A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis
Abstract
Background
Low-grade endometrial stromal sarcoma (LG-ESS) is a rare tumor that lacks a prognostic prediction model. Our study aimed to develop a nomogram to predict overall survival of LG-ESS patients.
Methods
A total of 1172 patients confirmed to have LG-ESS between 1988 and 2015 were selected from the Surveillance, Epidemiology and End Results (SEER) database. They were further divided into a training cohort and a validation cohort. The Akaike information criterion was used to select variables for the nomogram. The discrimination and calibration of the nomogram were evaluated using concordance index (C-index), area under time-dependent receiver operating characteristic curve (time-dependent AUC), and calibration plots. The net benefits of the nomogram at different threshold probabilities were quantified and compared with those of the International Federation of Gynecology and Obstetrics (FIGO) criteria-based tumor staging using decision curve analysis (DCA). Net reclassification index (NRI) and integrated discrimination improvement (IDI) were also used to compare the nomogram's clinical utility with that of the FIGO criteria-based tumor staging. The risk stratifications of the nomogram and the FIGO criteria-based tumor staging were compared.
Results
Seven variables were selected to establish the nomogram for LG-ESS. The C-index (0.814 for the training cohort and 0.837 for the validation cohort) and the time-dependent AUC (> 0.7) indicated satisfactory discriminative ability of the nomogram. The calibration plots showed favorable consistency between the prediction of the nomogram and actual observations in both the training and validation cohorts. The NRI values (training cohort: 0.271 for 5-year and 0.433 for 10-year OS prediction; validation cohort: 0.310 for 5-year and 0.383 for 10-year OS prediction) and IDI (training cohort: 0.146 for 5-year and 0.185 for 10-year OS prediction; validation cohort: 0.177 for 5-year and 0.191 for 10-year OS prediction) indicated that the established nomogram performed significantly better than the FIGO criteria-based tumor staging alone (P < 0.05). Furthermore, DCA showed that the nomogram was clinically useful and had better discriminative ability to recognize patients at high risk than the FIGO criteria-based tumor staging.
Conclusions
A prognostic nomogram was developed and validated to assist clinicians in evaluating prognosis of LG-ESS patients.
Abbreviations
-
- AIC
-
- Akaike information criterion
-
- AUC
-
- area under the time-dependent receiver operating characteristic curves
-
- C-index
-
- concordance index
-
- DCA
-
- decision curve analysis
-
- ESS
-
- endometrial stromal sarcoma
-
- HG-ESS
-
- high-grade endometrial stromal sarcoma
-
- Hys – BSO
-
- hysterectomy without bilateral salpingectomy and ovariectomy oophorectomy
-
- Hys + BSO
-
- hysterectomy with bilateral salpingectomy and ovariectomy oophorectomy
-
- Hys NOS
-
- hysterectomy, not specified
-
- ICD
-
- International Classification of Diseases
-
- IDI
-
- integrated discrimination improvement
-
- L/E/OTH
-
- local surgery, exenteration, and other surgery types
-
- LG-ESS
-
- low-grade endometrial stromal sarcoma
-
- NRI
-
- net reclassification index
-
- OS
-
- overall survival
-
- SEER
-
- Surveillance, Epidemiology and End Results
-
- TP
-
- total points
-
- VIF
-
- variance inflation factor
-
- WHO
-
- World Health Organization
1 BACKGROUND
Low-grade endometrial stromal sarcoma (LG-ESS) is a rare malignant mesenchymal tumor but the second most common type of uterine sarcomas [1], representing approximately 1% of all uterine malignancies [2], and is diagnosed by histology [3]. LG-ESS and high-grade ESS (HG-ESS) were previously considered to be subtypes of endometrial stromal sarcoma (ESS) [4]. However, in 2014, the updated World Health Organization (WHO) classification of soft tissue sarcoma recognized LG-ESS and HG-ESS as entities with distinct histopathological characteristics [5]. Many studies have shown that LG-ESS and HG-ESS differ greatly in prognosis [4, 6, 7]. All these findings suggest that LG-ESS should be studied independently, rather than analyzing ESS without recognizing pathological type [8-11]. However, current prognostic evidence for LG-ESS is still mainly based on studies of ESS, most of which had small sample sizes. Clinical measures for the prognosis assessment of LG-ESS need to be determined. LG-ESS is more common than HG-ESS [6, 7, 12], and some patients with LG-ESS have poor outcomes [4], suggesting that careful evaluation of their prognosis is essential. However, there is no individual prediction model to evaluate the prognosis of LG-ESS patients.
The International Federation of Gynecology and Obstetrics (FIGO) staging system is most frequently used to evaluate the prognosis of ESS patients. However, its major limitations include low accuracy, disregard of other factors (such as age), and poor performance in predicting individual survival risk [13-15]. Therefore, a personalized prediction model is needed for patients with LG-ESS.
The nomogram has been widely used as a predictive method in oncology in recent years [15-18]. It meets requirements for an integrated model, plays a part in the drive towards personalized medicine [15], and is convenient for clinicians to use in prognosis prediction [15, 19, 20]. In the present study, using a large LG-ESS dataset from the Surveillance, Epidemiology and End Results (SEER) database, we aimed to establish a nomogram to predict prognosis of LG-ESS patients.
2 MATERIALS AND METHODS
2.1 Patient selection
Women diagnosed with ESS between January 1988 and November 2015 were initially identified from the SEER database, in which all deposited cases came from the United States, using SEER*Stat 8.35. LG-ESS was defined as grade I/II, well and moderately differentiated tumors [6, 21]. The inclusion criteria were as follows: (i) the International Classification of Diseases (ICD) code O-3 morphology 8930/3 or 8931/3; (ii) SEER site recodes of ICD-O-3 included the corpus uteri/uterus not specified; (iii) active follow-up to ensure reliable patient status; (iv) ESS as the only or first primary tumor that was confirmed by histology. The exclusion criteria were as follows: (i) missing information on ethnicity, American geographic region, marital status, lymph node status, tumor stage (determined using the FIGO criteria-based tumor staging), surgery type, lymphadenectomy, adjuvant radiotherapy, or adjuvant chemotherapy; (ii) patient died within 1 month or was followed up less than 1 month since initial diagnosis.
2.2 Cohort definition and variable recode
The patients were divided into the training and validation cohorts with a ratio of 7:3 using the R function “createDataPartition” to ensure that outcome events were distributed randomly between the two cohorts. The training cohort was used to screen variables and construct the model. The validation cohort was used to validate the results obtained using the training cohort. Eleven variables from the SEER database were included: age (at diagnosis), ethnicity, geographic region, marital status (at diagnosis), tumor size, lymph node status, tumor stage, surgery type, lymphadenectomy, radiotherapy, and chemotherapy. Ethnicity, region, marital status, tumor size, and surgery type were recorded. Surgery types included hysterectomy with bilateral salpingectomy and ovariectomy oophorectomy (Hys + BSO); hysterectomy without bilateral salpingectomy and ovariectomy oophorectomy (Hys – BSO); hysterectomy, not specified (Hys NOS); local surgery, exenteration, or other surgery types (L/E/OTH). The tumor stage was recoded based on the 2009 FIGO staging criteria: localized stage corresponded to FIGO stage I, and regional stage corresponded to FIGO stages II and III, and distant stage corresponded to FIGO stage IV [21, 22]. Univariate and multivariate Cox regression analyses were performed for all 11 variables, and variables with P < 0.05 in both univariate and multivariate Cox regression were identified as independent risk factors.
2.3 Statistical analysis
The stepwise regression based on the Akaike information criterion minimum was used to select variables for inclusion in the nomogram [15]. The 5-/10-year overall survival (OS) probabilities were estimated using the nomogram. Concordance index (C-index) and area under the time-dependent receiver operating characteristic curve (time-dependent AUC) calculated by bootstrapping were used to evaluate discriminative ability. Calibration plots were used to evaluate calibrating ability. C-index and AUC values vary from 0.5 to 1.0, where 0.5 represents random chance and 1.0 indicates a perfect fit. Typically, C-index and AUC values greater than 0.7 suggest a reasonable estimation.
The net reclassification index (NRI), integrated discrimination improvement (IDI), and decision curve analysis (DCA) were used to evaluate the clinical benefits and utility of the nomogram compared with a FIGO criteria-based tumor staging alone. NRI and IDI are two alternatives to AUC to assess improvement in risk prediction and measure the usefulness of a new model [23, 24]. DCA is a method for evaluating the clinical benefit of alternative models [25, 26] and was applied to nomograms by quantifying net benefits at different threshold probabilities. The curves of treat-all-patients scheme (representing the highest clinical costs) and the treat-none scheme (representing no clinical benefit) were plotted as two references. Risk stratifications with the nomogram and the FIGO criteria-based tumor staging were compared using Kaplan-Meier method and the Cox model. The cut-off point for risk stratifications was selected using X-tile [27].
OS was the endpoint of interest in the present study. It was calculated from diagnosis to death of all causes or to date of last follow-up in November 2015. Data from patients alive at the last follow-up were censored. Inclusion of covariates in the nomogram followed Harrell's guideline (the number of events should exceed the number of covariates by at least 10 folds) [15]. Meanwhile, the variance inflation factor (VIF) was assessed among the covariates in the nomogram, and VIF > 4.0 was interpreted as indicating multicollinearity. Variables with VIF > 4.0 were not included in the final model analysis. Statistical differences of distribution in age between the training and validation cohorts were evaluated using the Wilcoxon-test, and other variables were analyzed by using the Chi-square test. All P values were two-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the R programming language and environment (http://www.r-project.org/).
3 RESULTS
3.1 Characteristics of patients and disease
A total of 1172 patients were identified as having LG-ESS and randomly divided into a training cohort and a validation cohort by a ratio of 7:3. The median follow-up was 127 [interquartile range (IQR): 62-189] months in the whole population, 127 (IQR: 62-190) months in the training cohort, and 128 (IQR: 60-186) months in the validation cohort. The demographic and clinical characteristics of these LG-ESS patients are summarized in Table 1. In the whole population, training, and validation cohorts, the median ages of patients with LG-ESS were 48 [interquartile range (IQR): 42–53], 48 (IQR: 43-54), and 47 (IQR: 42-53) years, respectively. Patients of white ethnicity (75.5%), those from the Pacific coast/Asian region (51.4%), and married women (63.3%) constituted the majority of the whole LG-ESS population. Moreover, the whole population had a relatively low rate of lymph node metastasis (12.6%). However, a third of the population received lymphadenectomy, and 9.0% of them underwent positive lymphadenectomy. More than 70% of patients had local tumor invasion. Hys + BSO (76%) was the main type of surgery underwent by LG-ESS patients. The training and validation cohorts were comparable in terms of demographic and clinical characteristics (P > 0.05).
| Characteristic | Whole population [cases (%)] | Training cohort [cases (%)] | Validation cohort [cases (%)] | P value |
|---|---|---|---|---|
| Total | 1172 | 821 | 351 | |
| Ethnicity | 0.185 | |||
| White | 885 (75.5) | 630 (76.7) | 255 (72.6) | |
| Black | 147 (12.5) | 102 (12.4) | 45 (12.8) | |
| Asian/Alaska Indian | 140 (11.9) | 89 (10.8) | 51 (14.5) | |
| American geographic region | 0.526 | |||
| Eastern | 325 (27.7) | 236 (28.7) | 89 (25.4) | |
| Northern | 170 (14.5) | 115 (14.0) | 55 (15.7) | |
| Pacific/Asian | 602 (51.4) | 415 (50.5) | 187 (53.3) | |
| Southern | 75 (6.4) | 55 (6.70) | 20 (5.7) | |
| Marital status | 0.362 | |||
| Single/unmarried | 232 (19.8) | 167 (20.3) | 65 (18.5) | |
| Married | 742 (63.3) | 510 (62.1) | 232 (66.1) | |
| Divorced/separated | 137 (11.7) | 96 (11.7) | 41 (11.7) | |
| Widowed | 61 (5.2) | 48 (5.8) | 13 (3.7) | |
| Tumor size | 0.151 | |||
| < 5 cm | 324 (27.6) | 223 (27.2) | 101 (28.8) | |
| 5-10 cm | 335 (28.6) | 235 (28.6) | 100 (28.5) | |
| ≥10 cm | 156 (13.3) | 121 (14.7) | 35 (10.0) | |
| Not specified | 357 (30.5) | 242 (29.5) | 115 (32.8) | |
| Lymph node metastasis | 0.950 | |||
| No | 1024 (87.4) | 717 (87.3) | 307 (87.5) | |
| Yes | 148 (12.6) | 104 (12.7) | 44 (12.5) | |
| Tumor stage * | 0.774 | |||
| Local | 832 (71.0) | 581 (70.8) | 251 (71.5) | |
| Regional | 229 (19.5) | 159 (19.4) | 70 (19.9) | |
| Distant | 111 (9.5) | 81 (9.9) | 30 (8.5) | |
| Surgery | 0.902 | |||
| No surgery | 22 (1.9) | 17 (2.1) | 5 (1.4) | |
| Hys + BSO | 891 (76.0) | 619 (75.4) | 272 (77.5) | |
| Hys − BSO | 193 (16.5) | 139 (16.9) | 54 (15.4) | |
| Hys NOS | 30 (2.6) | 21 (2.6) | 9 (2.6) | |
| L/E/OTH | 36 (3.1) | 25 (3.0) | 11 (3.1) | |
| Radiotherapy | 0.629 | |||
| No | 996 (85.0) | 695 (84.7) | 301 (85.8) | |
| Yes | 176 (15.0) | 126 (15.3) | 50 (14.2) | |
| Chemotherapy | 0.980 | |||
| No | 1125 (96.0) | 788 (96.0) | 337 (96.0) | |
| Yes | 47 (4.0) | 33 (4.0) | 14 (4.0) | |
| Lymphadenectomy | 0.185 | |||
| No | 782 (66.7) | 538 (65.5) | 244 (69.5) | |
| Yes | 390 (33.3) | 283 (34.5) | 107 (30.5) |
- LG-ESS: low-grade endometrial stromal sarcoma; Hys + BSO: hysterectomy with bilateral salpingectomy and ovariectomy oophorectomy; Hys – BSO: hysterectomy without bilateral salpingectomy and ovariectomy oophorectomy; Hys NOS: hysterectomy, not specified; L/E/OTH: local surgery, exenteration, and other surgery types.
- * The International Federation of Gynecology and Obstetrics (FIGO) criteria-based tumor staging was used to determine tumor stage.
3.2 Nomogram variable screening
According to the stepwise regression results, the model containing age, marital status, tumor size, tumor stage, chemotherapy, radiotherapy, and lymphadenectomy had minimal AIC value in the training cohort. The VIF values were all < 4, indicating that no collinearity existed between screened variables. In the univariate regression analysis, eight variables (age, marital status, tumor size, tumor stage, surgery type, chemotherapy, radiotherapy, and lymphadenectomy) were significantly associated with OS. In the multivariate Cox regression analysis, age, marital status, tumor size, tumor stage, chemotherapy, and lymphadenectomy were identified as independent prognostic factors for LG-ESS (Table 2).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (years) | 1.064 | 1.050-1.078 | <0.001 | 1.052 | 1.034-1.071 | <0.001 |
| Race | ||||||
| White | 1.000 | 1.000 | ||||
| Black | 1.359 | 0.819-2.253 | 0.235 | 1.476 | 0.821-2.653 | 0.193 |
| Asian/Alaska Indian | 0.484 | 0.212-1.105 | 0.085 | 0.621 | 0.263-1.466 | 0.276 |
| American geographic region | ||||||
| Eastern | 1.000 | 1.000 | ||||
| Northern | 0.807 | 0.442-1.472 | 0.484 | 0.571 | 0.298-1.093 | 0.091 |
| Pacific/Asian | 1.029 | 0.656-1.616 | 0.901 | 1.041 | 0.640-1.691 | 0.872 |
| Southern | 1.088 | 0.527-2.247 | 0.820 | 1.375 | 0.635-2.977 | 0.420 |
| Marital status | ||||||
| Single/unmarried | 1.000 | 1.000 | ||||
| Married | 0.963 | 0.571-1.623 | 0.886 | 1.051 | 0.588-1.879 | 0.866 |
| Divorced/separated | 1.032 | 0.487-2.187 | 0.935 | 1.634 | 0.743-3.598 | 0.222 |
| Widowed | 4.413 | 2.349-8.291 | <0.001 | 2.260 | 1.015-5.032 | 0.046 |
| Tumor size | ||||||
| < 5 cm | 1.000 | 1.000 | ||||
| 5-10 cm | 2.171 | 1.254-3.760 | 0.006 | 2.915 | 1.624-5.232 | <0.001 |
| ≥10 cm | 3.241 | 1.748-6.011 | <0.001 | 3.122 | 1.594-6.115 | <0.001 |
| Not specified | 1.571 | 0.898-2.747 | 0.113 | 1.808 | 0.996-3.281 | 0.051 |
| Lymph node metastasis | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 1.107 | 0.682-1.796 | 0.681 | 0.682 | 0.397-1.170 | 0.165 |
| Tumor stage * | ||||||
| Local | 1.000 | 1.000 | ||||
| Regional | 1.830 | 1.149-2.914 | 0.011 | 1.536 | 0.895-2.638 | 0.120 |
| Distant | 4.034 | 2.563-6.349 | <0.001 | 3.059 | 1.752-5.344 | <0.001 |
| Surgery | ||||||
| No surgery | 1.000 | 1.000 | ||||
| Hys + BSO | 0.291 | 0.127-0.668 | 0.004 | 1.047 | 0.411-2.665 | 0.924 |
| Hys − BSO | 0.134 | 0.049-0.370 | <0.001 | 0.824 | 0.259-2.628 | 0.744 |
| Hys NOS | 0.456 | 0.128-1.621 | 0.225 | 0.716 | 0.156-3.296 | 0.668 |
| L/E/OTH | 0.441 | 0.134-1.446 | 0.176 | 0.633 | 0.175-2.290 | 0.486 |
| Radiotherapy | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 1.673 | 1.087-2.576 | 0.020 | 1.518 | 0.932-2.473 | 0.093 |
| Chemotherapy | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 9.351 | 5.781-15.130 | <0.001 | 7.461 | 4.182-13.311 | <0.001 |
| Lymphadenectomy | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 0.649 | 0.421-1.000 | 0.050 | 0.587 | 0.367-0.938 | 0.026 |
- LG-ESS: low-grade endometrial stromal sarcoma; Hys + BSO: hysterectomy with bilateral salpingectomy and ovariectomy oophorectomy; Hys – BSO: hysterectomy without bilateral salpingectomy and ovariectomy oophorectomy; Hys NOS: hysterectomy, not specified; L/E/OTH: local surgery, exenteration, and other surgery types.
- * The International Federation of Gynecology and Obstetrics (FIGO) criteria-based tumor staging was used to determine tumor stage.
3.3 Nomogram construction and validation
We constructed a nomogram for LG-ESS according to the variables screened. The top four factors ranked by the standard deviation (SD) along nomogram scales for the nomogram model were age (SD: 14.71), tumor size (SD: 12.91), chemotherapy (SD: 10.61), and tumor stage (SD: 8.51). Figure 1 shows an example of using the nomogram to predict survival probability of a given patient. The total score was determined based on the individual scores calculated using the nomogram; most patients in the present study had total risk points ranged from 260 to 400.
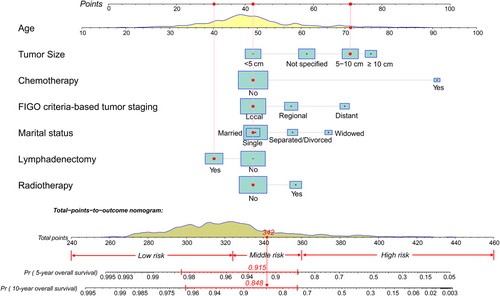
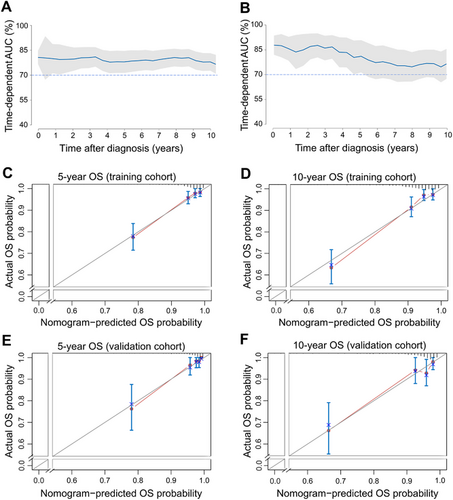
The C-index value was 0.814 [95% confidence interval (CI) = 0.769-0.860] in the training cohort and 0.837 (95% CI = 0.781-0.893) in the validation cohort. The time-dependent AUC was > 0.7 for the prediction of OS within 10 years in both the training and validation cohorts (Figure 2), indicating favorable discrimination by the nomogram. The calibration curves of the nomogram showed high consistencies between the predicted and observed survival probability in both the training and validation cohorts (Figure 2). In summary, the nomogram for LG-ESS had considerable discriminative and calibrating abilities.
3.4 Clinical value of the nomogram compared with the FIGO criteria-based tumor staging
The changes in C-index, NRI, and IDI were used to compare the accuracy between the nomogram and the FIGO criteria-based tumor staging alone. While using the nomogram in the training cohort, the C-index was 0.163 (95% CI = 0.107-0.209, P < 0.001), the NRI for the 5- and 10-year OS were 0.271 (95% CI = 0.078-0.44) and 0.433 (95% CI = 0.276-0.595), and the IDI values for 5- and 10-year OS were 0.146 (95% CI = 0.08-0.247, P < 0.001) and 0.185 (95% CI = 0.121-0.282, P < 0.001) (Table 3). These results were validated in the validation cohort (Table 3), indicating that the nomogram predicted prognosis with greater accuracy than the FIGO criteria-based tumor staging.
| Training cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|
| Index | Estimate | 95% CI | P value | Estimate | 95% CI | P value |
| NRI (vs. the FIGO criteria-based tumor staging) | ||||||
| For 5-year OS | 0.271 | 0.078-0.440 | 0.310 | 0.030-0.584 | ||
| For 10-year OS | 0.433 | 0.276-0.595 | 0.383 | 0.137-0.616 | ||
| IDI (vs. the FIGO criteria-based tumor staging) | ||||||
| For 5-year OS | 0.146 | 0.080-0.247 | <0.001 | 0.177 | 0.086-0.377 | <0.001 |
| For 10-year OS | 0.185 | 0.121-0.282 | <0.001 | 0.191 | 0.119-0.356 | <0.001 |
| C-index | ||||||
| The nomogram | 0.814 | 0.769-0.860 | 0.837 | 0.781-0.893 | ||
| The FIGO criteria-based tumor staging | 0.652 | 0.599-0.704 | 0.685 | 0.616-0.754 | ||
| Change | 0.163 | 0.107-0.209 | <0.001 | 0.152 | 0.125-0.194 | <0.001 |
- FIGO, the International Federation of Gynecology and Obstetrics.
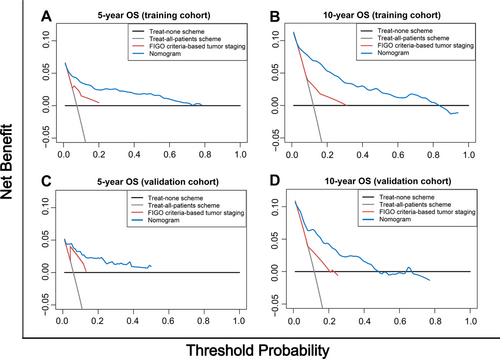
The clinical benefits of the nomogram were compared with those of the FIGO criteria-based tumor staging. DCA curves showed that the nomogram could better predict the 5- and 10-year OS, as it added more net benefits compared with the FIGO criteria-based tumor staging for almost all threshold probabilities in both the training and validation cohorts, and with both the treat-all-patients scheme and the treat-none scheme (Figure 3).
3.5 Risk stratification based on the nomogram
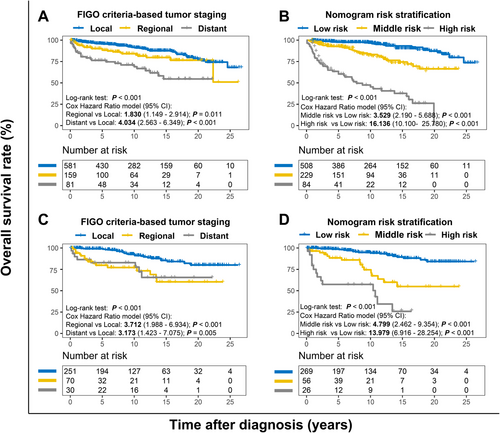
We finally made a risk stratification based on total points calculated using the nomogram. Patients with LG-ESS were divided into three risk groups[27]: low risk (total points < 325), middle risk (325 ≤ total points < 360), and high risk (total points ≥ 360). The Kaplan-Meier OS curves showed great discrimination among the three risk groups, whereas the FIGO criteria-based tumor staging had limited ability to recognize high-risk patients in both the training and validation cohorts (Figure 4).
4 DISCUSSION
LG-ESS is a relatively rare tumor, with little clinical evidence on its prognosis. Therefore, we constructed a nomogram to predict the prognosis of patients with LG-ESS. The validation of the nomogram showed that it had good discriminative and calibration capabilities. Seven variables were selected by the stepwise regression based on AIC minimum and incorporated into the nomogram. Measured by standard deviation along nomogram scales, age was the most important prognostic factor, followed by chemotherapy, tumor size, and tumor stage.
Previous studies on ESS had proposed some factors potentially affecting the OS of patients with LG-ESS, such as lymph node metastasis and adjuvant therapies. These factors were fully considered in the present study. Machida et al. [8] and Yoon et al. [28] reported that lymph node metastasis was associated with short OS among ESS patients. However, Seagle et al. [6] and Shah et al. [10] demonstrated that lymph node metastasis was not a significant prognostic factor for OS in LG-ESS patients, which was consistent with our results. Seagle et al. [6] proposed that the analysis of lymph node metastasis may be underpowered when the sample size was small. It should also be noticed that lymphadenectomy was not performed in all LG-ESS patients [4, 10, 29, 30], indicating that some lymph nodes were not sampled and might resulted in a false-negative inference. In addition, lymph node invasion was not the major route of metastasis in LG-ESS patients [30], and the patients with lymph node metastasis may have slow disease progression because of the tumor's indolent growth[12]. These features of LG-ESS may differ from gastric and colorectal cancers whose lymph node metastasis occurs more frequently and is associated with poor prognosis [31, 32]. Due to above reasons, lymph node metastasis may be a weak predictor for OS in patients with LG-ESS.
Adjuvant therapy was another controversial factor. Adjuvant radiotherapy was shown to improve the local control of ESS in many retrospective studies [9, 33-36]. In the present study, the inclusion of adjuvant radiotherapy decreased the AIC value of the nomogram, suggesting its value in predicting the OS of LG-ESS patients. Two retrospective studies found that adjuvant chemotherapy was not associated with survival of patients with LG-ESS [37, 38]. However, a National Cancer Database study showed that it was a harmful factor for OS [6]. In the present study, our nomogram also demonstrated that adjuvant chemotherapy was an important detrimental factor (Table 2, Figure 1), whose effect may exceed that of adjuvant radiotherapy. Given these results, we caution against the excessive administration of adjuvant chemotherapy for patients with LG-ESS. The present study also identified marital status as a prognostic factor by using the nomogram, with widows more likely to die. Up to now, only one study has reported that marital status was associated with survival of ESS patients [8], although an epidemiological study has reported an association between marital status and risk of uterus sarcoma [39].
Notably, surgery is the initial treatment for LG-ESS. However, it was not incorporated in the nomogram because it increased the AIC value of the nomogram. This should not be interpreted as meaning that surgery has no benefit on survival. In previous studies, hysterectomy plus bilateral salpingectomy was generally regarded as an important measure to improve patients’ prognoses [30], whereas some researchers argued that the administration of surgery, including its extent and type, should be decided on an individual basis, such as patient's symptoms [4]. In the present study, most patients had undergone surgery, making its effects hard to be analyzed properly.
In the present study, the analysis of only LG-ESS cases provided an opportunity to reconsider the factors that could be integrated into the prognostic nomogram. The nomogram integrates multiple factors, including demographic and clinicopathological characteristics, into a quantitative model and has been shown to perform better than some conventional staging systems, such as American Joint Committee on Cancer (AJCC) and FIGO staging systems, in predicting prognosis and making clinical decision [13, 40]. Traditionally, the FIGO criteria-based tumor staging has been the initial choice for predicting prognosis of patients with LG-ESS. Generally, the stages of this system are strongly associated with OS. However, different prognoses were observed among patients at the same stage. This prognosis heterogeneity could be explained by that age, marital status, adjuvant therapy, and other factors are not considered in the FIGO criteria-based tumor staging. Therefore, we compared the nomogram, which involves more variables, with the conventional FIGO criteria-based tumor staging. The positive NRI and IDI of the nomogram versus the staging system indicated that the nomogram had better predictive capability than the FIGO criteria-based tumor staging alone. In addition, DCA proved that our nomogram predicted survival with better clinical benefit and utility than the conventional staging system.
We divided patients into low-, middle-, and high-risk groups according to their nomogram TPs. The Kaplan-Meier method and Cox hazard ratio model demonstrated significant differences in OS among the three risk groups with better discrimination than the conventional staging system (Figure 4). In particular, the nomogram had a greater ability to recognize the high-risk population than the conventional staging system. Due to their poor outcomes, particular attention should be paid to patients with TP > 360.
The nomogram demonstrated potential value in clinical practice. We analyzed a large set of samples using the data from 18 medical centers registered in the SEER database, which represent the populations of different areas. We followed the recommendation of the Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement [41] to use bootstrapping and cross-validation methods in calculating C-index, time-dependent AUC, and calibration curves. The favorable results were replicated well in the validation cohort. Overall, our nomogram may be a useful method of evaluating prognosis of patients with LG-ESS to date.
Although the nomogram performed well, the present study had some limitations. For instance, SEER did not release the data about surgical margins and hormonal treatment. Therefore, the present study did not evaluate these variables. Multicenter clinical validation is also needed to evaluate the external utility of our nomogram.
5 CONCLUSIONS
In summary, given its increased accuracy, good clinical utility, and more precise prognosis prediction compared with conventional staging system, our nomogram may be used to predict survival of patients with LG-ESS.
ETHICS DECLARATIONS
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
CONSENT FOR PUBLICATION
Not applicable.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGEMENTS
We thank Professor Roger Marshall in the University of Auckland, New Zealand for his help in using the R package regplot. He has no responsibility for the content of the manuscript.
FUNDING
This study was supported by grants no. 81670123 and no. 81670144 from the National Natural Science Foundation of China (NSFC).
AUTHOR CONTRIBUTIONS
Study design: Jie Wu, Huibo Zhang, Bin Xu and QiBin Song; data collection: Lan Li, Mengxue Hu and Liang Chen; manuscript preparation: Jie Wu, Lan Li, Mengxue Hu and Liang Chen; data analysis & interpretation: Jie Wu and Huibo Zhang; all authors confirm that they contributed to manuscript reviews—revising it critically for important intellectual content—and read and approved the final draft for submission. All authors are also responsible for the manuscript content.
Open Research
DATA AVAILABILITY STATEMENT
The data of this study are available in the SEER database (https://seer.cancer.gov/). Data downloading and processing are as described in Materials and Methods.




