Abnormal cortical sensorimotor activity during “Target” sound detection in subjects with acute acoustic trauma sequelae: an fMRI study
Abstract
The most common consequences of acute acoustic trauma (AAT) are hearing loss at frequencies above 3 kHz and tinnitus. In this study, we have used functional Magnetic Resonance Imaging (fMRI) to visualize neuronal activation patterns in military adults with AAT and various tinnitus sequelae during an auditory “oddball” attention task. AAT subjects displayed overactivities principally during reflex of target sound detection, in sensorimotor areas and in emotion-related areas such as the insula, anterior cingulate and prefrontal cortex, in premotor area, in cross-modal sensory associative areas, and, interestingly, in a region of the Rolandic operculum that has recently been shown to be involved in tympanic movements due to air pressure. We propose further investigations of this brain area and fine middle ear investigations, because our results might suggest a model in which AAT tinnitus may arise as a proprioceptive illusion caused by abnormal excitability of middle-ear muscle spindles possibly link with the acoustic reflex and associated with emotional and sensorimotor disturbances.
Introduction
Tinnitus and hearing loss are frequent consequences of acute acoustic trauma (AAT). Tinnitus is defined as an illusory or phantom auditory percept because it is perceived in the absence of any objective physical sound source. Tinnitus is often described by AAT subjects as a perception of a high-pitch continuous sound (such as whistling or ringing) and sensation of aural fullness at the onset of AAT. Noise-induced tinnitus percept after an AAT is almost immediate or develops very rapidly. Repetitive exposure to noise usually increases the periodicity and/or the intensity of tinnitus, which can become chronic. Tinnitus is a common feature of military life, due to exposure to impulse noise associated with the use of firearms. It was demonstrated that attention tasks are sensory enhancers of tinnitus (Knobel and Sanchez 2009, 2008; Paltoglou et al. 2009) and furthermore that auditory attention deficits were observed in subjects with tinnitus (Jacobson et al. 1996; Cuny et al. 2004; Hallam et al. 2004; Jastreboff 2007).
In humans, the neural correlates of AAT sequelae, namely tinnitus, have previously been determined using fMRI but have mainly concentrated on the neuronal correlates of the tinnitus perception itself (Kovacs et al. 2006; Smits et al. 2007; Leaver et al. 2011). Here, we did not focus on tinnitus itself, but we have imaged the neuronal correlates of AAT sequelae during an auditory “oddball” attention task during which tinnitus could not be perceived and using frequency tones well perceived, not affected by AAT hearing loss (i.e., <2 kHz). Using such a task, we anticipated that we may identify neural correlates of anomalies, other than hearing loss and tinnitus perception itself associated with AAT, such as previously described in the literature. Additionally, we hypothesized that undetected brain activity dysfunctions caused by AAT may be revealed in our experimental conditions, and could suggest new possibilities for the origin of AAT tinnitus whose mechanistic origin is still a matter of debate.
Materials and Methods
The study was performed according to the sixth revision of the Declaration of Helsinki (WMA 2008), approval by the local medical ethic committee (comité de protection des personnes) was obtained and reference as N°05-CRSS-1/CPPsud-est. Subjects gave written informed consent before the start of the study.
Participants
We compared and examined two groups of subjects: subjects with a history of AAT and aged-matched healthy volunteers without tinnitus. AAT subjects were 19 military subjects aged 30 ± 8 years, who had been exposed to artillery impulse noise and who had experienced one or several AAT during gunfire practice rounds. All presented with high-pitch tinnitus in the right, left, or in both ears. All traumatic events occurred at least 6 months prior to the study, nevertheless not exceeding 2 years. The AAT had been diagnosed by the physician of the regiment following audiometry within 24 h posttrauma. All the subjects had continuous tinnitus at least during the first 24 h posttrauma. At the time of the study, subjects were still exposed to gunfire noise, none of the subjects reported particular aural fullness and tinnitus was perceived either occasionally (generally after target practice rounds or after exposure to intense noise of other origin), either frequently/permanently. Subjects with permanent tinnitus did not receive any treatment and could roughly cope with their tinnitus. None of the subjects had a history of neurological disorders. The age- and sex-matched control subjects (n= 19) were military subjects not exposed to impulse noise, free from any history of AAT, and not reporting tinnitus.
AAT subjects with occasional tinnitus represented another sub-control group for frequent/permanent tinnitus subjects, because they did not perceive their tinnitus before entering into the scanner.
Two subjects in each of the AAT and control groups were left handed.
Questionnaire and audiological assessment
We used a French translation (Meric et al. 2000) of the Tinnitus Reaction Questionnaire (TRQ) (Wilson et al. 1991) to assess the degree of coping/habituation or handicap/distress associated with the tinnitus, when present. TRQ scores refer often to intensity of the percept. The TRQ score was the result of the summation of grades (range from 0, “not at all” to 4 “almost always”) of 26 questions with a maximum score of 104. In clinical practice, a score superior to 50 has to be taken into account with proposition of psychological therapy. The TRQ also allows assessment of the level of anxiety (Andersson et al. 2003). We also used a standardized questionnaire to assess the periodicity of the tinnitus.
Prior to collecting audiograms, otoscopy was performed by an ENT specialist. Examinations were normal in all subjects. Audiograms were acquired (Békésy method) with frequency sweeps from 250 to 8000 Hz and sound levels were increased and decreased stepwise by 2.5 dB. Figure 1 displays the audiograms of the AAT group with subgroups of tinnitus (occasional and frequent/permanent) and of the control group for the left and right ears. As expected, high frequency hearing thresholds were higher and V shape (noise notch) in the AAT group than in the control group. Noise notch was more bilateral among the frequent/permanent tinnitus subjects. It is usually a mark of more severe traumas (Nottet et al. 2006). Using analysis of variance (ANOVA) and tests corrected for multiple comparisons, differences were significant at 4 kHz and 5 kHz (P= 0.02) between controls and frequent/permanent tinnitus AAT subjects, and at the significance limit at 4 kHz (P= 0.07), between controls and AAT subjects with occasional tinnitus. Importantly, there was no statistically significant difference between AAT group and control group at frequencies lower than 2 kHz, which were used in the auditory attention task described below.
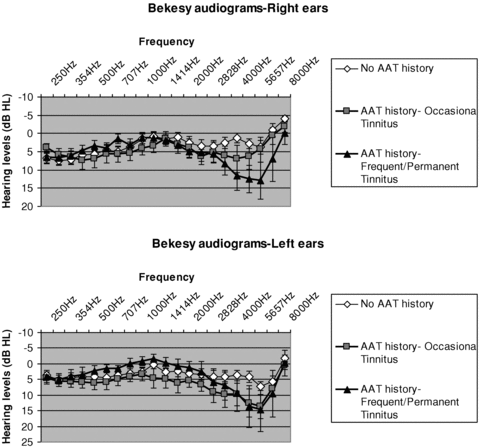
Hearing levels of participants: right and left audiograms (Békésy method) in the AAT group (occasional and frequent/permanent tinnitus) and control group. Hearing loss is observed at high frequency in the AAT group.
fMRI task and experimental procedures
We used sounds in the 250–1000 Hz frequency range, hearing levels were not significantly different between groups in this frequency range.
An auditory “oddball task” was applied. Three types of auditory stimuli were used: “Standard” (probability of occurrence P= 0.80, n= 348), “Target” (probability of occurrence P= 0.10, n= 48), and “Novel” (probability of occurrence P= 0.10, n= 48). Each “Target” and “Novel” stimulus was preceded by 4–7 randomly chosen “Standard” stimuli to ensure a minimum interval of 4.5 sec between two sequential nonstandard stimuli. Stimuli were produced by digitally synthesized sound files (Sound Forge 7.0, Sony Pictures Digital Inc., TX). All three types of stimuli lasted 130 msec. The “Standard” stimulus was a sound with frequencies increasing linearly from 250 to 1000 Hz, while the “Target” stimulus was a sound with frequencies decreasing linearly from 1000 to 250 Hz. “Novel” stimuli consisted of different 130 msec noises (e.g., onomatopoeia sound effects used in cartoons). Interstimulus intervals lasted 800 msec during which subjects could hear in background the scanner noise.
All stimuli were presented during a silent gap and baseline recorded in silent gaps without stimulus presentation.
Participants were instructed to respond as quickly as possible with their right thumb (pushing a button) at the occurrence/recognition of every “Target” stimulus. The task thus demanded strong attention associated with a muscular reflex. During auditory stimulus presentation, subjects were instructed to watch a gray screen with a fixation point (black cross).
Presentation® software (http://www.neurobs.com/presentation) was used to present stimuli, to register the subject's responses and to analyze the behavioral tests (i.e., reaction times, intrasubject reaction times variability, error rates).
Before the actual start of the scans, subjects were trained outside the scanner in order to familiarize to stimuli and handling of the system. All subjects were able to perceive sounds and operate the response keys correctly. By contrast, tinnitus could not be perceived because masked by the experimental environment.
In order to ensure comfortable hearing of stimuli in the noisy MRI environment, we performed some acoustic measures inside the scanner before optimizing the setup for the transmission of the auditory stimuli. The mean acoustic sound pressure level (SPL) during fMRI scans was 80 dB SPL with a very narrow spectral peak of 120 dB SPL at 1.12 kHz. To reduce scanner noise, a passive sound-attenuating cylinder was inserted into the bore of the scanner. It was composed of two layers of 5-mm-thick sound-attenuating material (Plastison®, www.serenata.tm.fr) fixed on a rigid cylindrical support (Sonotube®, http://sonotube.com). Furthermore to improve hearing of the stimuli, imaging slices were acquired in three stacks. Acquisition of each stack took 800 msec. Stacks were separated by a silent gap of 130 msec (gradient system “off”), during which period the auditory stimuli were presented. Subjects wore earplugs and stimuli were transmitted by home-made prototype earphones inserted in industrial hearing protectors (Bilsom®). The frequency range of the stimuli (250–1000 Hz) was below the peak frequency of the echo-planar imaging (EPI) sequence.
fMRI protocol
Blood oxygen-level-dependent (BOLD) images were acquired on a 3-Tesla whole body MR scanner (Brucker Medspec S300, Ettlingen, Germany) using gradient-echo planar imaging (EPI). Images of the whole brain, including cerebellum and brainstem, were obtained.
A total of 39, 3-mm-thick axial slices were acquired in three stacks of 13 slices each, in interleaved mode. Slice orientation was axial, parallel to the anterior and posterior commissures (AC and PC). In-plane resolution was 3 × 3 mm2. Main parameters of the T2*-weighted EPI sequence were: Time Repetition (TR) = 2400 msec, Time Echo TE = 30 msec, flip angle = 80°, spectral bandwidth in the readout direction = 172 kHz, field of view = 216×216 mm2, acquisition matrix = 72 × 72. Voxel size was 3 × 3 × 3 mm3. After acquisition of six dummy images permitting to the spin system to reach a stationary state, 172 brain volumes were acquired for each subject during each functional run. Interleaved with the acquisition of two identical functional runs, a high resolution (1 mm3), T1-weighted, sagittal, anatomical image was acquired. Main T1-weighted MPRAGE sequence parameters were: TR = 12 msec, TE = 4.6 msec, TI = 900 msec, recovery time = 2.5 sec, field of view (FOV) = 256 × 256 × 176 mm3, acquisition matrix = 256 × 256 × 176, two segments. At the end of the examination, two conventional gradient-echo MR images were further acquired (TE = 5.5 msec and TE = 14.6 msec), in order to enable eventual correction of the geometrical distortions (Cusack and Papadakis 2002)
fMRI Data processing and analysis
Image data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, London, http://www.fil.ion. ucl.ac.uk/spm). All functional volumes were time corrected taking into account the specific timing of slice acquisition in this study. We therefore adapted the SPM software (“spm_slice_timing.m” function). The B0 field map was further computed using the SPM FieldMap toolbox (www.fil. ion.ucl.ac.uk/spm/toolbox/fieldmap/) (Hutton et al. 2002). Geometric distortion and motion corrections were performed within a realigned framework (Andersson et al. 2001). Eventually, images were co-registered with the anatomical image. Spatial normalization of anatomical images was performed using the T1-weighted template from the Montreal neurological institute (MNI). Parameters corresponding to this spatial transformation were further applied to the functional images to align them within the standardized MNI space. Normalized data were smoothed with an isotropic Gaussian kernel of 6-mm full-width-at-half-maximum.
Individual statistical analyses of the variations of the BOLD signal were based on the application of the general linear model for the four following regressors of interest: “Target” with correct button-press within 1200 msec poststimulus, correctly ignored “Novel” stimuli within the 0–1200 msec window, Stimuli with errors in button-press and “Standard” stimuli. Onsets of these different regressors were derived from the stimuli presentation sequence and from the response registration files acquired for each subject. Realignment parameters were introduced in the general linear model as regressors of no interest. Event-related responses to these stimuli were modeled using the canonical hemodynamic response function together with its first temporal derivative. Thus, variations around the peak latency of 6 sec could be taken into account (Calhoun et al. 2004). The following contrasts were calculated for each subject: response to standard versus baseline, target stimuli versus baseline, target stimuli versus standard ones, novel stimuli versus standard ones.
The contrast images were used in a random effects analysis, permitting inferences about condition effects across subjects that generalize to the population. Differences between groups were evaluated using a two-sample t-test to derive statistical parametric maps (SPMs) of t-statistics. Statistical t-maps were then thresholded at P < 0.001 and extent >20 voxels. We further retained only those clusters for which the probability corrected for multiple comparisons (using the false discovery rate method) was <0.05.
Other statistical tests
Comparisons of audiological data and behavioral data between AAT subjects and control subjects were performed using nonparametric Mann–Whitney tests because of the inequality of variance. Correlation coefficient used between quantitative and ordinal variables was the Spearman's rho. The level of significance was set at P= 0.05. Data are presented as mean ± standard error of mean (SEM).
Results
Questionnaires
All subjects reported that they could comfortably hear the stimuli under the MRI conditions. TRQ scores were below 50, indicating limited distress induced by tinnitus.
Descriptive tinnitus data are summarized in four subgroups (Table 1) according to the subject's TRQ score (two categories) and the periodicity of tinnitus (two categories). Regarding the TRQ score, a bimodal distribution was observed (not presented). Subjects were grouped according to this bimodal distribution as either low TRQ score group or high TRQ score group (medians were seven and 28, respectively). Regarding the periodicity, grouping relied on whether subjects had tinnitus occasionally (i.e., only after target practice rounds or exposure to noise), or frequently/permanently.
| Tinnitus Intensity/handicap (TRQ) median score | ||||
|---|---|---|---|---|
| 28 | 7 | Total | ||
| Tinnitus periodicity | Frequent/permanent | 3 | 8 | 11 |
| Occasional | 3 | 5 | 8 | |
| Total | 6 | 13 | 19 | |
No significant correlation was found between TRQ score and periodicity of tinnitus (r=−0.30, P= 0.208).
Behavioral task
Behavioral results of the auditory “oddball” task are shown in Figure 2. Mean reaction times were not significantly different between groups either in the audio laboratory or in the MR scanner. However, the mean intrasubject variability of the reaction times in AAT subjects was significantly larger than in controls, both in the laboratory (P= 0.017) and in the MR scanner (P= 0.030). The responses of the AAT, but not of the control subjects were affected in MRI conditions, as indicated by a significantly increased error rate (P= 0.012). Nevertheless, the error rate was lower than 10% in all conditions in both groups.
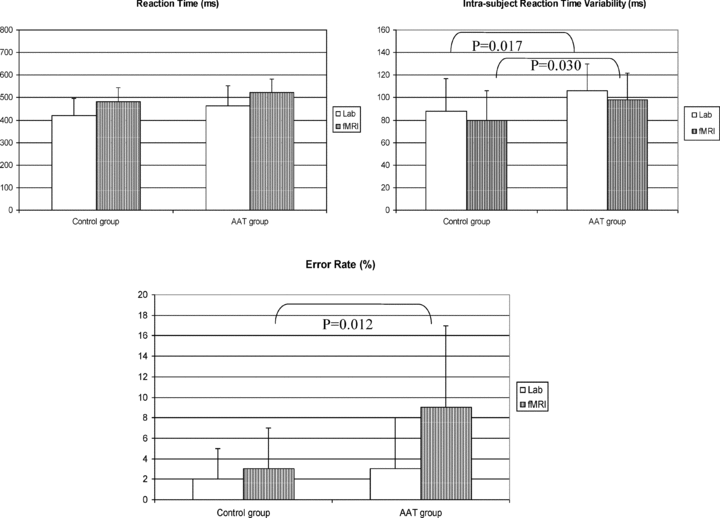
Behavioral auditory task results in the AAT group and in control group. Task was performed in the audiolab and in the MR scanner. Intrasubject reaction-time variability and error rate were significantly higher in the AAT group.
fMRI
A synopsis of statistically significant differences between the AAT group and the control group is presented in Table 2. No significant abnormal activation could be elicited for the “Novel” sounds. With “Target” sounds, several distinct regions displayed abnormal activations in the AAT group compared to the control group (see “Target vs. baseline” and “Target vs. Standard” in Table 2). Significant hyperactivations (Fig. 3) were found in a variety of structures involved in emotional response including the prefrontal cortex, the anterior and middle cingulate gyrus, and the insula. We also found abnormalities in regions generally considered as important for motor preparation and motor feedbacks such as premotor cortex (BA6), supplementary motor area (SMA), and in deep gray matter such as substantia nigra. Hyperactivity was also observed in the BA19 area, which corresponds to the visual associative peristriate cortex.
| Regions of interest | Brodmann area BA | MNI coordinates | Voxel of peak intergroup difference in t | ||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | t Standard versus baseline AAT > controls | t Target versus baseline AAT > controls | t Target versus standard AAT > controls | ||
| Frontal lobes | |||||||
| Left inferior frontal gyrus (pars triangularis) | BA 46 | −38 | 40 | 8 | 4.39 | ||
| Right anterior insula | 40 | 18 | 0 | 5.42 | 5.02 | ||
| Right SMA | BA 6 | 2 | 12 | 52 | 4.62 | ||
| Left precentralis gyrus | BA 6 | −56 | 2 | 22 | 5.01 | 5.88 | |
| Bilateral SMA | BA 6 | 0 | −8 | 54 | 5.00 | 5.10 | |
| Right posterior insula | 36 | −10 | 16 | 4.49 | 4.51 | ||
| Parietal lobes | |||||||
| Right Rolandic operculum | BA 43/40 | 42 | −18 | 18 | 3.82 | 4.29 | |
| Left Rolandic operculum | BA 43 | −44 | −8 | 12 | 3.56 | 4.42 | |
| Right inferior parietal lobule | BA 40 | 36 | −32 | 22 | 5.23 | 4.61 | |
| Left inferior parietal lobule | BA 40 | −50 | −50 | 40 | 5.71 | ||
| Left precuneus | BA 7 | −2 | −56 | 40 | 5.32 | ||
| Left superior parietal lobule | BA 7 | −30 | −60 | 46 | 4.78 | ||
| Left precuneus | BA 7 | −2 | −68 | 44 | 4.68 | ||
| Occipital lobes | |||||||
| Right lingual gyrus | BA 19 | 26 | −56 | −6 | 5.41 | ||
| Left middle occipital gyrus | BA 19 | −26 | −72 | 30 | 4.85 | ||
| Right cuneus | BA 19 | 8 | −72 | 28 | 4.33 | ||
| Right superior occipital gyrus | BA 19 | 20 | −84 | 38 | 4.26 | ||
| Cingulate gyrus | |||||||
| Right anterior cingulate gyrus | BA 32 | 2 | 40 | 10 | 5.06 | 4.13 | |
| Right anterior cingulate gyrus | BA 32 | 6 | 38 | 22 | 5.13 | ||
| Left anterior cingulate gyrus | BA 32 | −2 | 36 | 20 | 4.80 | 4.38 | |
| Anterior cingulate gyrus (bilateral) | BA 24 | −4 | 20 | 24 | 4.91 | 4.67 | |
| Left middle cingulate gyrus | BA 32 | −6 | 16 | 36 | 5.12 | 4.41 | |
| Right middle cingulate gyrus | BA 31 | 14 | −36 | 40 | 4.61 | 4.42 | |
| Left posterior cingulate gyrus | BA30 | −2 | −56 | 12 | 4.87 | ||
| Deep gray matter | |||||||
| Left putamen | −22 | 14 | 2 | 4.61 | |||
| Right caudate nucleus (head) | 12 | 14 | 6 | 4.25 | |||
| Left globus pallidus | −18 | 0 | 6 | 4.31 | |||
| Right substantia nigra | 8 | −20 | −10 | 5.81 | |||
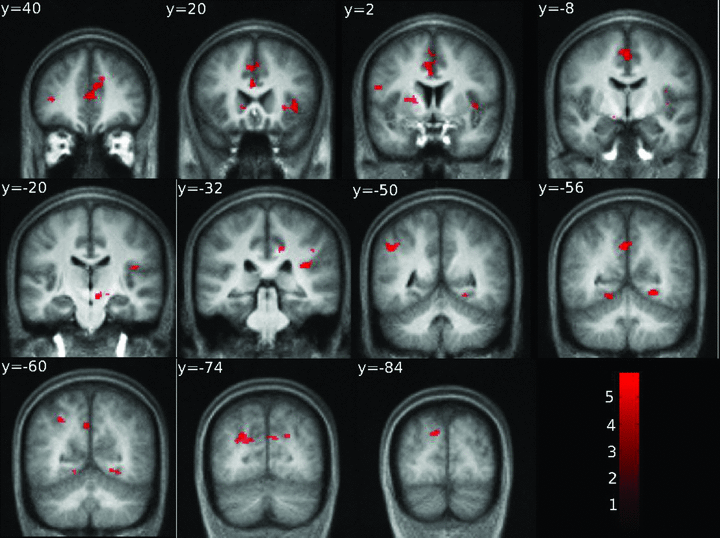
Overall view of the differences of contrast between the AAT group (N= 19) and the control group (N= 19); AAT > controls, in the “target sounds vs. baseline” contrast using an auditory oddball task. Significance assessed at P < 0.001, uncorrected and extent > 20 voxels. Activations are superimposed on the mean anatomical image from the control group. Left hemisphere is left on the image.
Interestingly, in the AAT group, significant hyperactivations were found in the limited region of the Rolandic operculum (Brodmann area [BA] 43), extending into the inferior parietal loblule (BA 43/40). At the MNI coordinates (42, −18, 18) corresponding to the maximal response, hyperactivation was correlated with the combination of tinnitus periodicity and TRQ score classes (Spearman's rho, r= 0.66, P < 0.001) (Fig. 4). A similar trend was observed when subjects were classified according to tinnitus periodicity alone, less to TRQ score alone, but the difference did not reach significance (P= 0.14 and P= 0.35, respectively; Mann–Whitney U test), possibly due to the small number of cases. No such correlation of overactivation at combination of tinnitus periodicity and TRQ score was found with other MNI coordinates.
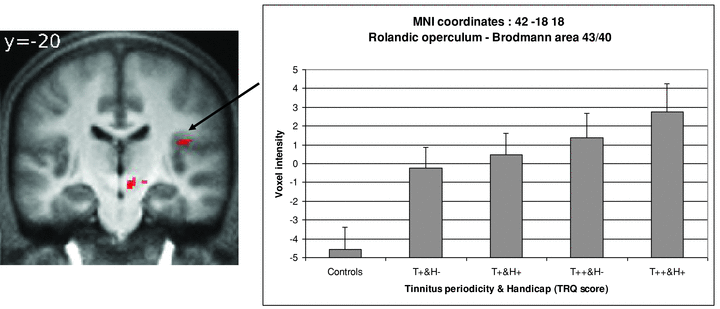
fMRI image and graph of mean voxel intensities at MNI coordinates 42, −18, 18 (BA 43/40) of the significant difference between AAT subjects and Control subjects for the contrast “Target vs. Baseline.” Mean voxel intensities were presented according to tinnitus periodicity and handicap. Occasional tinnitus = T+; Frequent/Permanent tinnitus = T++; Low TRQ scores = H−; High TRQ scores = H+. There is a significant correlation between voxel intensities and the combination of tinnitus periodicity and the subjective distress/handicap (TRQ) (Spearman's rho: r = 0.66, P < 0.001).
We have recently localized the cortical representation of the middle-ear superficial proprioception (i.e., small movements of tympanic membrane due to variations of pressure) in a specific limited region of Brodmann area 43 at the caudal edge of the somatosensory cortex (Job et al. 2011). The superposition of the cortical representation of tympanic membrane movement due to air pressure variation in BA 43 (green voxels) and of the hyperactivations found in the present study in BA 43 and BA43/40 (red voxels) shows that these regions are very close (Fig. 5). The hyperactivity zone observed in AAT subjects (red voxels) extended more deeply within the lateral sulcus than the hyperactivity caused by tympanic movement.
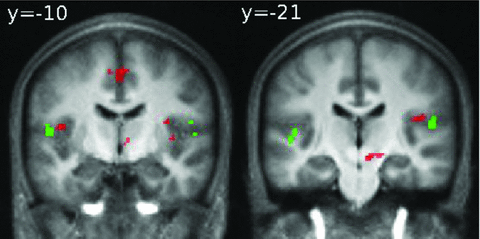
Hyperactivation in the Rolandic operculum (BA 43/40) in the AAT group during auditory oddball task (red voxels) for the contrast “Target sound vs. baseline” with superimposition of cortical activations in BA 43 found in Job et al. (2011) study for the contrast “tympanic movement due to air pressure variations vs. no pressure variations” in normal hearing subjects (green voxels).
Note that in the present study, in which the tinnitus itself was masked by the scanner noise, no significant abnormal activation was observed in the primary auditory cortex of AAT subjects.
Discussion
We observe perturbed emotional or sensorimotor responses in AAT subjects responding to target stimuli, with associated hyperactivation of a brain region involved in middle-ear movements.
A lack of difference was observed with “standard” or “novel” stimuli. Differences between groups only appeared with “target” stimuli. Compared to “standard” or “novel” stimuli, “target” stimuli require a rapid motor response with, in all probability, increased drastically tension and stress. Rapid motor action and stress such as a reflex may therefore sensitize the detection of emotional, sensorimotor, and proprioceptive anomalies in AAT subjects, in our experimental conditions. Dysfunctions observed were also consistent with a “salience” brain network dysfunction (Seeley et al. 2007). “Target” sound (memorized sound) was salient stimulus, for the subjects had to quickly react after detection of this specific sound compare to novel and standard sounds. Very recently “Salience” brain network have been found abnormal in tinnitus subjects (De Ridder et al. 2011)
Attentional emotional network dysfunction
The hyperactivation that we observed in the anterior cingulate cortex, the insula and the precuneus affects structures which are components of a general emotional limbic network, consistent with previous studies demonstrating emotional disorders in subjects with clinical tinnitus (Jastreboff et al. 1996; Roberts et al. 2010). Similar limbic structures have been previously linked to tinnitus distress in an EEG study (Vanneste et al. 2010) or whole head magnetoencephalography (Schlee et al. 2008). Thus, activation of limbic structures may be a general feature of stress responses.
Explanation of the meaning of a widespread cingulate gyrus hyperactivation at anterior, middle, and posterior sites (i.e., BA 32, 24, 31, and 30) is nevertheless complex because the cingulate gyrus region is involved in many functions that overlaps, in the autonomous function regulation, in some somatic functions (e.g., motor tone, movement feedbacks…) and in emotional/behavioral responses (e.g., sensitivity to pain, memory tasks…). Afferences of the cingulate cortex come from associative areas of the frontal, parietal, and temporal lobes, subiculum, septal nucleus, and thalamus (medial-dorsal and anterior). For example, anterior thalamus itself receives his afferences from the mamillary bodies, connecting memory with emotion. Slight dysregulations at the level of the mamillo-thalamic tract might also result in dysfunctions of the cingulate gyrus, which could reflect altered sound memory during the auditory task due to more stressful conditions for AAT subjects (i.e., exposure to scanner noise).
Premotor dysfunction
In AAT subjects, we have detected abnormal activations in deep gray matter, including substantia nigra, and parts of the premotor cortex. Both structures are involved in movement preparation in response to a stimulus (Schwarz et al. 1984a, 1984b; Boecker et al. 2008) and in spasticity (Laplane et al. 1977; Baykushev et al. 2008). In our study, target sound perception presumably triggered ear and thumb muscles preparation or feedback regulation requires in muscle reflex. Nevertheless, one premotor cortex hyperactivation was somatotopically localized in the mouth/jaw region rather than thumb region; it could suggest a role for a muscle involved in swallowing or orofacial activity, for instance, tensor tympani muscle. A conservative hypothesis is that such sensorimotor disturbances were one of the consequences of the emotional stress experienced by the AAT subjects. A similar explanation may apply in the case of the cross-modal anomalies that we observed in the visual associative cortex (Valsecchi and Turatto 2009).
Brodmann area 43 dysfunction
We found hyperactivities in BA 43 and BA 43/40 in AAT subjects, correlating with tinnitus periodicity and handicap. In a previous study, we have demonstrated activation of a limited region in BA 43 at the caudal edge of the somatosensory cortex in response to movements of tympanic membrane caused by gentle pressure variations. Besides the fact that BA 43 is clearly related to gustation and swallowing, this particular BA 43 region was demonstrated to correspond to pressure activities in oropharynx (Haslinger et al. 2010) and to middle-ear pressure sensitivity (Job et al. 2011). In our study, the hyperactivation of BA 43 and BA 43/40 was located close to the previously identified region although deeper in the sulcus. deep sensitivity (i.e., muscles, tendons, joints) in the somatosensory cortex is known to be represented mainly within the depth of the sulci (Krubitzer et al. 2004). It is therefore likely that AAT subjects present dysfunction of the deep sensitivity of the middle ear.
In osteoarticular and muscle systems, proprioception is mediated by intrafusal fibers of muscle spindle. Intrafusal fibers of muscle spindles possess neuronal primary endings that are highly sensitive to weak stretches. The fusimotor system controls joint muscles for tension, balance, and coordination of the joint movements (feedback regulation) (Roll et al. 1989). Like any other skeletal muscle, middle ear muscles (i.e., tensor tympani and stapedius) possess muscle spindles (five on average) as well as a fusimotor system (one to three intrafusal fibers) (Kierner et al. 1999). Conceivably, high acoustic pressures of firearms could cause, besides hearing loss, stretch/contraction microlesions on muscles and joint tendons of the middle ear due to exaggerated acoustic reflexes, with resulting deleterious effects on fusimotor system of middle ear muscle spindles, and proprioception dysfunction. Action of middle ear muscles was required for the auditory task in the MRI noisy environment. Movement preparation or achievement probably triggers widespread muscle tone response, which may account for a sensitizing effect of the motor activity involved in “target” conditions, in revealing middle ear proprioceptive anomalies in AAT patients. The emotional and sensorimotor anomalies associated with AAT may be aggravating co-factors, ultimately generating abnormal physical constraints along the tympano-ossicular chain through, for instance, tension in the temporo-mandibular region (Allin 1975; Al–Ani and Gray 2007). Additionally, anxiety and stress activate the sympathetic system that innervates the muscle spindles (Nozzoli et al. 1987). The relative importance of mechanical dysregulation or of emotional hyperreactivity in middle ear proprioceptive dysfunction is a matter of conjecture and may vary from subject to subject.
In general, proprioceptive dysfunction causes mild confusion and a reduction of the accuracy of task performance (Verschueren et al. 1999) that may also explain the differences in performance observed between AAT and control groups during the behavioral task. Our results led us to envisage besides an emotional disturbance, the possibility of a relationship between middle ear proprioceptive dysfunction and tinnitus in AAT subjects.
Mechanistic origin of tinnitus
The mechanistic origin of tinnitus is still a matter of debate. Cochlear cell damage (Liberman and Dodds 1987) is widely considered as a most likely origin for AAT tinnitus. It is widely assumed that cochlear cell damage triggers changes in the central auditory system, which is then interpreted as tinnitus by the higher processing stages in the brain (Jastreboff 1990; Roberts et al. 2010). Thus, hyperactivity and synchronization of neural firing in the dorsal cochlear nucleus, inferior colliculus or the auditory cortex in acoustic traumas has been reported (Rajan and Irvine 1998; Kaltenbach 2000; Eggermont 2003, 2006; Norena and Eggermont 2003) and attributed to an imbalance of exitability between the cochlear inner hair cells (IHC) and the outer hair cells (OHC) (Jastreboff 1990; Shiomi et al. 1997; Job et al. 2007). Additionally, following damage of cochlear cells, central representations of intact lesion-edge frequencies have been found enlarged, and one theory of tinnitus holds that this process could be related to the tinnitus sensation (Muhlnickel et al. 1998; Dietrich et al. 2001; Lockwood et al. 2002; Moller 2003). Such data brought support for a cochlear origin for tinnitus but alternative possibilities have been raised. Thus, it is unclear whether hyperactivity along the auditory pathway is a direct consequence of cochlear cell damage or results from hyperactivity in other neuronal pathways (Shore et al. 2008) and it has been argued that an auditory map reorganization, cannot satisfactorily explain the emergence of tinnitus perception (Weisz et al. 2005). Additionally, not all available data fit with an exclusive role of cochlear damages. Several studies have shown that hearing loss, which is directly related to cochlear cell damage, is not a clear predictor of the occurrence and severity of tinnitus, despite the fact that tinnitus is more prevalent in subjects with hearing loss (Jastreboff and Hazell 2004; Verret et al. 2005; Nottet et al. 2006).
Besides cochlear damage, other factors, such as somatosensory disturbances may be involved in tinnitus (Levine 1999, 2003; Sanchez et al. 2002; Levine et al. 2007). However, evidence for a somatosensory origin has been lacking in the case of AAT tinnitus. Tinnitus has also been proposed to be analogous to phantom pain (Tonndorf 1987; Moller 1997; Folmer et al. 2001; De Ridder et al. 2007). Patients with severe tinnitus actually share similar emotional disturbances proposed to be similar to chronic pain sufferers (Axelsson and Ringdahl 1989; Heller 2003). Finally, an influence of anxiety/mood states on noise-induced tinnitus onset after noise exposure has been also demonstrated (Job et al. 2004), suggesting a role for the autonomous sympathetic system (Hoehn–Saric and McLeod 1988; Critchley et al. 2004). Very recently parasympathetic stimulation in rats has demonstrated to abolish the tinnitus-like signal in conditioned animals when coupled to simultaneous auditory stimulation (Engineer et al. 2011).
Could AAT tinnitus be a proprioceptive illusion?
Tinnitus is defined as an illusory percept. In osteoarticular and muscle systems, illusory percepts can be triggered by activation of the fusimotor systems (Goodwin et al. 1972; Roll et al. 1989). In limb muscles, for instance, low-frequency vibration applied to a specific muscle tendons activate muscle spindle endings via the fusimotor system and induce illusory sensation of specific gesture(Calvin–Figuiere et al. 1999). Interestingly, the induction of kinesthetic illusions generates hyperactivations in the precentral gyrus (BA 6), inferior parietal lobule (BA 40), and cingulate cortex (BA 32, BA 24) (Romaiguere et al. 2003), which we also find hyperactive in AAT subjects. Based on these data and previous localization of tympanic membrane movements at the caudal part of postcentral gyrus in the Rolandic operculum (BA 43), it could be possible to suggest that tinnitus may arise as a proprioceptive illusion. Such a possibility may seem inconsistent, because tinnitus is a sound, not a movement. However, movements of the tympano-ossicular chain are normally caused by sound. Thus, it would seem logical to us that illusory movements of the same chain generated by abnormal fusimotor activity could be interpreted as sounds by the brain.
It may seem paradoxical that we detected brain anomalies in AAT subjects only with “target” stimuli. We hypothesized that if the dysfunction is related to fine dysregulation of the acoustic reflex, a reflex activity such as found in “oddball task” (muscular responses when hearing targets) could reveal this type of dysfunction. It would be logical that the dysfunction as to reach a certain level to make the illusory percept clearly perceived (i.e., from occasional perception to permanent perception).
In our study, subjects with AAT sequelae were nonclinical tinnitus subjects, frequent/permanent tinnitus subjects had no severe handicap according to TRQ scores and consequently it might explain a nonmassive cortical overactivity in Broadman area 43 and 43/40, if it relates to tinnitus.
Possibly, recordings of very fine parameters of acoustic reflex or Eustachian tube function should be of interest to support a middle ear hypothesis.
In any case, direct experiments are clearly needed to test, for instance, whether specific vibrations applied to tendons of middle-ear muscle do generate tinnitus and which of the muscles, the stapedius, the tensor tympani, or both are involved in the illusory percept. If confirmed, the identification of a proprioceptive origin for tinnitus could open a new field of therapeutic approaches to this distressing pathology.
Furthermore, in the treatments of tinnitus, it could raised up the problem of middle-ear implants and their impacts on middle-ear muscle spindles activities. Depending on the location and the constraint applied to each of the middle-ear muscles, the illusory percept would be modified.
Conclusion
Our results actually illustrated the neuronal correlates of the hyperreactivity to auditory modality associated with AAT, and suggested associated sensorimotor anomalies affecting nonauditory pathways. Interestingly, our data also indicated abnormal overactivity in a brain region that corresponds to middle ear proprioception. We propose further investigations in this brain area because our results might suggest a model in which AAT tinnitus could arise as a proprioceptive illusion, associated with (or caused by) widespread emotional and somatosensory dysfunctions.
Acknowledgments
We thank Dr Greg O’Beirne for comments on the English manuscript and Alain Roux, Denis Preté, and Alexandre Krainik for their helpful technical assistance. This study was supported by a grant from the French government (DGA/PEA 010809/project no. 05Co002-05).




