1 BACKGROUND
Cancer is a major health burden and the leading cause of death worldwide [1], especially in China [2]. According to estimation, 18.1 million new cancer cases and 9.6 million cancer deaths occurred in 2018 across 20 world regions [1]. Deficiency of immune function contributes through all stages of cancer development: from early neoplastic transformation to progression and metastasis [3]. Generally, tumor cells evolve various mechanisms to escape from the host immune attack, by avoiding the immune recognition and shaping an immunosuppressive microenvironment [4]. Remodeling anti-tumor immunity has been widely investigated. Various strategies for treating cancer by remodeling anti-tumor immunity have been developed [5]. Among them, immune checkpoint modulation is currently the most successful strategy [6, 7].
Immune checkpoints are regulators of the immune system. They're essential for self-tolerance, but cancer cells can also use them to avoid immune attack [8]. Many inhibitory and stimulatory immune checkpoint molecules were discovered, including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (LAG-3), and CD-137 [5, 9, 10]. PD-1 is an inhibitory immune checkpoint molecule which is expressed on various immunologic cells. When engaged by its ligand programmed cell death ligand 1 (PD-L1), PD-1 inhibits the kinase signaling pathway that activates immune cells [11]. Anti-tumor effect of the PD-L1/PD-1 pathway blockade has been demonstrated in tremendous clinical trials across various cancers [12]. Several antibodies against PD-L1/PD-1 have been approved for clinical use worldwide [13]. However, the pharmacokinetic data of anti- PD-L1/PD-1 antibodies in Chinese cancer population are still limited [14, 15].
Toripalimab is a humanized IgG4 monoclonal antibody against PD-1. It is one of the first monoclonal antibodies against PD-1 that were approved by the China Food and Drug Administration (CFDA) into clinical trials [16]. Based on the efficacy and safety data, it has been approved for the treatment of unresectable or metastatic treatment-refractory melanoma in December 2018 [17]. Reported results from clinical trials have demonstrated its manageable safety profile in several cancer types, such as melanoma [18], urologic cancer [18], and gastric cancer [19]. It also showed promising anti-tumor activity in Asia-specific acral and mucosal melanoma and comparable efficacy with other anti-PD-1 antibodies in gastric cancer [18, 19].
Here, we report the result of a phase I study of toripalimab, focusing on its safety, pharmacokinetics, and pharmacodynamics in Chinese patients with treatment-refractory, solid malignant tumors.
2 MATERIALS AND METHODS
2.1 Patient selection
This is a single-institutional, open-labeled, first in-human, phase I, dose escalation study conducted in Sun Yat-sen University Cancer Center (Guangzhou, Guangdong, China) between 15th March 2016 and 24th January 2018 (ClinicalTrials.gov identifier NCT02857166). Eligible patients had treatment-refractory, advanced, solitary malignant tumors. The inclusion criteria were as follows: (1) with at least one measurable lesion (Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1); (2) age of 18-75 years; (3) with a life expectancy ≥3 months; (4) with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (5) interval since latest anti-tumor treatment ≥4 weeks; (6) has adequate organ functions. The exclusion criteria were as follows: (1) has a history of autoimmune disease; (2) received previous immunotherapy; (3) has active infections; (4) received systemic immunosuppressive therapy. This study was approved by institutional review board of Sun Yat-sen University Cancer Center. Each participant signed an informed consent before participating to this study.
2.2 Study design and procedures
The procedures of this study are summarized in Figure 1. Briefly, patients fulfilling the inclusion and exclusion criteria would be assigned sequentially to receive 0.3 mg/kg, 1 mg/kg, 3 mg/kg, 10 mg/kg, and 240 mg toripalimab via intravenous infusion every 2 weeks. The primary objectives were to evaluate the safety and dose-limiting toxicity (DLT) of toripalimab. The secondary endpoints included anti-tumor activity, pharmacokinetics, immunogenicity, and pharmacodynamics.
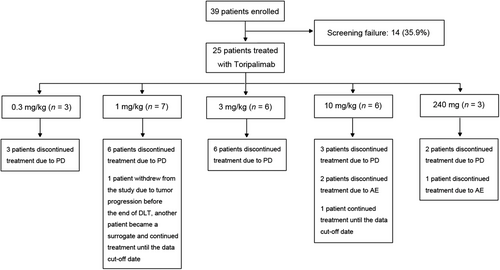
Consort diagram of the study. Twenty-five out of 39 screened patients were enrolled to receive toripalimab treatment. The study adopted the standard 3 + 3 design. Abbreviations: PD, progressed disease; AE, adverse event; DLT, dose-limiting toxicity
- Hepatic DLT definition
- - Alanine transaminase (ALT) or aspartate transaminase (AST) between 5 × upper limit of normal (ULN) and 10 × ULN, and persisting for at least 2 weeks
- - ALT or AST > 10 × ULN
- - Total bilirubin > 5 × ULN
- - ALT or AST > 3 × ULN, and total bilirubin > 2 × ULN
- Hematological DLT definition
- - Grade 3 thrombocytopenia with bleeding
- - Grade 4 thrombocytopenia
- - Grade 3 neutropenia with fever
- - Grade 4 neutropenia
- Other non-hematological DLT definition
- - Grade 2 uveitis or ophthalmodynia, not responding to topical treatment and not improving to grade 1 within 14 days, or need systemic treatment
- - Grade 3 non-dermatological TRAE, excepting those could be well controlled by hormone replacement therapy or those without significant sequelae
- - Grade 4 TRAE
- - Grade 3 or higher transfusion reaction would be discussed before a DLT was concluded
Tumor responses were evaluated according to RECIST version 1.1. Imaging assessment was performed every 6 weeks. Patients with progressive disease could continue receiving toripalimab treatment if they were considered to have potential benefit from further toripalimab treatment by investigators.
2.3 Pharmacokinetics and immunogenicity
For detection of drug serum concentration, serum samples were collected before receiving the first dose to sixth dose of toripalimab, and at the end of infusion up to 27 days after infusion. Intensive serum sample collection was arranged after the first dose and sixth dose of toripalimab. Toripalimab serum concentration was determined with an electrochemiluminescence method (biotinylated His-tag human PD-1 extracellular domain [Shanghai Junshi Biosciences, Shanghai, China] served as a capture, and ruthenylated His-tag human PD-1 [Shanghai Junshi Biosciences, Shanghai, China] served as a detector) using MESO™ QuickPlex SQ120 (Meso Scale Discovery, Maryland, USA). The method was validated with lower limit of quantification at 2.56 ng/mL.
For immunogenicity analysis, serum samples were collected before the first dose and every 14 days prior to dosing. An enhanced chemiluminescence method was developed and validated for the detection and quantitation of anti-toripalimab antibodies in human serum. In this assay, anti-drug antibodies (ADA) were compounded between biotinylated and ruthenylated toripalimab. For all assays, the samples were diluted at 1:10 in master mix and then incubated with labeled drug to allow for the formation of molecular complexes. The complexed samples were loaded into the wells of streptavidin-coated carbon-electron plate (Meso Scale Discovery, Maryland, USA). After the unbound materials were removed by washing, read buffer (Meso Scale Discovery, Maryland, USA) was added and the bound complexes were detected by reading chemiluminescence signals using MESO™ QuickPlex SQ120 (Meso Scale Discovery, Maryland, USA). Samples that produced signals higher than the screening cut point (SCP) S/N = 1.05 were considered positive samples and were subjected to the confirmation process in a competition assay in the presence of unlabeled toripalimab. The screening confirmation cut point (CCP) was determined to be 10.8%. If the response of the test sample was above CCP, it was reported as an ADA-positive sample. The corresponding toripalimab trough concentration was also evaluated in ADA-positive samples for neutralizing activity.
2.4 Pharmacodynamic and immunological assessment
The ability of toripalimab to occupy PD-1 receptor on peripheral T lymphocytes and peripheral blood mononuclear cells was assessed with flow cytometry using a FACS-Canto cytometer (BD Biosciences, Franklin Lakes, New Jersey, USA). All whole-blood samples were analyzed within 48 h of collection. A cocktail of mouse anti-human IgG4 pFc’-PE (Southern Biotech, Birmingham, Alabama, USA), APC mouse anti-CD45RA (BD Biosciences, Franklin Lakes, New Jersey, USA), FITC mouse anti-CD3ɛ (BD Biosciences, Franklin Lakes, New Jersey, USA), and PerCP/Cy5.5 anti-human CD8a (BioLegend, San Diego, CA, USA) was used. The results were analyzed with FlowJo (BD Biosciences, Franklin Lakes, New Jersey, USA) and GraphPad PRISM 5.0 (GraphPad, San Diego, CA, USA). Occupation above 80% was considered as full receptor occupation.
2.5 Immunohistochemistry assay
Archived tumor samples obtained prior to toripalimab treatment were used for PD-L1 and CD8 double immunohistochemistry staining by a rabbit anti-human PD-L1 antibody (1:800;SP142, spring bioscience, California,USA) and mouse anti-human CD8 antibody (1:30;CA1817T, Biorad, California, USA). PD-L1 expression on the membrane of both tumor cells and tumor-infiltrating immune cells was assessed by 2 certified pathologists. A positive PD-L1 expression was defined as ≥5% membrane staining of any intensity on tumor cells or tumor-infiltrating immune cells. Positive CD8 staining was defined as “++” or above by pathologists.
2.6 Statistical analyses
Safety analysis included all patients who received at least one dose of toripalimab, and efficacy analysis included patients who received at least one dose of toripalimab and at least one assessment of anti-tumor efficacy after the first dose. The confidence intervals of objective response rate (ORR) and disease control rate (DCR) were determined by using the exact Clopper and Pearson method. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Patients and treatments
A total of 25 patients were enrolled between 15th March 2016 and 27th September 2016 and treated with toripalimab. There were 3 (12.0%) patients in the 0.3 mg/kg group, 7 (28.0%) in the 1 mg/kg group, 6 (24.0%) in the 3 mg/kg group, 6 (24.0%) in the 10 mg/kg group, and 3 (12.0%) in the 240 mg group, respectively (Figure 1). Because 1 patient in the 1 mg/kg group was taken off the study due to tumor progression before the end of DLT observation period, thus, 1 substitute was enrolled in this group.
The baseline characteristics of the enrolled patients are listed in Table 1. The median age was 52 years (range: 26-65 years). All of these patients were diagnosed with treatment-refractory, stage IV malignant tumors at enrollment. The cancer types they suffered from included nasopharyngeal carcinoma (6/25, 24.0%), esophageal squamous cell carcinoma (6/25, 24.0%), gastric adenocarcinoma (5/25, 20.0%), melanoma (3/25, 12.0%), pancreatic adenocarcinoma (2/25, 8.0%), bile duct adenocarcinoma (1/25, 4.0%), pharyngeal squamous cell carcinoma (1/25, 4.0%), and lingual squamous cell carcinoma (1/25, 4.0%). Additionally, 16 (64.0%) patients had at least 2 prior lines of chemotherapy. Until data cut-off time of 24th January 2018, the median treatment period was 57 days (range: 1-550 days).The median dose intensity explored was 1.500 mg/kg/week (range: 0.128-5.000 mg/kg/week). The median follow-up time was 5.0 months (range: 1.5-19.8 months).
| Characteristic | No. of patients (%) |
|---|---|
| Gender | |
| Male | 20 (80.0) |
| Female | 5 (20.0) |
| ECOG PS | |
| 0 | 4 (16.0) |
| 1 | 21 (84.0) |
| Cancer type | |
| Nasopharyngeal carcinoma | 6 (24.0) |
| Esophageal squamous cell carcinoma | 6 (24.0) |
| Gastric adenocarcinoma | 5 (20.0) |
| Melanoma | 3 (12.0) |
| Pancreatic adenocarcinoma | 2 (8.0) |
| Bile duct adenocarcinoma | 1 (4.0) |
| Pharyngeal squamous cell carcinoma | 1 (4.0) |
| Lingual squamous cell carcinoma | 1 (4.0) |
| Previous lines of chemotherapy | |
| 1 | 9 (36.0) |
| 2 | 5 (20.0) |
| > 2 | 11 (44.0) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
3.2 Safety
All patients treated with toripalimab were included for safety analysis. TRAEs of any grade were observed in 22 (88.0%) patients. The common TRAEs that occurred in more than 10% patients were fatigue (16/25, 64.0%), rash (6/25, 24.0%), proteinuria (4/25, 14.0%), anorexia (4/25, 14.0%), diarrhea (3/25, 12.0%), pruritis (3/25, 12.0%), and hypothyroidism (3/25, 12.0%). No grade 3 or higher TRAEs were observed. No DLT, treatment-related serious adverse events (SAEs), or treatment-related death was observed. The details of TRAEs are shown in Table 2, and the grades of TRAEs are listed in Supplementary Table S1. Immune-related AE (irAE) occurred in 4 (16.0%) patients, including 2 patients with hypothyriodism, 2 with rash, and 2 with pruritis (Supplementary Table S2). Among them, only 1 patient with rash, and 1 patient with rash and pruritis received topical steroid treatment. No systemic steroid treatment was administered.
| Adverse events* | Total n (%) | 0.3 mg/kg n (%) | 1 mg/kg n (%) | 3 mg/kg n (%) | 10 mg/kg n (%) | 240 mg n (%) |
|---|---|---|---|---|---|---|
| Fatigue | 16 (64.0) | 3 (100) | 4 (57.1) | 6 (100) | 3 (50.0) | 0 (0) |
| Rash | 6 (24.0) | 1 (33.3) | 1 (14.3) | 1 (16.7) | 2 (33.3) | 1 (33.3) |
| Proteinuria | 4 (16.0) | 0 (0) | 2 (28.6) | 1 (16.7) | 0 (0) | 1 (33.3) |
| Anorexia | 4 (16.0) | 0 (0) | 1 (14.3) | 2 (33.3) | 1 (16.7) | 0 (0) |
| Diarrhea | 3 (12.0) | 0 (0) | 1 (14.3) | 1 (16.7) | 1 (16.7) | 0 (0) |
| Pruritis | 3 (12.0) | 0 (0) | 1 (14.3) | 1 (16.7) | 0 (0) | 1 (33.3) |
| Hypothyroidism | 3 (12.0) | 0 (0) | 1 (14.3) | 1 (16.7) | 1 (16.7) | 0 (0) |
| Nausea | 2 (8.0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (16.7) | 0 (0) |
| Leukopenia | 1 (4.0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| ALT elevation | 1 (4.0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) |
| Tinnitus | 1 (4.0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Fever | 1 (4.0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) |
| Dysphonia | 1 (4.0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Hyperthyroidism | 1 (4.0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) |
| Facial edema | 1 (4.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) |
| AST elevation | 1 (4.0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) |
- * All toripalimab-related adverse events were grade 1 or 2.
- Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
3.3 Efficacy
One patient in the 1 mg/kg group discontinued treatment due to AE before assessment of anti-tumor efficacy of toripalimab. The other 24 patients were included for efficacy analysis. Three (12.5%) patients, including 1 melanoma patient from the 10 mg/kg group, 1 nasopharyngeal carcinoma patient from the 0.3 mg/kg group, and 1 esophageal squamous cell carcinoma patient from the 3 mg/kg group experienced partial remission (PR). Two (8.3%) patients achieved stable disease (SD). Nineteen (79.2%) patients had progressive disease (PD). The ORR was 12.5% (95% confidence interval [CI]: 2.7%-32.4%), and DCR was 20.8% (95% CI: 7.1%-42.2%). Notably, the melanoma patient with PR experienced 100% shrinkage of target lesions, and non-complete remission (CR)/non-PR of non-target lesions, and she was still under toripalimab treatment when the study ended. She was on toripalimab treatment for 2 years, and maintained PR when she stopped toripalimab treatment. The spider plots and waterfall plots are shown in Supplementary Figure S1.
3.4 Pharmacokinetics
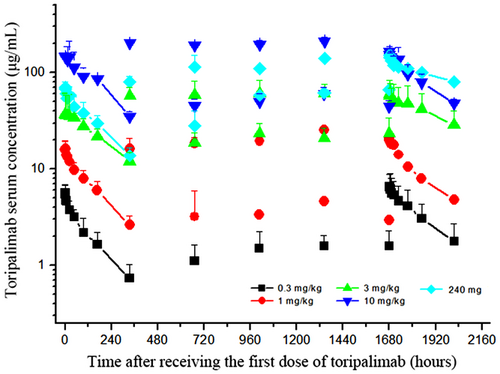
The mean serum concentration-time profiles of toripalimab after single dose and multiple doses are shown in Figure 2. Toripalimab serum concentration reached maximum within 2 h after infusion and was then decreased slowly. A dose-dependent linear pharmacokinetic profile was observed. After six consecutive doses, toripalimab reached steady state for trough concentrations, with accumulation factor ranged from 1.4 to 1.9. The measured serum half-life of toripalimab was 150-222 h after a single infusion and 188-525 h after multi-dose infusions (Figure 2). The pharmacokinetic parameters following single dose and multiple doses are shown in Table 3.
| 0.3 mg/kg | 1 mg/kg | 3 mg/kg | 10 mg/kg | 240 mg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic parameter | C1 | C6 | C1 | C6 | C1 | C6 | C1 | C6 | C1 | C6 |
| Cmax (µg/mL) | 5.8 ± 1.3 | 6.6 ± 2.2 | 16.5 ± 3.4 | 20.8 | 48.2 ± 18.9 | 63.5 ± 21.1 | 205.8 ± 35.8 | 257.8 ± 110.4 | 71.6 ± 8.7 | 152.6 |
| Tmax (h) | 1-2 | 0-2 | 0-2 | 2 | 0-96 | 0-6 | 0-24 | 0-2 | 0-2 | 0 |
| AUC0-t (µg/mL × h) | 655 ± 199 | 1099 ± 448 | 2148 ± 408 | 3163 | 7722 ± 1472 | 13723 ± 6478 | 34708 ± 6561 | 49361 ± 24835 | 11400 ± 5886 | 34212 |
| AUC0-inf (µg/mL × h) | 816 ± 260 | 1584 ± 718 | 2908 ± 678 | 4614 | 10967 ± 2331 | 26948 ± 9658 | 50817 ± 18764 | 104767 ± 76949 | 17243 ± 5420 | 95200 |
| t1/2 (h) | 151 ± 24 | 188 ± 5 | 160 ± 30 | 211 | 200 ± 36 | 338 ± 79 | 212 ± 84 | 331 ± 125 | 222 ± 27 | 525 |
| CI (mL/h/kg) | 0.39 ± 0.11 | 0.21 ± 0.10 | 0.36 ± 0.08 | 0.22 | 0.29 ± 0.08 | 0.12 ± 0.04 | 0.22 ± 0.06 | 0.14 ± 0.09 | 14.76 ± 4.05 | 2.52 |
| Vd (mL/kg) | 85.4 ± 31.3 | 57.0 ± 24.5 | 81.2 ± 15.1 | 66.0 | 80.5 ± 14.1 | 60.7 ± 27.0 | 61.1 ± 13.5 | 58.1 ± 29.0 | 4640.9 ± 890.6 | 1909.8 |
- Abbreviations: Cmax, maximum concentration; Tmax, peak time; AUC, area under the curve; CI, consistency index; Vd, apparent volume of distribution.
- The data of Tmax are presentd as range, and the data of other Pharmacokinetic parameters are presentd as mean ±standard deviation.
Anti-drug antibody was detected in 1 (33.3%) patient in the 0.3 mg/kg group, 3 (42.9%) in the 1 mg/kg group, and 1 (16.7%) in the 3 mg/kg group, but not detected in the 10 mg/kg group and the 240 mg group. However, none of the ADA-positive patients had significantly accelerated toripalimab clearance or reduced toripalimab trough concentration, indicating the lack of neutralizing activity.
3.5 Pharmacodynamics
Most patients, including patients from the 0.3 mg/kg group, maintained complete PD-1 receptor occupancy on activated T cells (CD3+CD45RA− cells) through the observation period (Supplementary Figure S2). Similar trends of PD-1 receptor occupancy on activated CD8+ cells (CD3+CD8+CD45RA− cells) and activated CD4+ cells (CD3+CD8−CD45RA− cells) were observed. No significant changes were observed for lymphocyte subgroups or activation status after toripalimab treatment (Supplementary Figure S3).
3.6 PD-L1 expression and presence of TIL
PD-L1 and CD8 double immunohistochemistry staining was performed on available tumor biopsies from 15 patients. Among them, 9 (60%) patients were PD-L1 positive, and 6 (40%) had CD8+ cells. Notably, all 3 responders were CD8 positive, and 2 of them were also PD-L1 positive.
4 DISCUSSION
We report the safety, efficacy, pharmacokinetic and pharmacodynamic profiles of toripalimab, an anti-PD-1 antibody, in 25 Chinese patients with refractory cancers from a dose-escalation phase I trial.
The pharmacokinetic and pharmacodynamic profiles of anti-PD-1 antibodies in Chinese patients were only reported by limited studies [15, 18]. Chinese patients generally have a smaller body mass index compared with western patients. In addition, Chinese patients have different genetic backgrounds from western patients. Considering those ethnic differences, it is important to explore the pharmacokinetic and pharmacodynamic profiles of anti-PD-1 antibodies in Chinese patients. The measured serum half-life of toripalimab was 150-222 h after a single-dose infusion, and 188-525 h after multiple-dose infusion. These results are consistent with a previous report of toripalimab [18]. The half-lives of toripalimab are numerically shorter than those of nivolumab and pembrolizumab [15, 20-22]. These differences could be resulted from ethnic or drug differences. Although there were pharmacokinetic differences, according to previous reports, the efficacy results of the three anti-PD-1 antibodies in refractory gastric adenocarcinoma were comparable [19, 23-25].
Toripalimab is a novel anti-PD-1 antibody, which differs from well-described ones: nivolumab and pembrolizumab. Three-dimensional structure analysis by X-ray crystallography has shown differential antigen-binding sites on PD-1 for toripalimab, nivolumab, and pembrolizumab [26]. Toripalimab binds to the FG loop of the PD-1 receptor, whereas pembrolizumab binds to the C'D loop and nivolumab binds to the N terminal loop of PD-1. In an in vitro antigen recall study, toripalimab and nivolumab promoted T-cell proliferation similarly, whereas toripalimab induced a stronger interferon-γ cytokine production [16]. Both pembrolizumab and toripalimab were approved in China in 2018 for the 2nd-line treatment of metastatic melanoma, with comparable clinical efficacy (ORR 16.7% for pembrolizumab [27] and 17.3% for toripalimab [18]), while nivolumab was approved in China earlier in 2018 for 2nd-line treatment of advanced non-small cell lung cancer.
In the present study, anti-drug antibody to toripalimab was detected in 20% of patients (8.3% in another study [18]). However, none of them showed neutralizing activity. There were also no significant differences in the rates of AEs between ADA-positive and ADA-negative patients.
We observed that no DLTs occurred in any toripalimab treatment group, and the maximum tolerated dose was not determined. All types of TRAEs were reported previously, no new safety concern was raised. There was no grade 3 or higher TRAE, no treatment-related SAE, and no treatment-related death. Toripalimab had a well-manageable safety profile. Actually, its safety profile had also been proved by several larger phase II trials [18, 19].
In the present study, one PR with 100% shrinkage of target lesions and with non-PR and non-CR status of non-target lesions was observed in a melanoma patient from the 10 mg/kg group. In addition, one pharyngeal carcinoma patient in the 0.3 mg/kg group and one esophageal carcinoma patient in the 3 mg/kg group experienced PR. For the patient with 100% shrinkage of target lesions, the toripalimab treatment was stopped after a 2-year period of treatment. Then, after further maintenance of PR for another one year without any anti-tumor therapy, the disease progressed. This patient showed durable response to toripalimab. PD-L1 immunohistological staining was performed in tumor slides. PD-L1 was positive, with a membrane expression percentage of > 20%. The CD8 staining was “+++”. Furthermore, all three responders were CD8 positive, and 2 of them were also PD-L1 positive. PD-L1 has been proposed to be a predictive marker for benefit from anti-PD-1 treatment [19]. Other effective indicators for benefit from toripalimab had also been reported, including tumor-infiltrating lymphocytes and tumor mutational burden [18, 19]. Efficient biomarkers are helpful for patient selection in future clinical trial design and clinical treatment. In addition, a variety of combinations of toripalimab with other treatments are already under exploration in clinical trials.
5 CONCLUSIONS
This phase I clinical trial showed the safety, efficacy, pharmacokinetic and pharmacodynamic profiles of toripalimab. Toripalimab was well tolerated with mainly grade 2 or lower TRAEs. Additionally, no DLT or treatment-related death was observed. The serum half-life of toripalimab was 150-222 h after a single infusion and 188-525 h after multi-dose infusions. Durable response was observed in certain patients. Toripalimab is a promising anti-PD-1 antibody, and many clinical trials in various malignancies are ongoing.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This clinical trial was approved by institutional review board of Sun Yat-sen University Cancer Center (Number A2016-008-01). Each participant signed an informed consent before participating to this study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
COMPETING INTERESTS
SY declares employment with Shanghai Junshi Bioscience Co., Ltd. All remaining authors declare that they have no competing interests.
FUNDING
This study was sponsored by Shanghai Junshi Biosciences Co., Ltd. and supported, in part, by National Key R&D Program of China (2018YFC1313300); Science and Technology Program of Guangdong (2019B020227002); CAMS Innovation Fund for Medical Sciences (2019-I2M-5-036); National Natural Science Foundation of China (81930065); Natural Science Foundation of Guangdong Province (2014A030312015); Science and Technology Program of Guangdong (2019B020227002); Science and Technology Program of Guangzhou (201904020046, 201803040019, 201704020228); Guangdong Basic and Applied Basic Research Foundation (2019A1515110171).
AUTHORS' CONTRIBUTIONS
RHX, FHW, and SY designed the study. XLW, CR, YZ, HYZ, BYZ, ZQW, MZQ, DSZ, HYL, FW conducted the study and collected the data. XLW, CR, FHW, SY analyzed the data and interpreted the results. XLW, CR, SY and RHX wrote the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We gratefully thank the patients and their families for participating in this study.




