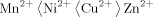Formation Constants of 3d Metal Ions With N-m-Tolyl-p-Substituted Benzohydroxamic Acids
Abstract
The thermodynamic metal ligand stability constants of hydroxamic acids with 3 d bivalent metal ions have been determined at 25° and 35°C in different mole fraction of dioxane. The stabilities of the complexes mostly follow the order of besicities of the ligands and also the electron affinities of the metal ions as measured by their ionisation potential. The stability constants of the 3 d transition metal complexes follow the order