Evaluation of breast skin and tissue stiffness using a non-invasive aspiration device and impact of clinical predictors
Clinical trials: NCT05301998.
Abstract
A personalized 3D breast model could present a real benefit for preoperative discussion with patients, surgical planning, and guidance. Breast tissue biomechanical properties have been poorly studied in vivo, although they are important for breast deformation simulation. The main objective of our study was to determine breast skin thickness and breast skin and adipose/fibroglandular tissue stiffness. The secondary objective was to assess clinical predictors of elasticity and thickness: age, smoking status, body mass index, contraception, pregnancies, breastfeeding, menopausal status, history of radiotherapy or breast surgery. Participants were included at the Montpellier University Breast Surgery Department from March to May 2022. Breast skin thickness was measured by ultrasonography, breast skin and adipose/fibroglandular tissue stiffnesses were determined with a VLASTIC non-invasive aspiration device at three different sites (breast segments I–III). Multivariable linear models were used to assess clinical predictors of elasticity and thickness. In this cohort of 196 women, the mean breast skin and adipose/fibroglandular tissue stiffness values were 39 and 3 kPa, respectively. The mean breast skin thickness was 1.83 mm. Only menopausal status was significantly correlated with breast skin thickness and adipose/fibroglandular tissue stiffness. The next step will be to implement these stiffness and thickness values in a biomechanical breast model and to evaluate its capacity to predict breast tissue deformations.
1 INTRODUCTION
Breast cancer is the most common cancer in France (Institut National du Cancer, 2019). Mammography, ultrasound and magnetic resonance imaging (MRI) are used for breast cancer screening and diagnosis and are also performed during the work-up before breast cancer surgery. These imaging exams are accurate, but are performed in different positions (supine or prone). This leads to a breast deformation that is very different from the intraoperative situation. Therefore, the surgeon must mentally reconstruct the three-dimensional (3D) anatomy of the breast before planning the surgery. The use of a personalized geometrical 3D model of the breast that includes a representation of the internal structure surfaces and of the tumor could help the surgeon to better plan the incisions and dissection of the breast tissue. It would also allow improving the preoperative information given to the patients and better explaining the surgical outcomes and scarring in oncologic and reconstructive/plastic surgery settings (Overschmidt et al., 2018). The challenge is to find a way to transform the 3D model from the prone/supine position to the sitting or half-sitting position during breast surgery (Han et al., 2014). This could be achieved with the help of a biomechanical 3D breast model that should ideally integrate also the biomechanical properties of all breast components.
Building an anatomical idea patient-specific biomechanical model requires to define constitutive laws for each tissue (skin and adipose/fibroglandular tissues in the case of breast) and to estimate their corresponding biomechanical properties. The choice of the breast tissue properties has strong consequences on the numerical simulations (Eder et al., 2014). These parameters can be identified from ex vivo or in vivo measurements of these tissues (Mira et al., 2018). Ex vivo measurements usually result in stiffer parameters that do not allow enough deformations under gravity loading (Mira et al., 2018). Few studies have estimated in vivo the breast skin constitutive parameters (Coumaré et al., 2015; Han et al., 2012; Sutradhar & Miller, 2013). One study reported significant stiffness variations in the breast adipose and glandular tissues (Han et al., 2012). These differences can be explained by the influence of various factors, particularly age, menstrual cycle phase, breastfeeding, pregnancy (Coumaré et al., 2015; Sutradhar & Miller, 2013). Moreover, in daily practice, body mass index (BMI), breastfeeding, history of radiotherapy, and menopausal status seem to influence the breast tissue constitutive parameters, although few studies with small cohorts evaluated their impact (Coumaré et al., 2015; Han et al., 2012).
In vivo measurements were mostly obtained using magnetic resonance elastography or a method that combines MRI and finite element modeling. These techniques assume a linear constitutive law for the tissues and stiffness is estimated using the Young modulus. However, such techniques are not easily available in the clinical daily practice. The Translational Innovation in Medicine and Complexity (TIMC) laboratory, Grenoble Alps University, France, developed non-invasive in vivo suction techniques based on volume measurements, called the VLASTIC method (Elahi et al., 2018, 2019). This method has been extended to assess the stiffnesses of bilayer materials (Connesson et al., 2023). It was then used in a clinical pilot study to measure the patients' tongue stiffness (Kappert et al., 2021) and breast skin (upper layer) and adipose/fibroglandular tissue (lower layer) stiffness in seven volunteers (Briot et al., 2022).
The aim of the present study was to use the VLASTIC device (Elahi et al., 2018) to measure the breast tissue stiffness in a large cohort of women. Breast skin thickness also was determined by ultrasonography. The secondary objective was to evaluate clinical predictors of stiffness and thickness.
2 METHODS
2.1 Study design and participants
This prospective study was carried out at the breast surgery department of Montpellier University Hospital, France. All women were older than 18 years of age and were recruited at the obstetrics and gynecology departments of Montpellier University Hospital. The participants were healthy women who went to the hospital for a routine control or pregnancy follow-up. They gave their written informed consent before inclusion in the study. The exclusion criteria were skin disease, skin lesions, and application of any cream within 12 h before the measurements. The following data were retrieved from the patients' files or during the interview: age, BMI, bra cup size, menstrual cycle phase, contraception, menopausal status, past history of breast radiotherapy and/or surgery, number of pregnancies and breastfeeding. This study was approved by the French Committee for the Protection of Human Subjects (N° ID-RCB: 2021-AO2385.35).
2.2 Procedure
2.2.1 Breast skin and adipose/fibroglandular tissue stiffness
Elasticity, considered as a bilayer material, was assessed using the bilayer VLASTIC suction method. This method was thoroughly described and validated by Connesson et al. (2023), and then tested in seven volunteers by Briot et al. (2022).
The VLASTIC device is made of a 1 mL syringe used to cyclically withdraw a known volume (Vsyringe) from a system composed of a manometer, a valve, a connection tube, and a 3D printed resin cup of aspiration diameter Di (Figure 1). This cup rests onto the tested material (breast skin, here). This device is used to measure the variations in negative pressure P and Vsyringe during quasi-static cyclic tests. A parallel calibration procedure is used to evaluate the system deformation (to exclude the deformation of the tested material) during a suction cycle, thus enabling to postprocess Pressure-Tissue Volume curves for the tested material.
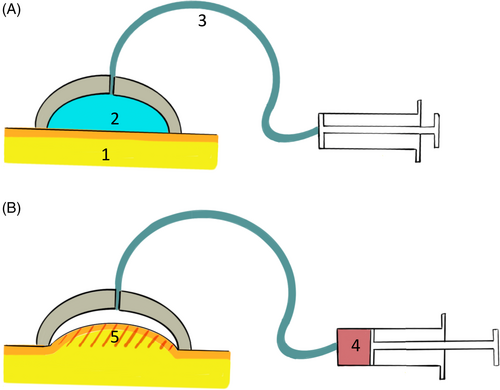
To extract distinct young moduli values for the upper and lower layer of the material, the exploited principle is the following: during a Pressure-Tissue Volume measurement, a suction cup extracts information from the underneath material structure. The thumb rule is that information is extracted up to a depth of about the suction aperture diameter D. For a suction diameter smaller than the upper layer thickness, only the upper layer mechanical behavior impacts the measured Pressure-Tissue volume curve (Zhao et al., 2011). By performing measurement with increasing suction diameter, the lower layer contribution increases progressively. The method developed in Connesson et al. (2023) uses a set of 7–9 curves obtained with different suction diameter Di from 4 to 30 mm and aims at identifying both the layer elasticity modulus explaining simultaneously the whole set of curves. The inverse identification process thus starts by a first guess of the skin and adipose/fibroglandular elasticity moduli. The expected pressure-Tissue volume curves for each suction diameter Di are then computed using Finite Element models (ANSYS software). A cost function is therefore built using all the experimental and simulated curves. The skin and adipose/fibroglandular elasticity moduli are then iteratively modified to minimize the cost function using a classical Levenberg–Marquardt method. Upon convergence, the noise and cost function properties are analyzed to evaluate the associated indifference parameter region.
For the measurements, participants were asked to lay in supine position, the arms along the body. Measurements were done in one breast (either right or left), at sites in the first three breast segments (I–III). Each participant's breast was marked at the midpoint of each segment (site 1, site 2, and site 3) (Figure 2). The cup was held in position using medical plasters.
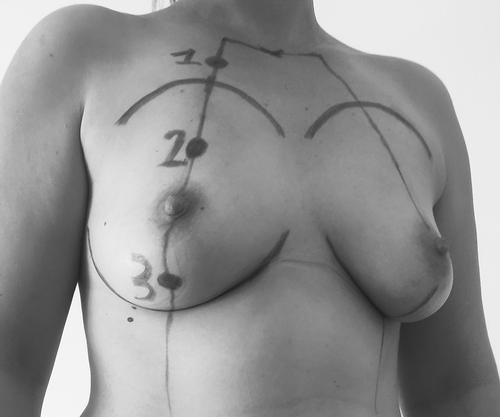
Seven cups were used (4–6–8–10–12.5–15–20 mm) and a total of 21 measurements per participant were obtained. Four successive tests (five aspiration cycles per test) were done for each measurement.
2.3 Breast skin thickness
Skin thickness was measured at the three sites described above with a GE Healthcare ultrasound system (Venue Go, GE Healthcare, United States) and Aquasonic translucent gel. Skin thickness was measured from the ultrasound image by the same experienced investigator. The mean value of five measurements for each site was recorded.
2.4 Statistical analysis
Quantitative variables were described using mean, standard deviation (SD) and median, quartiles and maximum/minimum; qualitative variables using numbers and percentages. After assessing the distribution of thickness and stiffness values, the linear correlation between data was assessed using the Pearson's correlation coefficient.
Three multivariable models were derived to assess predictors of skin thickness and stiffness, and adipose/fibroglandular tissue stiffness. Among the clinical predictors, variables with a univariate p-value <0.20 were included in the multivariable analysis. When the log-linearity criteria were met, quantitative variables were preferred to qualitative variables. Variables with too many missing data or too rare modalities were excluded. The backward method was used to obtain a parsimonious model, and the selection criterion was a multivariable p-value <0.10.
3 RESULTS
In total, 200 women were included in the study between March and May 2022. Four women were excluded from the final analysis because breast tissue stiffness could not be analyzed due to the poor quality of measurements. The characteristics of the 196 remaining participants are presented in Table 1. The mean (SD) BMI was 24.5 kg/m2 (±5.4 kg/m2). The mean number of pregnancies was 1.14 (±1.39). Five (2.5%) women were pregnant at the study time (12, 25, 28, 35, and 36 weeks of amenorrhea, respectively). Six (3%) and 14 (7%) women had history of radiotherapy and breast surgery, respectively. Among the 46 menopausal women, seven (3.6%) were taking hormone replacement therapy. The mean time necessary for the measurements was 45 min. No adverse event was observed.
| Characteristics | Values |
|---|---|
| Age (N = 196) | |
| Mean (± SD) | 41.32 (±12.80) |
| 18–24 years old (N, %) | 6 (3.03) |
| 25–44 years old (N, %) | 128 (65.30) |
| 45–64 years old (N, %) | 46 (23.46) |
| >64 years old (N, %) | 13 (6.63) |
| Body mass index (N = 196) | |
| Underweight (N, %) | 5 (2.55) |
| Healthy weight (N, %) | 120 (61.22) |
| Overweight (N, %) | 41 (20.92) |
| Obesity (N, %) | 30 (15.31) |
| Smoking status (N = 196) | |
| Never smoked (N, %) | 147 (75.00) |
| Current smoker (N, %) | 32 (16.33) |
| Former smoker (N, %) | 17 (8.67) |
| Pregnancies (N = 196) | |
| None (N, %) | 88 (44.90) |
| At least one pregnancy (N, %) | 108 (55.10) |
| Breastfeeding (N = 196) | |
| None (N, %) | 134 (68.37) |
| At least one (N, %) | 62 (31.63) |
| Contraception (N = 145) | |
| No (N, %) | 118 (75.1) |
| Yes (N, %) | 27 (17.20) |
| Menstrual cycle (N = 118) | |
| Day 1–Day 14 (N, %) | 62 (52.54) |
| >Day 14 (N, %) | 56 (47.45) |
| Menopausal status (N = 196) | |
| Non menopausal (N, %) | 150 (76.5) |
| Menopausal, <5 years (N, %) | 24 (12.24) |
| Menopausal, >5 years (N, %) | 22 (11.22) |
| Breast size cup (N = 196) | |
| A (N, %) | 5 (2.55) |
| B (N, %) | 106 (54.08) |
| C (N, %) | 57 (29.08) |
| D (N, %) | 22 (11.22) |
| E and > (N, %) | 6 (3.06) |
3.1 Breast skin and adipose/fibroglandular tissue stiffnesses
The stiffness results are presented in Table 2 and Figure 3. Overall, the mean breast skin and adipose/fibroglandular tissue stiffness values were significantly different: 39.28 versus 3.02 kPa (all sites included). The mean stiffness values (skin and adipose/fibroglandular tissue) were similar at site 2 and site 3.
| Characteristics | Site 1 (N = 196) | Site 2 (N = 196) | Site 3 (N = 196) |
|---|---|---|---|
| Skin thickness, mm | |||
| Mean ± SD | 1.78 (±0.26) | 1.84 (±0.23) | 1.87 (±0.24) |
| Median (Min; Max) | 1.73 (1.22; 2.80) | 1.83 (1.19; 2.52) | 1.87 (1.32; 2.72) |
| Skin stiffness, kPa | |||
| Mean ± SD | 42.33 ± 17.33 | 37.07 ± 13.30 | 38.19 ± 14.40 |
| Median (Min; Max) | 39.97 (8.69; 148.12) | 34.60 (6.79; 101.81) | 34.64 (7.73; 93.44) |
| Fatty/fibroglandular stiffness, kPa | |||
| Mean ± SD | 3.51 ± 1.32 | 2.77 ± 0.91 | 2.79 ± 1.12 |
| Median (Min; Max) | 3.37 (0.84; 9.32) | 2.59 (0.61; 7.21) | 2.50 (0.56; 8.36) |
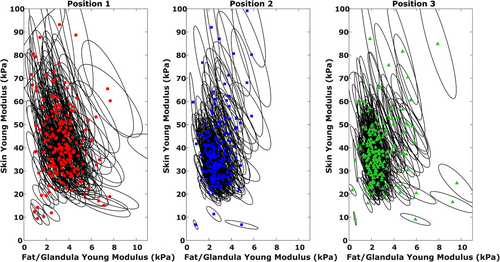
3.2 Breast skin thickness
The mean breast skin thickness values (Table 2) were similar at the three sites: 1.78 ± 0.26 mm for site 1, 1.84 ± 0.23 mm for site 2, and 1.87 ± 0.24 mm for site 3.
Breast skin thickness and stiffness were negatively correlated at the three sites (Pearson correlation coefficient: −0.37, p < 0.0001 at site 1; −0.27, p = 0.0002 at site 2; −0.28, p < 0.0001, at site 3).
3.3 Clinical predictors of breast skin thickness and breast skin/adipose/fibroglandular tissue stiffness (Table 3)
| Variable | Skin thickness (mm) | Fatty/fibroglandular stiffness (kPa) | ||
|---|---|---|---|---|
| Beta ± SD | p-value | Beta ± SD | p-value | |
| Age > 44 yo versus 18–44 yo | 0.100 ± 0.047 | 0.035 | ||
| Body mass index (kg/m2) | 0.005 ± 0.003 | 0.052 | ||
| Past breastfeeding (at least one vs. none) | 0.052 ± 0.031 | 0.090 | ||
| Menopausal status | ||||
| Menopausal <5 years versus non menopausal | −0.093 ± 0.043 | 0.034 | −0.157 ± 0.056 | 0.006 |
| Menopausal >5 years versus non menopausal | −0.195 ± 0.045 | <0.0001 | −0.277 ± 0.061 | <0.0001 |
- Note: Values are the regression parameter beta and its standard deviation, SD. Results of parsimonious models after backward variables selection.
The following factors were not analyzed in the univariate analysis because of the too small number of concerned participants: history of radiotherapy, history of breast surgery, hormone replacement therapy in postmenopausal women, current pregnancy.
After univariate analysis, age (p = 0.02), BMI (p = 0.07), past breastfeeding (p = 0.15), and menopausal status (p < 0.0001) were included in the multivariable model concerning skin thickness. The multivariable analysis showed that only menopause influenced skin thickness. Specifically, skin thickness was lower in menopausal women (reduction by 0.1 and 0.2 mm in women in menopause for <5 years and for ≥5 years, respectively).
After univariate analysis, menopausal status (p = 0.19) and menstrual cycle phase (p = 0.07) were included in the multivariable model concerning skin stiffness. However, they were not significantly correlated with tissue stiffness.
After univariate analysis, age (p = 0.18), smoking status (p = 0.2), past pregnancy (p = 0.04), past breastfeeding (p = 0.002), menopausal status (p = 0.003), menstrual cycle phase (p = 0.03), and bra cup size (p = 0.18) were included in the multivariable model concerning adipose/fibroglandular tissue stiffness. This analysis found that only menopause was an independent predictors of adipose/fibroglandular tissue stiffness. Specifically, stiffness was decreased by ~0.16 and ~0.28 kPa in women in menopause for <5 years and for ≥5 years, respectively.
4 DISCUSSION
Skin stiffness and thickness were evaluated in several studies in the literature, but mainly in the upper limb and abdomen (Kim et al., 2013; Luebberding et al., 2014). The present study determined breast skin thickness and breast tissue stiffnesses in 196 women. It also showed that menopausal status was the only clinical predictor of breast skin thickness. Age and menopausal status were clinical predictors of adipose/fibroglandular tissue stiffness.
The mean Young modulus value for breast skin was 39.28 kPa, with an important variation among participants (from 6.7 to 148 kPa), as previously reported in the literature (Han et al., 2014; Hendriks et al., 2006; Sutradhar & Miller, 2013). This could be explained by several factors. The first one is the used measurement techniques. Sutradhar and Miller (2013) and Han et al. (2014) estimated in vivo skin properties with a cutometer. Sutradhar and Miller (2013) found that in 23 female volunteers (aged from 29 to 75 years), the skin elastic modulus ranged between 15 and 480 kPa (mean value: 334 kPa ± 88). The cutometer probe is very sensitive and can be influenced by a change in room temperature or humidity, and especially by the pressure exerted by the operator (Bonaparte et al., 2013; Dobrev, 2000). This becomes more important as the number of operators increases. The Young modulus can be estimated also by combining finite element analysis and imaging data. This method, using a neo-Hookean strain energy density model, resulted in softer materials, as indicated by the skin shear modulus value range (2.47–5.78 kPa) (Han et al., 2014). However, it should be noted that these studies involved small cohorts. Second, data heterogeneity could be due to measurement errors. In our study, a number of parameters could have influenced the results, such as temperature, presence of gel inside the cup, or the woman's movements. The third factor is the inter- and intra-individual variability (Mira et al., 2018). Like in our study, Han et al. (2012) found significant inter-individual variability in the shear modulus value (between 0.22 and 43.64 kPa). Unlike other studies (Coltman et al., 2017; Coumaré et al., 2015; Lorenzen et al., 2003), we did not find any effect of age and menstrual cycle on breast skin stiffness. However, these studies involved small cohorts and did not include a multivariate analysis. It also has to be noted that in our study, only 8% of the women were over 60 years and 20% over 50 years. So, a larger cohort of women over 60 years old may be necessary to show an impact of age on breast skin stiffness.
In our study, the mean Young modulus value for adipose/fibroglandular tissue stiffness was 3.02 kPa (0.56–9.32 kPa). Several groups studied the elastic modulus of adipose and glandular tissues and reported values between 0.1 and 271 kPa (Gamage et al., 2021; Han et al., 2012; Krouskop et al., 1998; Rajagopal et al., 2008; Wellman et al., 1999). Higher values were obtained in ex vivo measurements (Krouskop et al., 1998; Wellman et al., 1999). Eder et al. (2014) found that many of the material models are too stiff, thus not allowing enough deformation under gravity loading. The most accurate values of the elastic modulus were reported by Rajagopal et al. (2008): 0.48 and 0.78 kPa in two young volunteers. These values were identified by comparing the breast MRI geometry in prone position under gravity or immersed in water. The identified model was a homogeneous neo-Hookean material that described the whole breast (no distinction between skin and adipose/fibroglandular tissues) under large deformation. These Young moduli values are on the lower range of the adipose/fibroglandular tissue values determined in our study. This is somewhat surprising: an equivalent Young modulus describing both skin and adipose/fibroglandular tissues, as done by Rajagopal et al., should provide stiffer values than for adipose/fibroglandular tissues alone. Moreover, the deformation range in the study by Rajagopal et al. was larger than the one used in our study (very small deformations) and this should lead to over-estimate the identified Young modulus. More comparisons are required to conclude. In addition, the deformations implemented in our study were probably much lower than those encountered in daily practice.
In our study, only menopause was significantly correlated with adipose/fibroglandular tissue stiffness. The effect of menopause seems consistent and could be explained by the reduction of fibroglandular tissue and increase of adipose tissue. Lorenzen et al. (2003) showed that during the menstrual cycle, the elastic properties of glandular tissues can change by ~30% because of hormonal changes. We did not find any effect of the menstrual cycle phases. However, we only tested each patient once. The best methodology would have been to test women at each menstrual cycle phase, which was not feasible. Moreover, the effect of pregnancy and breast surgery/radiotherapy could not be analyzed because of the limited number of participants in these groups.
Our results on breast skin thickness are consistent with the literature (Coltman et al., 2017) as well as the weak correlation between breast skin thickness and stiffness (Coltman et al., 2017; Krueger et al., 2011; Smalls et al., 2006; Sutradhar & Miller, 2013). Menopausal status was the only clinical predictor of skin thickness. Results in the literature are controversial. Like in our study, Sutradhar and Miller (2013) did not find any effect of age, unlike Coltman et al. (2017) and Ulger et al. (2003). However, these two studies did not include a multivariate analysis and stated that age-related decline in skin thickness is mainly due to hormonal effects. The decline in thickness at non-breast skin sites after 45 years of age has been attributed to hormonal effects with the menopause-related reduction in estrogen (Farage et al., 2009; Hall & Phillips, 2005). However, we could not test the impact of other factors (e.g., previous radiotherapy) because of the small number of concerned women. It has to be noted that all patients were included in Spring. The season could not have had any impact on skin thickness as it has been showed by Uchegbulam et al. (2022).
The strengths of our study were the large cohort and the in vivo measurements using a non-invasive method. This study has some limitations. The first is related to the device. As said previously, several parameters could have influenced the results (temperature, the presence of gel inside the cup or woman's movements). Modifications are underway to improve the device efficiency. Second, important clinical factors, such as history of radiotherapy or breast surgery, could not be studied because of the limited number of concerned participants.
5 CONCLUSION
This study allowed us to determine the breast skin thickness and skin and adipose/fibroglandular tissue stiffnesses in a large cohort of women using a non-invasive method. It also showed that menopausal status was the only clinical predictor of breast skin thickness and adipose/fibroglandular tissue stiffness. The important heterogeneity in breast skin and tissue stiffnesses was mostly explained by inter-individual variations. The next step will be to implement these values in a biomechanical breast model and evaluate its capacity to predict breast tissue deformations (Eder et al., 2014).




