Optimizing biophysical properties of cellular niches to enhance stem cell-derived extracellular vesicle function in musculoskeletal regeneration
Yang Xu, Chung Yin Matthew Cheung, and Liling Liu contributed equally to this study.
Abstract
Extracellular vesicles (EVs) secreted by stem cells have become a promising cell-free approach in regenerative medicine, with significant potential for the repair and treatment of musculoskeletal tissues and disorders. However, the limited bioactivity and scalability of EV production pose significant challenges for commercial production and clinical translation. To overcome these challenges, researchers have started exploring how the cellular microenvironment can modulate EV characteristics and enhance their therapeutic efficacy. While the microenvironment's biochemical facets have been the primary focus of prior investigations, the influence of biophysical factors on EV characteristics remains relatively underexplored. This review consolidates the existing research investigating the effects of biophysical features of the cellular microenvironment on EV production and function, with a particular emphasis on applications in musculoskeletal regeneration. By providing a comprehensive understanding of how biophysical factors impact EVs, this review seeks to enhance the development of effective strategies that harness the power of EVs for large-scale production and their successful application in regenerative therapies for musculoskeletal disorders. Ultimately, such insights could greatly assist patients who require innovative, cell-free regenerative treatments, thereby propelling advancements in musculoskeletal tissue engineering and in regenerative medicine.
1 INTRODUCTION
Stem cells possess unique regenerative abilities due to their abilities to proliferate, differentiate, and self-renew.1-5 Emerging evidence suggests that the therapeutic benefits of stem cells are primarily mediated through paracrine mechanisms, involving the secretion of extracellular vesicles (EVs).6, 7 EVs are membrane-bound structures released by various cell types, including stem cells.8 As essential elements of the stem cells secretome, EVs can be taken up by recipient cells, enabling the transfer of a diverse array of bioactive molecules, such as proteins, lipids, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA), for intercellular communication.9-11 EVs form through either inward or outward budding of the cellular membrane and are classified into three main subtypes based on their origin: exosomes, microvesicles, and apoptotic bodies (Figure 1a).12, 13 Exosomes, for instance, are endosomal vesicles formed through multiple invaginations of the plasma membrane and are released into the extracellular space via multivesicular bodies (MVBs), typically ranging from 30 to 150 nm.14 In contrast, microvesicles, also known as plasma membrane-derived EVs (pmEVs), directly originate from the plasma membrane without involving the MVB pathway or exocytosis, ranging from 100 to 1000 nm.8 The third category of EVs, known as apoptotic bodies, is released through the membrane blebbing in cells that are undergoing programmed cell death or apoptosis and exceeding 1000 nm in size.12
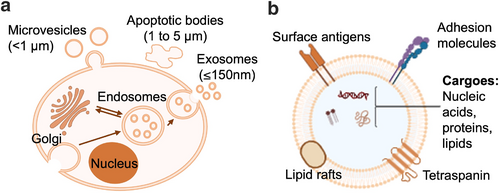
Biogenesis and structural characteristics of EVs. (a) Schematic representation of the biogenesis process and size range of three types of EVs: exosomes (30–150 nm) are formed through the endosomal pathway, microvesicles (<1000 nm) are directly released from the plasma membrane, and apoptotic bodies (1000–5000 nm) are generated during programmed cell death. (b) Structural characteristics of EVs, including microvesicles and exosomes. Both types consist of a lipid bilayer membrane that contains various bioactive molecules, such as proteins, nucleic acids, and lipids. EVs, extracellular vesicles.
Similar to their cellular counterparts (Figure 1b), stem cell-derived EVs have shown the capacity to promote proliferation and differentiation,15 immunomodulation,16 cell recruitment,17 pro-angiogenesis,16 and pro-survival functions.18 Therefore, EVs have gained recognition as a promising cell-free therapy for treating various diseases, offering advantages over cell-based approaches, such as reduced risks of genetic instability and malignant transformation (Figure 2).19 Furthermore, EVs exhibit superior permeability, systemic retention, and minimal immune clearance, making them a viable option for clinical translation.20, 21 In addition to the stem cell-derived EVs, EVs from other cell types have also demonstrated significant efficacy in treating musculoskeletal diseases. For example, EVs released by osteoblasts, endothelial cells, myocytes, and macrophages can also contribute to bone formation.22 Chondrocyte-derived EVs23 and skeletal muscle-derived EVs24 have also been shown to positively regulate cartilage and muscle regeneration. However, despite the considerable potential of EVs in regenerative medicine, several challenges must be addressed to facilitate their broader clinical application in EV-based therapies.12
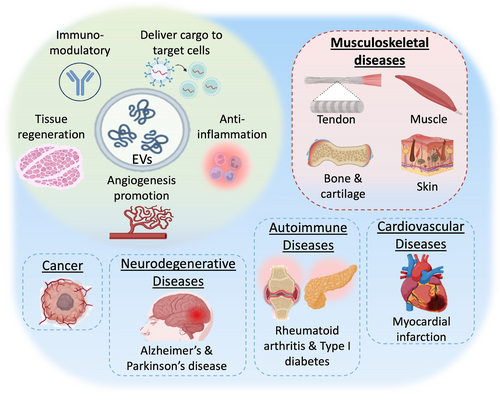
Roles of stem cell-derived EVs for disease treatment. Stem cell-derived EVs play critical roles in immune modulation, tissue regeneration, promotion of angiogenesis, anti-inflammation, and delivery of cargo to target cells, addressing various conditions including musculoskeletal diseases, cancer, neurodegenerative diseases, cardiovascular diseases, and autoimmune diseases. EVs, extracellular vesicles.
From a biological perspective, additional research is essential to better understand the molecular mechanisms underlying the therapeutic actions of exosomes.25 Furthermore, the assembly and packaging of EVs differ based on factors such as cell type, cell condition, and environmental stimuli, causing the standardization of EV production for clinical use to be challenging.26 Practically, comprehensive safety assessments are necessary, along with the development and standardization of manufacturing and quality control processes for clinical-grade EVs, particularly concerning large-scale production and optimal efficacy.25, 27 This underscores the need to explore strategies to improve the production scale, therapeutic efficacy and stability of EVs.28-31
As a promising strategy for influencing the functions of cells and their secreted EVs, extensive research is being conducted on modulating the cellular microenvironment which comprises a complex and dynamic network of biochemical and biophysical elements.32 Biochemical signals, such as growth factors (GFs), their derivatives, small bioactive molecules, and genetic regulators, combined with biophysical factors like stiffness, pore size, porosity, and topography, can collectively influence the attachment, proliferation, differentiation and paracrine activities of stem cells, including EV production.33, 34 While extensive research has been conducted on the biochemical approaches to enhance EV bioactivity, the significant impact of biophysical factors on EVs remains relatively understudied.35, 36 In this review, a bibliometric analyses was performed using PubMed, Web of Science, and ClinicalTrials databases, where a total of 322 papers were identified in August 2024 from the primary search using the following search terms: (1) “stem cells”, “extracellular vesicles”, and “regenerative medicine”; (2) “stem cells”, “extracellular vesicles”, and “musculoskeletal diseases”; (3) “stem cells”, “extracellular vesicles”, and “3D culture”; (4) “stem cells”, “extracellular vesicles”, and “topography”; (5) “stem cells”, “extracellular vesicles”, and “mechanical stimulation”; (6) “stem cells”, “extracellular vesicles”, and “electrical stimulation”; (7) “stem cells”, “extracellular vesicles”, and “stiffness”; (8) “stem cells”, “extracellular vesicles”, and “viscoelasticity”. After carefully examining each paper, 65 non-duplicate original research articles investigated the effects of biophysical features of the cellular microenvironment on EV characteristics, such as production and efficacy, specifically for the application in musculoskeletal regeneration (Figure 3). Data were collected from the full text of the articles as follows: (i) the microenvironment modulations and details of modulations; (ii) the details of EVs: source and size; (iii) the effects of stimulations on EVs: yield, efficacy enhancement, and potential mechanisms. With this information, this review endeavors to consolidate current studies investigating the effects of the biophysical features of the stem cell microenvironment on EV characteristics, such as production and efficacy, specifically for the applications in musculoskeletal regeneration. By providing a comprehensive understanding of the influence of biophysical factors on EVs, this review seeks to enable the formulation of effective strategies for large-scale EV manufacturing and clinical translation.
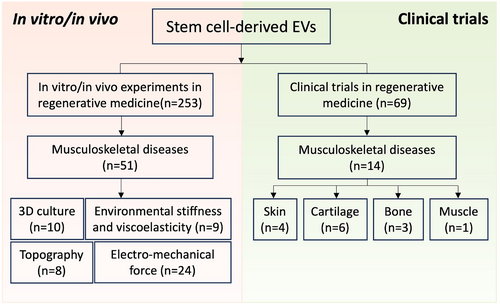
Literature review flowchart. The flowchart illustrates the systematic process of literature screening for in vitro, in vivo, and clinical trial studies involving stem cell-derived extracellular vesicles in the context of musculoskeletal tissue regeneration. (“n” refers to the number of relevant papers included in this review).
2 BIOCHEMICAL AND BIOPHYSICAL SIGNALS WITHIN THE CELL NICHE
Stem cells exist within a localized extracellular microenvironment or niche, where their behavior is closely regulated by biochemical and biophysical signals within the niche.37, 38 Therefore, a promising approach to enhance the bioactivity of stem cell-derived EVs is to exert precise control over stem cell microenvironments for modulated stem cell behaviors (Figure 4). Early studies suggested that the regenerative effects of mesenchymal stem cell (MSC)-EVs could be enhanced by modifying the biochemical cues of the cellular microenvironment. For example, hypoxic conditions can increase EVs production,39 or enhanced angiogenesis for promoting bone fracture healing.40 Supplementation with soluble factors, including GFs and cytokines within stem cell culture has led to the enhanced immunoregulatory effects of EVs for tissue regeneration.40 Biochemical modulation is relatively straightforward to implement and features targeted mechanisms with well-defined protocols. However, these methods can be costly and may entail side effects or toxicity. Additionally, regulating their effects over extended periods is challenging due to the short and variable half-lives of many biochemical factors.41-43
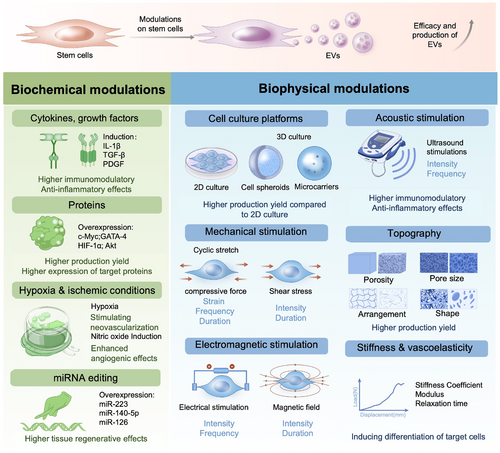
Modulation of biophysical and biochemical properties within the cell niche for enhanced yield and efficacy of stem cell-derived extracellular vesicles. Biochemical modulations encompass the application of for example, cytokines and growth factors, proteins, hypoxia and ischemic conditions, and gene editing techniques. Biophysical modulations include for example, cell culture platforms, mechanical stimulation, electromagnetic stimulation, acoustic stimulation, topography, stiffness, and viscoelasticity.
In contrast, biophysical cues present appealing alternatives. They are often more cost-effective and can be tailored to create specific microenvironments that mimic natural niches, allowing for precise control over cellular behaviors and functions.44 These biophysical cues, including surface topography, matrix stiffness, and mechanical forces have been thoroughly investigated and are crucial in regulating stem cell proliferation, self-renewal, and differentiation under various physiological and pathological conditions,45 including the regeneration of the injured musculoskeletal tissue. However, biophysical modulation also has notable limitations. For instance, different cell types may respond variably to the same biophysical cues, complicating the generalization of findings across different contexts.45 Furthermore, creating and maintaining specific biophysical environments can be technically demanding, requiring sophisticated equipment and expertise. Translating results from small-scale experiments to larger applications, such as in vivo models or clinical settings, can be complex and may not always yield consistent outcomes. Most importantly, the underlying mechanisms by which biophysical features influence cell behavior are not fully understood, making the design of effective strategies more challenging.
Biophysical cues within the cellular niche are essential in regulating stem cell fates, and their effects on the interaction between stem cells and EVs have garnered considerable attention.36, 46, 47 Modifying the biophysical properties of the cell environment, including the culture conditions, mechanical loading, electrical stimulation (ES), substrate topography, and substrate biomechanical features, will directly impact the bioactivity of cell-derived EVs (Table 1). While numerous reviews have shown that niche biophysical cues have the ability to induce both immediate intracellular responses and long-term alterations in cell phenotype,65-67 the reports on their effects on EV yield and function have been limited. However, this area of research remains largely unexplored. By understanding these advantages and limitations, researchers can better design experiments and applications that effectively utilize biophysical niche features to modulate cell behaviors, ultimately aiming for the scalable production of stem cell-derived EVs with enhanced therapeutic efficacy.
| EV source | Modulation conditions | Culture or scaffold conditions | Stimulation conditions | EV size | EV yield (relative to 2D control) | Efficacy | Potential pathways or mechanisms | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Cell culture platforms | Human BMSCs | N/A | A 3D hanging drop (3D-HD) spheroid of 2.5 × 104 MSCs/35 μL was cultured on a plate and incubated upside down in 3D-HD culture | N/A | N/A | 3-fold increase | No detectable difference | N/A | [48] |
| Human BMSCs | N/A | 3D printed porous Ti6Al4V titanium scaffolds | N/A | Average diameter around 200 nm | 2.2-fold increase | Higher osteogenic potency, extracellular matrix production, and mineralization | N/A | [49] | |
| Human dental pulp stem cells (DPSCs) | N/A | 3D Fibra-cell elastic scaffolds composed of 50% polyester fiber and 50% polypropylene | N/A | 172.9 ± 3.048 nm (The EV mean size did not differ among all groups) | 5- to 10-fold increase | No detectable difference | N/A | [50] | |
| Human adipose-derived MSCs | N/A | A cell-encapsulating 3D core–shell microfiber | N/A | N/A | 1000-fold increase | Higher levels of stemness markers; greater protein content (1023 proteins in 3D cultures vs. 605 proteins in 2D cultures) | N/A | [51] | |
| Human dermal microvascular endothelial cells | N/A | A 3D printed scaffold-perfusion bioreactor consists of small pillars that are 0.5 mm in size spaced 1 mm apart in a multi-layered shelf geometry | N/A | 40–200 nm | 100-fold increase | A trend of higher vascularization bioactivity (no statistical significance) | N/A | [52] | |
| Mechanical stimulation | Human skeletal muscle cells (SkMCs) | Cyclic stretch | PDMS elastic scaffold | 25% strain at 1 Hz for 2 days | N/A | About 11-fold increase | No detectable difference | N/A | [50] |
| Mouse BMSCs | Cyclic stretch | FX-5000T Flexercell tension plus unit | 0.5 Hz sinusoidal curve at 8% strain for 48 h | 40–260 nm (peak: 105 nm) | No difference | Inhibited osteoclast differentiation during both early (more potent) and late stages | Inhibited the NF-κB signaling pathway | [53] | |
| Human periodontal ligament cells (PDLCs) | Cyclic stretch | Silicon resin chambers coated with type I atelocollagen | 20% strain at a frequency of 0.167 Hz for 24 h | 30–100 nm (average: 50 nm) | About 30-fold increase | N/A | Inhibited LPS-induced NF-κB signaling pathway in macrophages | [54] | |
| Human PDLCs | Compression | A layer of glass with metal weights places a cell layer on top at 80% confluence in six-well plates | 1.5 g/cm2 static compression for 24 h | 30–150 nm | About 4-fold increase | Induced osteoclast differentiation as shown by increased expression levels of the osteoclast markers | Upregulated ERK phosphorylation in macrophages | [55] | |
| Human PDLCs | Compression | A layer of glass with metal weights places a cell layer on top at 80% confluence in six-well plates | 1 g/cm2 static compression for 24 h | 118.4 ± 47.8 nm | About 1.3-fold increase | Downregulated expression of sICAM-1 in hPDLCs, indicating possible downstream response of immune cells such as macrophage and T cells | Potential activation of NF-κB and ERK but requires further investigation | [56] | |
| Human periodontal ligament fibroblasts (PDLFs) | Compression | A layer of glass cover (1–3 pieces) were placed on top | 1 g/cm2 static compression for 4 h | 78.74 ± 18.09 nm | About 2-fold decrease | Increased the proportion of M1 macrophages | Exosomes derived from compression-treated PDLFs promoted M1 macrophage polarization via YAP-TEAD transcriptional axis | [57] | |
| Human DPSCs | Direct flow stimulation | Fibra-cel scaffoldTM (eppendorf) | 0.5 mL/min for 2 days | 172.9 ± 3.048 nm | 24-fold increase | Enhanced biological function to promote axonal sprouting | Regulated the paracrine effect of stem cells through YAP-mediated mechanosensing and activated Wnt signaling | [50] | |
| Human MSCs | Direct flow stimulation | Fibra-cel scaffoldTM (eppendorf) | 0.5 mL/min for 2 days | 167.1 ± 17.53 nm | 3.4-fold increase | No detectable difference | N/A | [50] | |
| Human osteocyte-like MLO-Y4 cells | Fluid shear stress | 10 μg/mL fibronecti-coated glass slides assembled into a custom parallel-plate flow chamber | 35 dyn/cm2 for two 10-min bouts of steady flow separated by a 15 min rest period | 200 nm | 20-fold increase | Increased in vitro expression of lysosomal-associated membrane protein 1 (LAMP1) and bone regulatory proteins in EVs | Induced Ca2+-mediated signaling | [58] | |
| Mouse bone marrow-derived hematopoietic progenitor cells (mHPCs) | Fluid shear stress | Cells seeded on 5 μg/cm2 bFN-coated glass slides subjected to pulsating fluid flow (PFF) using a parallel-plate flow chamber | Low intensity mechanical stimulation by fluid shear stress (0.7 ± 0.3 Pa, 5 Hz) for 2 min | At 10,000 rcf (ultra)centrifugation: 127.1 ± 11.6 nm; | Lower RCF generated about 5.8-fold higher EV yield | Reduced osteoclast formation by 2-fold compared to the control | N/A | [59] | |
| At 100,000 rcf (ultra)centrifugation: 91.7 ± 9.3 nm | No detectable difference | N/A | |||||||
| Mouse bone marrow-derived hematopoietic progenitor cells (mHPCs) | Fluid shear stress | Cells seeded on 5 μg/cm2 bFN-coated glass slides subjected to pulsating fluid flow (PFF) using a parallel-plate flow chamber | High intensity mechanical stimulation by fluid shear stress (2.9 ± 0.2 Pa, 1 Hz) for 2 min | At 10,000 rcf (ultra)centrifugation: 141.3 ± 18.2 nm | Higher RCF generated about 4.5-fold higher EV yield | No detectable difference | N/A | [59] | |
| At 100,000 rcf (ultra)centrifugation: 93.7 ± 10.5 nm | 2-fold increase in osteoclast formation compared to the control | N/A | |||||||
| Rat primary condylar chondrocytes | Fluid shear stress | Type I collagen-coated glass slides (Flexcell) | Fluid flow shear stress at 4, 8, and 16 dyn/cm2 for 1 h every 3 days for 21 days | 50–150 nm | Higher fluid shear stress increased yield from 1.3- to 2.5-fold relative to static conditions | Promoted calcification in vitro and exacerbated cartilage degeneration in vivo in osteoarthritic rats | Protein levels of MGP, TNAP, and NPP1 in exosomes were decreased | [60] | |
| Human dermal microvascular endothelial cells (HDMECs) | Dynamic shear stress | 3D printed scaffolds using a commercially available stereolithography apparatus | Inlet and outlet of the media circulating at 4 mL/min (average: 1.5 × 10−2 dyn/cm2) flow rate for 24 h | 97 ± 21 nm with ethanol treatment, | About 100-fold increase | EVs from HDMECs ECs were shown to have a trend of higher vascularization bioactivity (no statistical significance) | Long non-coding RNA mediated mechanism | [50] | |
| 89 ± 23 nm without ethanol treatment | |||||||||
| Electromagnetic stimulation | Circulating EV were isolated from serum of aged mice (21–24 months old) following neuromuscular electrical stimulation (NMES) | Electrical stimulation | N/A | Symmetric waveform, 9 mA, 150 μs pulse duration, 50 Hz, 5 s on/10 s off with 0.5 s ramp up/down | 50–100 nm | No difference | Enhanced gait functional recovery in aged mice skeletal muscle after acute injury | N/A | [61] |
| Human BMSCs | Static magnetic field | Cultured with 50 μg/mL magnetic Fe3O4 in a culture plate with a magnet underneath composed of a monolayer of small magnetic sheets | 50 μg/mL Fe3O4 nanoparticles and a 100 mT static magnetic field (SMF) for 5 days | 52–168 nm | About 1.8-fold increase | Enhanced angiogenesis and increased cord-like structures in HUVECs in vitro. It promoted bone regeneration and angiogenesis in vivo, resulting in higher BV/TV, trabecular number, collagen density, and vascular structure | Upregulated miR-1260a, which was found to enhance osteogenesis and angiogenesis through inhibition of HDAC7 and COL4A2 | [62] | |
| Human BMSCs | Magnetic field | Cultured with magnetic Fe3O4 in a culture plate assembled a permanent neodymium magnet underneath | 20 μg/mL Fe3O4 and a 100 mT SMF for 48 h | 80–160 nm (mean size: 130 nm) | No difference | Enhanced fibrogenesis and proliferation of NIH3T3 fibroblasts in vitro. In vivo, it facilitated tendon-bone healing, evidenced by increased maximal graft failure forces, greater tendon graft stiffness, reduced cross-sectional area of tibial bone tunnels, and augmented collagen and osteoid deposition | Exosomal miRNA-21-5p mediated EV on tendon-bone healing by targeting SMAD7 | [63] | |
| Human periodontal ligament stem cells (PDLSCs) | Magnetic field-induced stretching | 3D microscale magnetically stretched collagen hydrogel | A 7.5% (w/v) Fe3O4 nanoparticle/collagen solution was injected and crosslinked at both ends of the hydrogel. The ends were fixed to NdFeB magnets, allowing the dumbbell-shaped hydrogel to be stretched with 20% strain for 72 h | 97.7 ± 1.7 nm | No difference | In vitro osteogenesis was enhanced by increased expression of osteogenesis-related genes, proteins, and mineralized nodules. In vivo, notable new bone formation (∼80% coverage of defect) was observed, compared to the PBS control group (∼10% coverage of defect) | N/A | [64] | |
| Topography | Human BMSCs | Pore shape | 3D printed porous Ti6Al4V titanium scaffolds | Pore of either triangular or square shape | Average diameter around 200 nm | 1.7-fold increase in scaffolds with triangular shape compared to square shape | Higher osteogenic potency, extracellular matrix production, and mineralization | N/A | [49] |
| Human BMSCs | Pore size | 3D printed porous Ti6Al4V titanium scaffolds | Pore size of either 500 μm or 1000 µm | Average diameter around 200 nm | 2.2-fold increase in scaffold with 1000 μm pore size compared to 500 μm pore size | Higher osteogenic potency, extracellular matrix production, and mineralization | N/A | [49] |
3 CELL CULTURE PLATFORMS
Modifying the culture platforms of stem cells can have favorable effects on the properties of EVs for tissue regeneration. Conventional cell culture techniques employ a two-dimensional (2D) approach, wherein cells adhere to a planar surface, such as a flask or petri dish. This culture system, while widely used, fails to recapitulate the complex three-dimensional (3D) microenvironment found in vivo. In addition to traditional 2D culture, various emerging 3D cell culture platforms have been developed to more closely emulate the natural tissue architecture and cell-cell interactions. These 3D culture systems include self-assembled cell spheroids and tissue-engineered microcarriers. By providing a more physiologically relevant microenvironment, these 3D culture platforms can potentially modulate the biogenesis, cargo, and functional properties of stem cell-derived EVs, making them more suitable for regenerative applications.68
Spheroids, for instance, are cell aggregates that self-organize within a setting designed to prevent attachment to a planar surface. Techniques such as non-adhesive agarose hydrogels, rotary cell cultures, nanofiber addition, magnetic levitation, and microfluidic systems have been employed to facilitate spheroid formation. Spheroids find applications in drug testing and the development of in vitro disease models.69 Additionally, 3D structured cell-laden scaffolds and hydrogels have been developed to offer essential physicochemical and mechanical support, enabling cells to form extracellular matrix (ECM) in vitro, which can be slowly degraded, resorbed, or metabolized upon in vivo implantation.70, 71
Microcarriers are another crucial element of 3D-structured scaffolds, facilitating the attachment of adherent cells to create cell-microcarrier complexes that are suspended in a growth medium.72 Microcarriers typically consist of tiny beads ranging in size from 100 to 300 microns, engineered to remain suspended during stirring. Microcarriers with a larger surface area-to-volume ratio and optimized surface properties facilitate cell attachment, allowing for the formation of microcarrier–cell complexes. This configuration offers a substantial surface area for cell growth in suspension cultures, enabling scalable cell production in smaller volumes of medium.73 Additionally, these cell suspension culture systems enhance nutrient and gas exchange and allow for adjustable shear stress, which may further promote cell differentiation. After cultivation, microcarrier–cell complexes can be used directly as end products without cell removal. For example, it was shown that microcarriers cultured with MSCs improved trabecular bone formation relative to cell-free microcarriers in long bone defects.73, 74 Alternatively, these complexes can be used to produce cell-derived products such as supernatant or EV. Commercially available microcarriers are constructed from various materials, including glass, diethylaminomethyl (DEAE)-dextran, acrylic, polystyrene, collagen, and alginate.75
To address the burgeoning need for biopharmaceutical production, a multitude of bioreactor systems have been devised. The conventional stirred tank bioreactor persists as the most commonly used option in the industry, while alternative technologies, including airlift and filtration-based designs (e.g., spin, hollow-fiber, acoustic, and cross-flow filters), have been less frequently utilized. Other widely employed platforms include fixed and fluidized-bed systems (e.g., packed-bed and fluidized-bed bioreactors) as well as disposable bioreactors (e.g., CellCube (Corning), Cell Factory (Nunclon), Regenbio (REGEN.GEEK), and the Wave bioreactor (WaveBiotech)).76, 77 Additional details about the 3D cell culture market can be found in Figure 5.
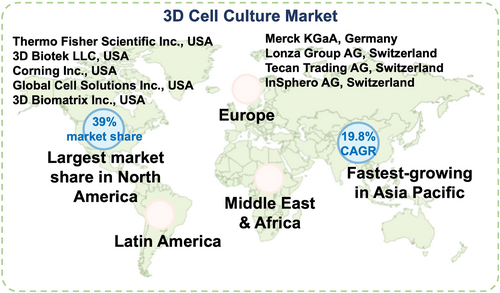
Global 3D cell culture market. At present, North America commands the global market, while the Asia-Pacific region is the fastest-region, driven by increased investments and developments in emerging economies. The major companies operating in the 3D cell culture market include Thermo Fisher Scientific Inc., Merck KGaA, 3D Biotek LLC, Lonza Group AG, Corning Inc., Tecan Trading AG, Global Cell Solutions Inc., 3D Biomatrix Inc., and InSphero AG. These companies offer a range of 3D cell culture products, such as scaffold-based systems, scaffold-free platforms, bioreactors, and microchips, which enable the creation of more physiologically relevant in vitro models.
Research has shown that transitioning from 2D to 3D environments, with an increased dimensionality of the ECM surrounding cells, can significantly impact various cell behaviors, such as cell proliferation, differentiation, mechano-responses, and cell survival in musculoskeletal regeneration.78-80 For instance, in a 3D self-assembled cell spheroid culture system, bone mesenchymal stem cells (BMSCs) exhibited enhanced multipotency compared to a 2D culture system. Notably, these cells demonstrated improved adipogenic and osteogenic differentiation capabilities, along with the ability to differentiate into ectodermal epithelial progenitor-like cells and neuron-like cells.81
Self-assembled spheroids derived from stem cells have shown strong angiogenic and vasculogenic abilities, indicating their potential to serve as effective vascularization units within porous scaffolds for bone and cartilage tissue engineering.82 The application of 3D-bioprinting technology to self-assembled cell spheroids has also been explored, especially regarding their potential to enhance the therapeutic effects of stem cells in cartilage regeneration and their application as chondral tissue implants.83, 84 Beyond spheroids, microcarrier systems that are based on biomimetic demineralized bone matrix have been shown to provide MSCs with exceptional osteogenic and angiogenic capacities.85 Moreover, 3D microenvironments have been found to modify the distribution of cell subpopulations in human tendon stem/progenitor cells (hTSPCs) and enhance tenogenesis through cell-cell interactions.86 Overall, 3D culture platforms have been found to augment the directional differentiation capacity of stem cells in musculoskeletal tissue regeneration.
Recognizing that 3D culture more closely mimics the physiological environment than traditional EV production using 2D culture on polystyrene,87 multiple studies have explored the yield and contents of EVs prepared in 2D versus 3D culture. For example, a study by Kim et al. contrasted the secretion of MSC derived-EVs across conventional 2D monolayer culture, 3D hanging droplet spheroids, and 3D aggregates formed via a poly (2-hydroxyethyl methacrylate) coating method. Quantitative analysis revealed a 3-fold augmentation in EV secretion favoring the 3D culture modalities over the 2D monolayer approach. Furthermore, decreasing the spheroid diameter resulted in up to a 6-fold increase in EV secretion, likely due to the increased effective surface area.48 Further highlighting the advantageous impact of 3D culture on EV secretion rates, hydrostatic cultivation of 3D-engineered tissues seeded with dental pulp stem cells (DPSCs) or MSCs led to a 5- and 10-fold increase in EV secretion compared to conventional 2D monolayer culture.50 Similarly, a recently published paper demonstrated that a 3D core-shell microfiber environment enriched particles by approximately 1009-fold compared to conventional culture methods.51 Additionally, the review further demonstrated that the secretion of EVs was significantly enhanced in a 3D continuous dynamic system utilizing different parent cells, with increases ranging from 4.44-fold to 100-fold.52 Overall, these findings provide strong evidence that 3D cell culture offers additional benefits in terms of EV production yield relative to conventional 2D cell culture.
Along with the increased production scale, liquid chromatography-mass spectrometry characterization results indicated that EVs derived from 3D cultures exhibited a higher number of proteins (1023 vs. 605) than those from 2D cultures.51 In a review by Ketki Holkar examining the influence of cell culture modalities on the regenerative potential of MSCs, particularly in bone regeneration, it was noted that growing MSCs in 3D microenvironments recapitulating the physiological parameters of native bone tissue could further enhance the MSCs' secretory profile. Moreover, EVs derived from MSCs cultured in collagen hydrogel exhibited a greater osteogenic potential than those from 2D-cultured MSCs.88 In the context of osteochondral regeneration, EVs derived from 3D cultures (3D-Exos) have shown superior performance compared to those from 2D cultures by activating transforming GF beta 1 and Smad 2/3 signaling. Additionally, the 3D microenvironment has been shown to enhance the secretion of angiogenic hypoxia-conditioned MSC-derived EVs.52 Therefore, 3D approaches and models may prove valuable for enhancing the secretion of therapeutic EVs from stem cells.
Generally, transitioning from traditional 2D cell culture to 3D geometries offers more physiologically relevant culture conditions, resulting in increased EV secretion yields and enhanced therapeutic potential for different disease models.89 However, while 3D culture increases the number and contents of EVs, there are certain drawbacks to consider. For example, the binding avidity of EVs to ECM constituents within the 3D scaffold can hinder their easiness of release, potentially necessitating additional processing, such as enzymatic treatment to break down the scaffolds. Unfortunately, such enzymatic treatments may impact the structural integrity and bioactivity of the harvested EVs.52 Furthermore, the yields of scaled-up MSC-derived EV production have shown inconsistencies, even when using similar culture techniques and consumables.75, 83 Consequently, scientists have explored various solutions to address these challenges and promote the use of EVs as regenerative nanomedicines. This includes regulating physical parameters to optimize EV production and release from 3D culture systems. By addressing the limitations of 3D culture, researchers aim to fully harness the benefits of this approach for enhancing the yield and therapeutic potential of EVs for the application of regenerative medicine.
4 MECHANICAL LOADING AND ELECTROMAGNETIC STIMULATION
Mechanical and electromagnetic stimulation exerts a significant role throughout the lifecycle of the musculoskeletal system.90, 91 The physiological loading of musculoskeletal tissues, characterized by localized stress and strain arising from muscle contractions and tendon and ligament tension, is an ubiquitous component in daily activities.92, 93 These extracellular mechanical stimuli are transduced into intracellular signaling cascades, modulating the expression of ECM genes, nuclear proteins, and transcription factors, which subsequently influence the cell secretome.94 The mechanoenzyme pathway constitutes a pivotal mechanism mediating intercellular communication and interactions between cells and their surrounding microenvironment. This pathway encompasses a cell's response to a mechanical load, resulting in the secretion of EV signals that mediate both local and distant intercellular communication.52 Thus, mechanical loading, including tension, compression, and shear force, as well as electromagnetic and acoustic stimulations, has the potential to modulate cellular EV secretion mechanisms, leading to enhanced EV production under specific loading conditions (Figure 4).95
4.1 Tension
Tension, characterized by a directional pull exerted upon an object, represents a rudimentary aspect of physiological processes, exemplified by muscle contraction—a result of cellular traction-induced movement.96 Cyclic tensile stretching, which induces elongation of the ECM, imposes strain on cells. In physiological settings, tension manifests cyclically, predominantly impacting fibroblasts in ligaments and tendons throughout the growth and development. Consequently, mechanical stretching has been extensively leveraged to promote tendon tissue regeneration.97 Applying mechanical stretching to engineered scaffolds or functional tendon constructs could facilitate cell infiltration and proliferation, maintain the mechanical strength of the tissue, preserve the bioactive GFs of tendon ECM,98, 99 and stimulate ECM deposition and collagen fiber organization.100-102 Moreover, such stretching activates mechanosensitive receptors, thereby promoting tenogenic differentiation.101, 103-105 For instance, uniaxial tensile loading (6%, 0.25 Hz) applied to tendon-derived stem cells (TDSCs) significantly upregulates tenogenic markers while suppressing expressions of osteogenic, adipogenic and chondrogenic markers by activating the Phosphoinositide 3-kinase (PI3K)/Protein Kinase B (AKT) signaling pathway.103 Similarly, cyclic tensile strain has also been widely used to promote osteogenic differentiation in MSCs across a spectrum of elongation percentages and cycles in several studies.90, 106-110 The clinical application of tensile strain, exemplified by distraction osteogenesis, a surgical procedure pioneered by the Russian physician Ilizarov in the early 1950s, demonstrates the capacity to induce and promote de novo bone formation within a distraction gap through intentionally creating a fracture between two ends of the bone segments and gradually pulling them apart.111, 112
While the profound influence of mechanical stimulation on cellular function is well-documented, the intricate interplay between mechanical agitation and EV biogenesis remains an emerging area of investigation. For instance, a recent study by Najrana et al. demonstrated a significant enhancement in exosome production by bronchial epithelial cells (BECs) subjected to 10% cyclic stretch at a frequency of 0.67 Hz for 24 h, resulting in a 2-fold increase compared to baseline levels, whereas production remained unchanged following 5% static stretch over the same period.113
In the musculoskeletal system, tensile stretch has been shown to strongly boost EV secretion. Skeletal muscle cells (SkMCs), seeded into 3D-printed Polydimethylsiloxane (PDMS) elastic scaffolds and subjected to cyclic tensile stretch at 1 Hz at 25% strain for 48 h, exhibited an 11-fold increase in EV yield compared to static SkMCs, while maintaining EV size.50 Furthermore, exosomes derived from the cells cultured within a 3D mechanically stretched environment demonstrate a more potent bioactivity. Yu et al. fabricated 3D microscale magnetically stretched collagen hydrogels to culture periodontal ligament stem cells (PDLSCs) and investigated the phenotypic and functional characteristics and in vitro and in vivo musculoskeletal bioactivity responses of exosomes derived from PDLSCs subjected to this unique 3D stretched strain environment (SM-Exo). SM-Exo significantly promoted BMSCs proliferation, with considerable upregulations of 1.5-to-5-fold in the expressions of osteogenic genes, such as Alkaline Phosphatase (ALP), Runt-Related Transcription Factor 2 (RUNX2), and Osteocalcin (OCN), compared to cells treated solely with pristine exosomes. In vivo studies corroborated these findings, demonstrating enhanced regeneration in defects treated with SM-Exo/Matrigel, as evidenced by histological analysis using Hematoxylin and Eosin (H&E) and Masson's trichrome staining. After a 6-week healing period, defects treated with SM-Exo/Matrigel exhibited significantly greater regeneration (∼80%) compared to those treated with Exo/Matrigel (∼35%), highlighting the superior regenerative potential of exosomes derived from mechanically stretched cells.64
In another study, exosomes were shown to play a pivotal role in maintaining homeostasis by modulating intracellular communication between osteoblasts and osteoclasts under mechanical loading. Exosomes derived from cyclic-stretch-treated BMSCs (CMS-Exos) demonstrated a potent inhibitory effect on osteoclast differentiation by attenuating the RANKL-induced nuclear factor kappa-B (NF-κB) signaling pathway, thereby mitigating osteoporosis induced by mechanical unloading in a hindlimb unloading (HU) mouse model.53 These findings offer insights to promote bone regeneration through the modulation of MSC-derived exosomes within a precisely engineered 3D mechanical stimulated microenvironment.
Albeit, in most cases, tensile stretch often leads to an increase in EV production by parent cells, exceptions still exist. For instance, exosome production from cyclically stretched periodontal ligament cells (PDL cells) increased 30-fold compared to unstimulated cultivation, but plateaued after approximately 36 h of cyclic stretch.54 Overall, these studies highlight the profound impact of cyclic tensile stress stimulations on the quantity and functional effects of EVs released by musculoskeletal stem cells, underscoring the critical need to investigate the optimal parameters of cyclic tensile stress to create a precisely controlled microenvironment that can preponderantly regulate stem cell differentiation towards tenogenic or osteogenic lineages and thus controlling EV secretion.
4.2 Cyclic compression
Compressive stresses, exerted perpendicular to the target area, induce directional compression that “squeezes” the target. This increased pressure within a more confined space can mediate intracellular communication and regulate cell growth and differentiation.114 Apart from cyclic tensile strain, compressive cyclic strain has also been shown to modulate osteogenic differentiation and accelerate osteogenesis within bone grafts.90, 115-118 For example, transient uniaxial compression at a mild magnitude (0.4%, 0.1 Hz, 2 h/day for 1 day) on human MSC (hMSC)-laden monetite calcium phosphate (CaP) scaffolds, which mimic the biomechanical properties of bone, effectively promoted osteogenic differentiation.119 However, evidence suggests that the osteogenic potential of hMSCs induced by dynamic compressive stress is contingent upon loading strain.119, 120 Subjecting hMSCs in electrospun 3D scaffolds to dynamic compressive strains at various levels exhibited distinct differentiation tendencies, where increased magnitudes of compressive strain upregulated chondrogenic gene expressions, while markers associated with bone and calcium deposition exhibited strain-dependent reductions.119 Current studies typically employ a compressive loading regimen characterized by a frequency of 0.5–1 Hz, a sinusoidal strain amplitude of 10%–15%, and a duration of at least 30 min per day. This mechanical stimulation protocol has been shown to significantly upregulate chondrogenic gene expression in cells after a minimum of 7 days of culture.119, 121, 122
The effect of compressive loading on EV biogenesis and release remains underexplored, with research hampered by inconsistencies in cell sources and stimulation methodologies. However, recent studies have begun to unveil this relationship. Hao et al. demonstrated that mechanical compressive effects induced by a microfluidic device boosted small extracellular vesicle (sEVs) production from human fetal bone marrow stem cells approximately 4-fold in 48 h. These stretch-derived sEVs were also shown to facilitate corneal epithelial wound healing, accelerating wound closure time by nearly 50%. Gene expression analysis revealed the upregulation of Interleukin-6 (IL-6), Transforming GF-beta 1 (TGF-β1), and Zonula Occludens-1 (ZO-1) genes in the scratched immortalized human corneal epithelial cells (iHCEC) monolayer treated with the stretch-derived sEVs compared to their static counterparts.123 Similarly, Huang et al. reported a significant enhancement in EV yield, approaching a 4-fold increase, following the application of 24 h of mechanical compressive stress to periodontal ligament stem cells. This was accompanied by upregulation of annexin A3 (ANXA3), an exosomal protein known to facilitate EV internalization. This upregulation triggered the phosphorylation of extracellular signal-regulated kinase (ERK) and subsequently induced osteoclast differentiation in the periodontal ligament. These studies highlight the potential of compression loading alongside tensile stretching, to regulate EV biogenesis and functionality for modulating bone hemostasis.55 However, the relationship between compressive stress and EV production is not always straightforward. While 1 g/cm2 compression on periodontal ligament cells (hPDLCs) for 24 h promoted a 1.3-fold increase in sEV production,56 the same magnitude of stress for 4 h resulted in a 50% decrease in EV secretion.57 This discrepancy may be attributed to factors including the specific nature of the compressive force applied, the heterogeneity of parent cell sources, and variations in experimental methodologies, such as EV isolation, purification, and quantification. Thus, further investigation is needed to establish an empirical relationship between compressive loading and EV product quality and quantity. Optimizing compression regimens for EV biogenesis and function will be crucial for leveraging these mechanical cues in therapeutic applications.
4.3 Shear stress
Shear stress, which represents the parallel force exerted on a cellular or extracellular structure induced by differences in the velocity of an adjacent body or fluid, constitutes a fundamental biochemical stimulus.124 Fluid flow models can be primarily categorized into two types: orbital and linear fluid flow.96 Flow-induced shear stress can be classified as steady, pulsatile, or oscillatory depending on the dynamic characteristics of fluid stress.114 Oscillatory flow is characterized by variations in shear direction and potentially its magnitude over time, while pulsatile flow exhibits regular changes in shear stress magnitude.114, 124 The systemic circulatory system, encompassing both blood and lymph flow, exerts continuous pulsatile or oscillating fluid shear stress on musculoskeletal cells. This shear stress is modulated by variations in mechanical loading, muscle contractions, blood pressure fluctuations, lymph flow, and other physical activity-related factors.125 In contrast to the direct deformation induced by tensile forces, cell membranes exhibit sensitivity to changes in shear stress, initiating a cascade of events that ultimately lead to cellular deformation. Chondrocytes and osteocytes, exposed to interstitial fluid flow, have prompted extensive investigations into the effects of shear stress stimulation on musculoskeletal stem cell differentiation.126-130 For example, Kim et al. showcased facilitated MSC osteogenic differentiation through the activation of transcriptional coactivator with PDZ-binding motif (TAZ) under a steady and extremely low shear stress environment generated by osmotic pressure-induced flow in a microfluidic chip.131
In addition to the magnitude of shear stress, the intricate interplay of fluid flow rate, viscosity, flow direction and flow pattern must be meticulously considered, as these parameters exert a profound influence on cellular responses, potentially modulating a wide range of cellular processes. Yue and colleagues unveiled a critical role for the rate of change in fluid shear stress (ΔSS) in orchestrating the lineage commitment of MSCs, directing them towards either osteogenic or chondrogenic differentiation pathways. Subjecting MSCs to 20 min of rapid ΔSS promoted chondrogenic differentiation, while slow ΔSS over the same duration facilitated osteogenic differentiation, exhibiting comparable ALP and glycosaminoglycan (GAG) secretion profiles to those induced by 5 days of osteogenic or chondrogenic chemical and 2 days of substrate stiffness induction. This suggests that the rate of fluid shear stress can be a powerful regulator of MSC differentiation, as effective as chemical or substrate stiffness cues.132, 133 However, the application of shear stress alone may not be sufficient; a multifactorial approach, incorporating the synergistic effects of shear stress and dynamic compression, may more closely mimic the intricate in vivo biophysical environment. For instance, a pin-on-ball bioreactor system that generates dynamic compression force and shear stress through simultaneous ball compression and oscillation on a cell-scaffold construct induced chondrogenesis, as evidenced by significant increases in the expression of chondrogenic gene markers, such as collagen II (COL 2), SRY-Box Transcription Factor 9 (SOX 9) and aggrecan (ACAN). Furthermore, the increased COL 2: COL 1 ratio suggested that the matrix was progressively composed mainly of the cartilage-specific COL 2.134
To investigate the hypothesis that 3D culture coupled with the application of mechanical stimuli mimicking pivotal physiological milieu can enhance EV biogenesis by modulating cellular responses, several studies have delved into the influence of shear stress or fluid flow on the yield and therapeutic potency of stress-derived EVs. Mild shear stress, ranging from 1 × 10−3 to 1 × 10−4 dyn/cm2, has been associated with the activation of plasma membrane ion channels, causing an influx of intracellular Ca2+ and upregulation of proteins associated to cytoskeleton reorganization and EV biogenesis, including β-catenin, ERK 1/2, and calpain.135
In a recent study, Kronstadt et al. investigated the impacts of flow-derived shear stress generated by a 3D-printed scaffold-perfusion bioreactor system on the production and bioactivity of EVs secreted from BM-MSCs. Applying a milder shear flow at a rate of 3 × 10−3 dyn/cm2, enhanced EV production by approximately 83-fold when compared with static culture and by 2.5-fold when compared with a higher flow rate of 1.2 × 10−2 dyn/cm2 without causing obvious cell detachment observed under a stronger flow rate condition. Notably, the EVs derived from the perfusion bioreactor improved wound healing in a diabetic mouse model, exhibiting increased CD31+ staining in wound bed tissue compared to animals treated with EVs generated from static cell culture, highlighting the potential of bioreactor-derived EVs for therapeutic applications. Altogether, this research underscores the role of shear loading in augmenting the secretion of MSC-derived EVs while preserving their pro-angiogenic bioactivity, effectively enhancing the therapeutic potency of EV-based formulations.136
Nevertheless, the expected EV production may differ significantly among different MSC subtypes. For instance, Guo et al. elucidated a 24-fold augmentation in the yield of DPSC-derived EV under an average shear stress of 0.5–5 dyn/cm2, whereas adipose MSCs exhibited only a 3.4-fold increase in EV production under the same conditions. Notably, MSC-derived EV secretion declined under stronger shear loading (5–30 dyn/cm2), suggesting the existence of cell-specific optimal shear levels, beyond which additional mechanical stress adversely affects EV yield.50 A pioneering study by Morrell et al. demonstrated that applying parallel bouts of steady fluid flow at 35 dyn/cm2 in two 10 min intervals to osteocyte-like Murine Long-Bone Osteocyte Y4 (MLO-Y4) cells resulted in simultaneous actomyosin contractions following the onset of flow-induced Ca2+ oscillations in osteocytes. This regimen boosted at least 20-fold increase in the yield of EV and upregulated the expression of the intracellular lysosomal-associated membrane protein 1 (LAMP1). This load-related increase in LAMP1 expression was significantly blunted in neomycin (a phospholipase C inhibitor)-injected mice, suggesting a critical role of EVs in Ca2+-mediated signaling in bone adaptation.58 In another study, Bratengeier et al. highlighted that the intensity of shear loading influences EV release with osteoclast-modulating effects. Mouse bone marrow-derived hematopoietic progenitor cells (mHPCs) were stimulated with low-intensity shear loading (PL) and high-intensity shear loading (SPL), respectively. The nuclear factor kappa-B ligand-induced osteoclastogenesis assay elucidated that the EV pellet derived from mHPCs after PL caused a reduction in osteoclast formation, whereas the SPL-derived EV pellet facilitated osteoclast formation. These findings provide compelling evidence that the intensity of mechanical loading exerts a significant influence on the release of EVs, subsequently altering their osteoclast-modulating effects, thus contributing to the delicate balance of bone homeostasis.59 Despite potential therapeutic benefits, exosomes derived from abnormal shear loading may activate pathological progression of osteoarthritis (OA). Culturing primary condylar chondrocytes under fluid flow shear stress (4, 8, and 16 dyn/cm2) for 1 h every 3 days produced roughly 1.3, 1.7 and 2.5-fold more exosomes compared with the control counterpart. The enhanced formation of exosomes induced from 16 dyn/cm2 fluid flow shear stress caused 3.5 times more in vitro calcified nodules formation and exacerbated in vivo cartilage degeneration in rats with temporomandibular joint (TMJ) OA, underscoring that exosomes derived from degenerative chondrocytes under aberrant mechanical environment can alternatively contribute to the progression of musculoskeletal pathogenesis.60 Hence, understanding the dynamic between shear stress and particular fluid shear stress induced by physiological fluid, and its influence on EV production offers potential therapeutic avenues and lays the foundation for understanding the mechanisms of musculoskeletal-related pathologies.
4.4 Electromagnetic stimulations
Electromagnetic fields (EMFs) epitomize the interaction between electric and magnetic fields. Endogenous electrical fields (ENEF) exert a profound influence on a wide range of physiological activities, encompassing cell proliferation differentiation, cell cycle, apoptosis, cytokine expression, neural conduction, and wound healing.137-140 Wang and Yasuda explored the piezoelectric effect in bone tissue, indicating that the piezoelectricity in bone may be ascribed to the piezoelectric effect of the crystalline micelles of collagen molecules.141, 142 This phenomenon, attributed to the non-centrosymmetrical organization of collagen molecules, underscores the intrinsic piezoelectricity in bone.143 Similarly, natural articular cartilage exhibits the capacity to convert external electromagnetic signals into mechanical signals and vice versa, owing to the piezoelectric nature of collagenous matrices, where charged particles flow across negatively charged proteoglycan (PG).137 Exogenous ES has emerged as a pivotal biophysical cue for modulating stem cell fate and advancing regenerative medicine by fostering a conducive and favorable microenvironment.137, 144-146 These signals have been shown to augment extracellular fibronectin adsorption, facilitate cell proliferation and migration, stimulate the endogenous synthesis of TGF-β1 through calcium ion signaling pathways, and improve chondrogenesis both in vitro and in vivo. Piezoelectric stimulation has also been found to enhance the regeneration of hyaline cartilage in rabbit osteochondral defect models, resulting in complete cartilage healing characterized by abundant chondrocytes and type II collagen 3 months post-surgery, in stark contrast to the limited healing observed in sham groups.147 These findings suggest that the potential of electroactive materials harnessing exogenous or endogenous electrical stimulations to customize the local electric environment for promoting musculoskeletal lineage differentiation is a significant research endeavor for tissue engineering.
The therapeutic efficacy of electric stimulations in exciting EV release and cargo loading has garnered growing attention across several disciplines, including cardiology, neurology, oncology, and orthopedics.61, 148-150 For instance, Yang et al. demonstrated a novel cellular nanoporation method where focal and transient ES were employed to amplify exosomes containing therapeutic mRNAs and targeting peptides. In comparison to conventional bulk electroporation and other stress-induced EV release strategies such as starvation, hypoxia, and heat, this nanoporation technique induced by transient electrical pulses on mouse embryonic fibroblasts (MEFs) demonstrated a remarkable increase in exosome production, achieving up to a 50-fold and 40-fold enhancement, respectively, compared to the aforementioned methods. Furthermore, this nanoporation approach resulted in a substantial surge in exosomal mRNA transcripts, exceeding a 103-fold increase. The orthotopic phosphatase and tensin homolog (PTEN)-deficient glioma mouse models revealed that the mRNA-containing exosomes restored tumor-suppressor function, enhanced inhibition of tumor growth, and prolonged survival, suggesting that electrical agitation is an effective means to enhance EV production and improve therapeutic efficacy to act as a functional mRNA cargo by increasing 2000 to 10,000-fold in loading efficiency.150
Similarly, low-level electric treatment moderately stimulated EV secretion in murine melanoma (B16F1) and murine fibroblast (3T3 Swiss Albino) cells. However, treatment with Rhosin hydrochloride, a Rho GTPase inhibitor, only suppressed the ET-mediated increase in EV secretion of B16F1, suggesting that sensitivity to the Rho GTPase inhibitor might vary between different cell types.151
In treating musculoskeletal-related injuries, Bean et al. illustrated that circulating EVs derived from aged animals subjected to neuromuscular electrical stimulations (NMES) exhibited a remarkable rejuvenating effect on the contractile activity of injured aged skeletal muscle. Exposing NMES (symmetric waveform, 9 mA amplitude, 150 μs pulse duration, 50 Hz frequency, 5 s on/10 s off with 0.5 s ramp up/down) to mimic repetitive muscle contractile activity to the knee of the hind limb of aged mice for two weeks decreased the EV surface tetraspanin expression profile and changed the biomolecular profile in lipid and sugar content. These findings suggest that NMES induces alterations in the structure and cargo composition of circulating EVs, thereby enhancing the efficacy of EV-based therapeutics for acute muscle injuries in aged mice.61 However, aberrant ES at a magnitude beyond the physiological range can inflict adverse impacts on cells, damaging the cell membrane and compromising normal physiological activity, which negatively affects cell and EV behaviors.149
Despite significant advancements in understanding the response of MSCs or multipotent stromal cells to ES, the precise relationship between this mechanical agitation and EVsecretion remains largely unexplored. While a few studies have demonstrated an enhancement in EV secretion following ES, the observed increase, typically ranging from 1.5- to 2-fold, falls short of meeting the dosage requirements for large-scale clinical applications. Consequently, extensive research is warranted to optimize ES regimens for the targeted modulation of EV lipid membrane components, nucleic acid contents, and functionality, ultimately paving the way for clinically relevant EV-based therapies.
Similarly, magnetic fields find a myriad of applications in musculoskeletal tissue engineering, which have been shown to accelerate the proliferation, migration, orientation, and differentiation of stem cells toward osteogenic, chondrogenic, and tenogenic lineages.63, 152-158 For instance, Arjmand et al. demonstrated that the osteogenic differentiation effects elicited by low-frequency pulsed electromagnetic fields (PEMFs) on adipose-derived mesenchymal stem cells (ADSCs) are comparable to the effects of osteogenic medium, suggesting that applying PEMF can serve as an alternative for osteogenic medium. Additionally, a synergistic effect was observed when PEMF was applied concurrently with osteogenic medium during the osteogenic differentiation of ADSCs seeded on a nanofibrous Poly(caprolactone) scaffold.153
In light of its wide applications in facilitating stem cell differentiation, magnetic stimulation is an advantageous method to enhance the exosome production and improve the biological functions of exosomes. For instance, Li et al. fabricated iron oxide nanoparticle (NP)-labeled exosomes (Exo + NPs) from NP-treated MSCs. These Exo + NPs significantly promoted proliferation, migration, and angiogenesis both in vitro and in vivo, suggesting the utility of magnetic field-guided navigation driven by NPs within the Exo + NPs. This targeted guidance significantly increased the accumulation of Exo + NPs at the injury site, leading to enhanced cutaneous wound repair. At week 5, nearly complete wound closure was observed in the Exo + NP group, in contrast to the PBS-treated control group, which exhibited only approximately 60% wound closure.159
Similar strategy to incorporate iron oxide nanoparticles to harness the power of magnetic stimulation in enhancing exosome yield has been reported within the context of musculoskeletal system. Wu et al. demonstrated that combining magnetic nanoparticles Fe3O4 with a static magnetic field (SMF) amplified more than 1.5-fold of exosomes derived from bone mesenchymal stem cells (BM-MSC-Fe3O4-SMF-Exo), which exhibited marked beneficial effects in promoting osteogenesis and angiogenesis in vitro and in vivo compared to pristine exosomes (BM-MSC-Exos). The quantitative analysis revealed that the newly formed bone, bone mineral density (BMD), bone volume fraction (BV/TV) ratio, and trabecular number were all at least 2-fold higher in the BM-MSC-Fe3O4-SMF-Exo compared to the PBS control group, introducing a new paradigm to promote bone regeneration for tissue engineering.62 While research on combinatory treatments involving biomechanical cues, optimized 3D matrices, and biophysical environments guided by magnetic stimulation is still in its nascent stages, the potential for additive effects presents a significant area for future investigation. Specifically, a comprehensive exploration of the impact of these combined strategies on EV lipid membrane components, nucleic acid contents, and functionality holds immense promise for advancing the field of EV-based therapeutics. Further investigation into the impact of magnetic fields on EV production by bone and cartilage tissue must be a priority.
4.5 Acoustic stimulation
Ultrasound, characterized by mechanical acoustic waves operating at frequencies exceeding the upper limit of human hearing, has been extensively studied for its potential to enhance the production of EVs. Ultrasound has been a mainstay in clinical practice and research for over half a century and has been utilized in the treatment of various human malignancies and pathologies.160 High-intensity focused ultrasound (HIFU) has been established as a non-invasive tool for the thermal or mechanical ablation of both benign and malignant tissues,160, 161 while low-intensity pulsed ultrasound has found applications in enhancing cancer treatment, accelerating bone fracture healing, chondrogenic differentiation facilitation, and tendon regeneration promotion.162-165
Yang et al. examined the molecular mechanisms underlying the anti-inflammatory effects of low-intensity ultrasound (LIUS). They found that LIUS considerably induced the expression of several EV biogenesis mediators, including a 2.9-fold upregulation of Ras-Related Protein Rab-11 (RAB11) and a 2.5-fold upregulation of Syntaxin-6 (STX6). This upregulation of exosome biogenesis in immunosuppressor cells exhibited a pronounced enhancement in the phagosome maturation pathway, while also delineating various signaling pathways with docking genes and emphasizing virus entry via endocytic pathways. These findings provide a robust mechanistic foundation for the development of ultrasound therapies across diverse domains, including cancer treatment, inflammatory diseases, tissue engineering, and tissue repair. This potential extends to stimulating stem cell differentiation, as evidenced by the enhanced expression of osteogenic, chondrogenic, and adipogenic markers.165-168
Furthermore, a comparative analysis of exosomes derived from LIUS-treated versus untreated bone marrow dendritic cells (BMDCs) revealed about a 10-fold increase in miR-16 and miR-21 in LIUS-treated exosomes. These enriched exosomes attenuated tumor necrosis factor α (TNFα)-induced inflammation in human umbilical vein endothelial cells (HUVECs) by blunting NF-κB signaling pathway. Since endothelial cell activation by proinflammatory cytokines produced by dendritic cells (DCs), such as TNF, initiates the progression of atherosclerosis, the inhibition of TNFα-elicited inflammation by exosomes derived from LIUS-treated BMDCs could have potential applications in atherosclerosis therapeutics.169
High-frequency acoustic waves have also been shown to promote exosome generation via a calcium-dependent mechanism. Exposure of mammalian cells (U87-MG and A549 cells) to cycles of acoustic irradiation amplified the number of exosomes by approximately 8- to 10-fold, while maintaining a high post-irradiation cell viability of approximately 95%. This iterative approach, involving irradiation and post-excitation incubation steps, facilitated the high-throughput production of a homogeneous population of exosomes. This advancement holds significant potential for accelerating the translation of exosome-based therapies into clinical practice.170 While ultrasound irradiation has been reported to enhance EV secretion by various cells, such as SPC-A1 cells (lung adenocarcinoma cell line) and GL261-luc2 cells (Luciferase-transduced GL261 murine glioma cells),171, 172 challenges persist regarding the reproducibility of experiments and the identification of suitable ultrasonic parameters tailored to different donor cells. While the regulatory effects of ultrasound-derived exosomes on musculoskeletal stem cell differentiation remain largely unexplored, further investigations are imperative to identify optimal ultrasonic parameters tailored to different donor cell types. Moreover, research aimed at triggering the accumulation of specific constituents within EVs holds significant promise for realizing the translational potential of this technology.
To summarize, the biophysical environment in which stem cells reside plays a critical role in influencing their behavior and the production and functionality of exosomes. Taken together, various factors such as tension, compression, shear stress, EMFs, and ultrasound collectively highlight the significance of the biophysical microenvironment in modulating the stem cell behavior and optimizing the yield and therapeutic potential of exosomes.
5 TOPOGRAPHY
Stem cells' behavior and secretory profile have been shown to be affected by modifying the surface topography of the cell culture substrate.52 The surface topology refers to the 3D structure of the material surface, which determines the physical cues presented to the cells.173 This encompasses factors such as the shape (e.g., pillars, pits, and gratings), dimension (e.g., curvature, feature size, spacing, and height), arrangement, and composition of the substrate. Various techniques have been utilized to create substrates with desired surface topographies at the micro- and nanometer scale, including two-photon polymerization,174, 175 soft lithography,176, 177 capillary force lithography,178 photolithography,179 Ultraviolet (UV)-assisted capillary molding,180 and micromachining.181, 182 These techniques allow for the precise control and manipulation of the surface topography, which can subsequently influence the behavior and secretory profile of stem cells cultured on these substrates. The specific surface features can provide physical cues that modulate cellular processes, including adhesion, migration, proliferation, and differentiation, ultimately impacting the stem cells' secretome and the production of EVs with therapeutic potential.
Studies have demonstrated that stem cells can perceive and respond to the shape features of their substrates, leading to distinct differentiation behaviors. For example, bone marrow stem cells displayed varying osteogenic differentiation behaviors when cultured on substrates coated with different shapes at the nanoscale. In particular, the stem cells showed higher calcium mineralization on quasi-spherical nanoparticles and nanotrioctahedra substrates than concave nanocube particles and porous nanoparticles substrates.183 At the nanoscale, the existence of nanotubes in the substrate was found to promote osteogenic differentiation by modulating epigenetic modifications.184 The feature of substrate pores also plays a vital role in the regenerative effects of stem cells. Polyelectrolyte complex silk fibroin/chitosan blended porous scaffolds have been shown to support chondrogenesis of MSCs in vitro.185 Additionally, collagen-hyaluronic acid scaffolds with larger mean pore sizes promoted greater cell proliferation, chondrogenic gene expression, and cartilage-like matrix deposition compared to those with smaller mean pore sizes.185 Furthermore, the arrangement of randomly oriented and interconnected pores on polystyrene substrates were superior in enhancing the secretion of factors related to bone remodeling compared to uniformly distributed homogenous pores on polyester substrates.186 Another commonly employed approach to guide stem cells toward a specific lineage involves patterned substrates that mimic the cells' native topographical features in their in vivo microenvironment.187 For example, human induced pluripotent stem cell (hiPSC)-derived MSCs seeded onto well-aligned fibers can differentiate into tenocyte-like cells by activating mechanosensitive signaling pathways. This suggests that a tendon-mimicking substrate alteration strategy may effectively promote hiPSC differentiation for tendon tissue regeneration.188 Additionally, the incorporation of mussel-inspired nanostructures into functionalized 3D-printed bio-ceramic scaffolds has demonstrated the potential to enhance tissue regeneration by modulating the paracrine signaling of adipose-derived MSCs.189 Moreover, except for the shape, pore, and native-mimic features, the fiber size of scaffolds was shown to influence cell adhesion, shape, spreading area, and ECM expression in annulus fibrosus-derived stem cells.190 In summary, the mentioned studies highlight the diverse effects of cell culture environment topography on stem cell behavior and emphasize its importance in tissue engineering and regenerative medicine.
While EVs have been recognized as important nano-cargo carriers for cell-cell communication, the effects and mechanisms by which surface topography regulates stem cell EV signaling are not well understood. However, recent studies have shed light on this topic and demonstrated the potential of surface topography in modulating stem cell EV-mediated communication. One investigation explored the potential of scaffolds with different pore features to improve the therapeutic efficacy of osteoblast-derived EVs. The results showed that EVs isolated from triangular pore scaffolds significantly enhanced mineralization in human BM-MSCs compared to those from square pore scaffolds (1.7-fold increase) and 2D cultures (2.2-fold increase).49 Interestingly, structures with higher permeability exhibited a significant increase in EV yield, particularly with larger pore sizes, showing a 2.2-fold increase.49 The increased EV production correlated with the presence of increased microtracks in the substrate of the breast cancer cell line culture environment, further supporting the potential effect of surface morphology changes on EV secretion by stem cells.191
These findings underscore the crucial role of surface topography in regulating stem cell behaviors and its potential effect on the secretion and functional properties of their secreted EVs in the context of musculoskeletal diseases. By strategically manipulating the shape, pore size, geometry, and other topographical features of the stem cell culture substrate, directed differentiation of stem cells can be promoted, and cell-cell communication through EVs can be modulated. These insights offer a rational foundation for the design and engineering of biomaterial substrates through the strategic modulation of topographical cues. This approach can precisely control the EV secretion of stem cells and enhance their therapeutic potential for regenerative medicine applications. The ability to optimize the surface topography of stem cell culture substrates allows for the targeted modulation of stem cell behaviors and the secretome, including the production and characteristics of the secreted EVs.
6 SUBSTRATE STIFFNESS AND VISCOELASTICITY
The mechanical properties within the cell niches are key factors that regulate the interactions between cells and their surrounding ECM.92, 192-194 Factors such as environmental stiffness, viscoelasticity, and rigidity have been shown to have a profound impact on various musculoskeletal tissues, including bone, cartilage, muscle, and tendon.195, 196 These mechanical properties can influence a wide range of cellular processes, such as adhesion, migration, proliferation, and differentiation, leading to alterations in gene expression and the secretion of EVs with distinct cargoes and functionalities.
Specifically, substrate stiffness refers to the rigidity of an object and its resistance to deformation when subjected to an applied force.65 Research has shown that the stiffness of the microenvironment matrix generates signals that direct lineage-specific differentiation of stem cells.38, 197 Soft materials with a storage modulus of less than 1 kPa are suitable for neural cells, while stiffer elastic materials with a storage modulus of 25–40 kPa are optimal for osteogenic differentiation. Intermediate substrate stiffness is optimal for promoting chondrocyte differentiation.198 For example, on 2D flat substrates, MSCs will undergo pronounced osteogenesis on stiff substrates with moduli around 40 kPa, while soft substrates (∼1 kPa) facilitate adipogenic differentiation. However, in 3D platforms, only cells in degradable (soft) microenvironments can spread and undergo osteogenesis.38 The expression of ALP, an early marker for osteogenesis, was found to increase in MSCs through both direct cell-cell contact and adhesion to stiffer substrates.199 Another study revealed that both chondrogenic and osteogenic markers were elevated in MSCs grown on substrates with stiffness less than 10 MPa.200 In the context of cartilage repair, hydrogels with greater substrate stiffness (storage modulus of 21 kPa) can enhance the chondrogenic differentiation of human MSCs, making them promising candidates for 3D culture and stem cell-based cartilage regeneration therapies.198
Viscoelasticity refers to a material's ability to dissipate mechanical energy and recover from deformation when subjected to an applied force, which can be either static or oscillating.66 In the context of musculoskeletal regeneration, the higher elasticity of 3D nano-composite constructs has been shown to promote stem cell growth and induce bone mineralization.201, 202 Additionally, surface viscoelastic behaviors of the substrate, such as mechanical response time, can effectively guide stem cells toward osteogenesis.203 Andrew et al. explored the influence of substrate viscoelastic properties on hMSCs and found that their ability to differentiate towards various lineages was augmented on substrates with high loss (viscous) modulus values.204
Simultaneously, the effects and mechanisms by which mechanical properties regulate stem cell EV signaling also need to be explored. The role of stiffness in regulating the paracrine signaling of MSCs has been demonstrated, as matrix stiffening has been observed to activate Rab8, a protein involved in regulating EV secretion.67 These findings suggest that substrate stiffness can modulate the paracrine capabilities of MSCs. Furthermore, research has shown that stem cell differentiation, and potentially the resulting production of tissue-specific EVs, can also be influenced by the mechanical stiffness of the microenvironment.205 Similarly, while the effect of substrate viscoelasticity on EVs has not yet been fully explored, the effect of substrate viscoelasticity on stem cell differentiation suggests that it may also have the potential to modulate and enhance the therapeutic effects of stem cell-derived EVs in regenerative medicine.
In summary, the mechanical properties within cellular niches, including stiffness and viscoelasticity, have a significant impact on cell behaviors and their paracrine signaling, particularly in the context of musculoskeletal regeneration. However, it remains unclear which mechanical parameter has the most significant contribution. Understanding and strategically manipulating these properties is needed to provide valuable insights for the utilization of stem cell derived-EVs in musculoskeletal regenerative medicine. Further research in this area will contribute to the development of efficacious strategies for improving tissue repair and regeneration.
7 CLINICAL APPLICATIONS OF STEM CELL-DERIVED EVS FOR DISEASES AND REGENERATION
The utilization of EVs as a novel cell-free therapeutic approach has sparked renewed optimism for tissue repair and disease therapy, as it holds potential solutions to address certain complications and limitations associated with stem cell therapy.206 While promising results have been reported in animal studies, there is currently a paucity of research investigating the effects of EVs in clinical settings. According to the information available on ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home), a comprehensive analysis reveals that there are currently 69 registered clinical trials that specifically investigate the therapeutic potential of EVs for regeneration. Among these trials, a subset of 14 trials specifically concentrates on the application of EV therapy for musculoskeletal diseases. The target tissues for clinical trials investigating the application of EVs in musculoskeletal diseases included skin (30.8%), cartilage (46.2%), bone (23.1%), and muscle (7.7%). Out of these studies, 66.6% were Phase II or Phase I and II clinical trials, and the total sample size reached 306 patients (Figure 6a). Remarkably, 13 (93.3%) specifically employed EVs derived from stem cells, particularly MSCs, which demonstrates the promising application of MSC-derived EVs in tissue regeneration.
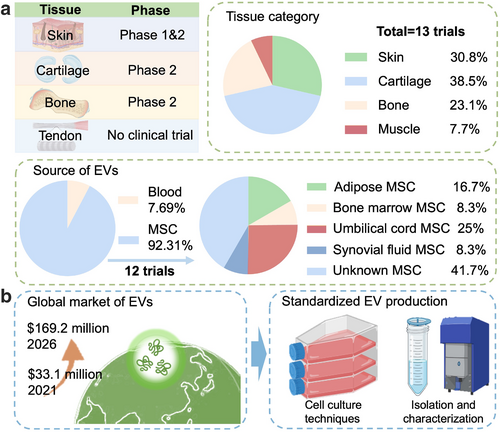
Clinical applications and market projections of EVs in musculoskeletal diseases. (a) The table provides an overview of the varying phases of clinical trials involving MSC-derived EVs for the treatment of diverse musculoskeletal conditions, encompassing skin, cartilage, bone, and tendon diseases. The accompanying pie charts depict the distribution of the 13 MSC-derived EVs clinical trials based on the target tissue category and the source of EVs. (b) The projected global market for exosome-based therapeutics is estimated to expand from $33.1 million in 2021 to $169.2 million by 2026, highlighting the increasing interest and investment in this emerging field. However, significant challenges remain in achieving large-scale production and overcoming obstacles in the clinical translation of EV-based therapies. These challenges encompass the standardization of cell culture techniques, EV isolation and characterization methods, and the optimization of EV manufacturing processes. EVs, extracellular vesicles.
The sources of the EVs in the MSC research varied, and the umbilical cord mesenchymal stem cells (UCMSCs) are the most frequently used (25%) compared with BMSCs, ADSCs, and synovial fluid mesenchymal stem cells (SF-MSCs) due to their high pluripotency and regenerative potency.207 However, the difficulties in acquiring UCMSCs restrict their clinical translation.207 BMSCs have been extensively studied, providing many foundational observations of MSC biology and therapeutic applications.207 Yet, the invasive procedure required to obtain bone marrow, along with the decreasing frequency and differentiation potential of BMSCs with donor age, has led researchers to explore alternative sources.207 In contrast, ADSCs are easily accessed due to their higher abundance in adipose tissue, making them an attractive alternative source of MSCs.207 While EVs derived from BMSCs, ADSCs, UCMSCs, and other MSC sources have demonstrated therapeutic efficacy in various pre-clinical models, in a few direct comparison studies to date, these sources have shown both similarities and noteworthy distinctions. For instance, Hoang et al. demonstrated that EVs from BMSCs were more effective at stimulating dermal fibroblast proliferation compared to EVs from ADSCs, whereas ADSC-EVs promoted greater keratinocyte proliferation208 and more robust angiogenic tube formation.209 However, the MSCs used in these studies differed in donor species, passage number, cryopreservation methods, and donor health status, leaving an incomplete understanding of the potential therapeutic advantages of one stem cell source over another. Therefore, identifying the optimal MSC tissue source for producing EVs with maximal therapeutic efficacy for specific clinical applications remains a significant challenge in the path to clinical translation.
According to these clinical studies, bone formation is a therapeutic target of MSC-derived EVs in several ongoing clinical trials. One Phase I and Phase II trial aims to develop a tissue-engineered bone grafting compound by combining concentrated autogenous adipose MSC- conditioned medium (CM), which is collected from the autologous fat tissue-derived stem cells culture medium, with a synthetic bone substitute (NCT04998058). This study aimed to evaluate the quality (density), quantity (volume), and maturation of bone formation achieved with these grafts. The hypothesis is that the paracrine effect associated with cultured autogenous GFs and cytokines, acting locally at physiological concentrations in the grafted sites, may enhance the cellular response and lead to more significant and faster bone formation. In a similar vein, another clinical trial focuses on the development of a biomedical cell product based on MSCs enriched with their own EVs (NCT04980261). The trial aims to evaluate the safety and efficacy of this biomedical cell product in treating 20 patients with segmental bone tissue defects. Safety evaluation involves monitoring adverse effects associated with the therapy at 1 month and 1 year after the surgery. The effectiveness assessment measures the percentage of patients with completely recovered segmental bone tissue defects. In addition to surgical interventions, ongoing clinical research involves the injection of MSC-derived secretome into the joints of 30 patients with RA (NCT05925647). The injections occur on the days 1, 2, 3, 4, 5, 6, 7, 14, 21, and 28 to evaluate the safety and effectiveness of MSC-derived secretome in treating this type of bone disease. The studies aim to provide critical insights into the safety and efficacy of MSC-derived EVs, secretome, and cell products in clinical settings, potentially leading to advancements in regenerative therapies for bone tissue repair.
MSC-derived EVs have gained considerable attention in the therapy of cartilage regeneration, particularly in the repair of denatured cartilage, such as meniscal tissue, in cases of OA. A Phase I clinical trial exemplifies this approach by exploring the administration of exosomes from allogeneic MSCs (CelliStem®OA-sEV) in individuals experiencing mild to moderate symptomatic OA (NCT05060107). In this trial, patients received a single intra-articular injection of MSC-derived exosomes (3~5 × 1011 particles), followed by a 12-month monitoring period to assess the safety and efficacy of the treatment. The effectiveness of MSC-derived exosomes in alleviating knee OA is evaluated by measuring pain reduction and disability improvement. While the trial is ongoing (estimated study completion date: October 5, 2023), it is presumed that the stimulation of musculoskeletal tissue regeneration induced by exosomes contributes to the improvement of OA symptoms. Another Phase I and Phase II trial aimed to compare the efficacy of CM from umbilical cord MSC culture secretome with Platelet-Rich Plasma (PRP) and hyaluronic acid for the treatment of knee OA (NCT05579665). Participants in each group received five injections of 3 cc PRP, 2 cc hyaluronic acid, or 2 cc CM from the umbilical cord MSC secretome at weekly intervals. Subjective outcome measures, including pain, stiffness, and physical functioning of the joints, were collected pre-treatment and 6 months after injection to evaluate clinical data. This trial aims to compare the effectiveness of these treatment modalities in managing knee OA symptoms. Similarly, another trial focuses on comparing the efficacy of umbilical cord MSCs and secretome between patients undergoing arthroscopy intervention and those without arthroscopy intervention for OA (NCT05211986). Additionally, a Phase II clinical trial examines the effect of synovial fluid-derived mesenchymal stem cells-derived exosomes (SF-MSC-EX) on tissue regeneration (NCT05261360). This trial recruited patients with degenerative meniscus damage in both knees. In the experimental group, synovial fluid-derived mesenchymal stem cells (SF-MSC) are applied intra-articularly to the right knee, while SF-MSC-EX were applied intra-articularly to the left knee. Knee functions, physical activity, pain level, radiological images and knee joint range of motion are evaluated and compared between the left and right knees. The goal was to estimate the effectiveness of SF-MSC-EX in promoting self-renewal and repair of meniscus tissue. The trial is estimated to be completed in 2025. Collectively, these clinical trials provide evidence of the potential therapeutic efficacy of MSC-derived exosomes in promoting cartilage regeneration. By exploring different approaches and treatment modalities, researchers aim to advance our understanding of the regenerative capabilities of MSC-derived EVs for the management of cartilage-related conditions such as OA.
There is another ongoing clinical trial that aims to investigate the potential of MSC-derived EVs in regenerating muscle atrophy associated with knee OA (NCT05211986). The study aims to recruit up to 18 participants who will undergo intramuscular administration of IMMUNA (IMM01-STEM) twice a week for a duration of 4 weeks. The trial will be divided into three dose cohorts: Cohort A will receive a dosage of 225 μg IMMUNA, Cohort B will receive 450 μg, and Cohort C will receive 900 μg of IMMUNA. IMMUNA is a secretome product derived from partially differentiated pluripotent stem cells and contains various regenerative molecules. Throughout the trial, participants received a total of eight injections of IMMUNA over the 4-week period. The functionality of the knee joint in these participants will be assessed using specific measures. Muscle strength will be measured through isometric knee extensor torque, physical function will be evaluated using the 6-min walk test, and the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index will be utilized for the evaluation of OA. These assessments will be conducted 3 days after the participants' last injection and subsequently on a monthly basis for a period of 3 months. By evaluating various parameters and utilizing established measurement tools, this study seeks to gather valuable data on the safety and effectiveness of IMMUNA as a therapeutic intervention for muscle regeneration.
Additionally, the regenerative potential of MSC-derived EVs in treating ulcer wounds, burn wounds, and aging skin has been demonstrated. One Phase I clinical trial (NCT04134676) initiated in late 2019 aims to assess the success rate, treatment duration, and differences in wound closure for chronic ulcers. Patients received treatment with CM derived from Wharton's Jelly mesenchymal stem cells, applied topically to the wound and covered with a transparent dressing for 2 weeks. Another Phase I pilot study (NCT05078385) aimed to evaluate the safety and efficacy of allogeneic MSC-derived EVs in deep second-degree burn wounds. The treatment involves delivering approximately 1 × 107 EV particles per cm2 of the treated area within 48 h of the burn injury, followed by additional administrations at 1 week and 2 weeks after the initial treatment. Tissue regeneration, pigmentation restoration, hair growth, and scar formation were evaluated at various intervals. Two ongoing clinical trials, encompassing Phase I and Phase II, are exploring the use of MSC-derived exosomes and secretome to address skin aging. One study (NCT05813379) focuses on the regenerative effects of MSC-derived exosomes, stimulating collagen production and reducing oxidative stress during skin regeneration. In another trial (NCT05508191), the effects of different devices, such as fractional CO2 laser and microneedle, on the delivery of ADSC-derived secretome are compared. These clinical trials highlight the potential of MSC-derived EVs, secretome, and exosomes in wound healing, scar reduction, and skin rejuvenation.
The clinical trials registered on ClinicalTrials.gov reveal the growing interest and potential therapeutic applications of MSC-derived EVs, secretome, and exosomes in the context of tissue regeneration for various musculoskeletal diseases. These trials cover a range of conditions, including wound healing, scar reduction, skin rejuvenation, cartilage regeneration, bone tissue repair, and muscle regeneration. Overall, the ongoing clinical investigations provide valuable insights into the therapeutic potential, safety, and efficacy of MSC-derived EVs in clinical settings. The successful translation of these EV-based therapies could pave the way for future advancements in regenerative medicine, as they harness the paracrine signaling and reparative capabilities of MSCs without the need for direct cell transplantation. As the field continues to evolve, the findings from these clinical trials will be vital in guiding the further development and optimization of EV-based regenerative therapies.
8 DISCUSSION AND CONCLUSION
The field of EVs has been rapidly expanding, driven by the increasing recognition of their potential in therapeutic applications. EVs inherit specific factors and genetic material from their parent cells, making them particularly valuable in regenerative medicine. In fact, the global market for EV therapeutics is projected to grow from $33.1 million in 2021 to $169.2 million by 2026, underscoring the growing investment and commercial interest in this field.210 Nonetheless, substantial challenges remain in achieving scalable production and overcoming barriers to the clinical translation of EV-based therapies. These include the standardization of cell culture techniques, EV isolation, and characterization methods (Figure 6b).
The current study presents a comprehensive review that highlights the impact of biophysical properties on the production of MSC-EVs and their effectiveness in treating musculoskeletal diseases. Yet, several important questions remain unanswered. While biophysical stimulations show promise, they also have limitations, including poorly defined effects and mechanisms of action, limited control, manufacturing inconsistencies, and challenges in large-scale fabrication.
Given that both biochemical and biophysical manipulation approaches have their respective advantages and drawbacks,211 integrating these cues in the production of stem cell-derived EVs could yield significant benefits. This integration may facilitate the development of next-generation protocols for scalable EV production. Most studies have focused on the combined effects of multiple niche properties, as biomaterial-related parameters—such as surface chemistry, viscoelasticity, (nano-)topography, and 3D architecture—are interrelated and cannot be easily isolated. This complexity makes it difficult to draw specific conclusions about the independent role of each biophysical feature.
To address this issue, employing a material platform that can decouple individual niche factors may be an efficient strategy. Such materials have been developed by tuning the crosslinking bath solution during the bioprinting of hydrogels composed of fibrinogen, alginate, and gelatin.66 Strategies used to modulate niche features vary significantly, complicating the comparison of results and the establishment of universal findings.
There is also a lack of direct comparisons regarding the impacts of biochemical and biophysical stimulations on stem cell-derived EV production and efficacy. For example, while some studies have explored different strategies to enhance the production yield of stem cell-derived EVs, such as Lai et al.’s immortalization of cells through c-Myc overexpression for scalable EV production.212 However, the relative effectiveness of these strategies compared to biophysical modulation approaches is still unknown. Although 3D culture methods are among the most commonly employed for scalable EV production, studies comparing different 3D culture platforms remain scarce.
In terms of the efficacy of stem cell-derived EVs, various approaches have proven effective for wound healing, bone regeneration, cartilage regeneration, and cardiac regeneration. However, reports indicate that EVs derived from different stem cell sources exhibit varying effects depending on the type of physical stimulation applied. For example, magnetic field exposure has been found to enhance the muscle regenerative properties of EVs,61 while cyclic stretch,53 compressive stretch,55 shear stress,58 and triangular pore stimulation49 promote their osteogenic potential.
Further investigations comparing the impacts of biochemical and biophysical niche features on EV production are essential for developing standardized protocols for efficient EV production. By addressing these limitations, researchers can enhance the scientific rigor and comparability of studies in this field.
In conclusion, our review provides a comprehensive understanding of how biophysical factors impact EVs, aiming to enhance the development of effective strategies that harness the potential of EVs for large-scale production and their successful application in regenerative therapies for musculoskeletal disorders. Ultimately, these insights could significantly benefit patients in need of innovative, cell-free regenerative treatments, advancing the fields of musculoskeletal tissue engineering and regenerative medicine.
AUTHOR CONTRIBUTIONS
DMW supervised the project. YX, LL, and DMW conceived and designed the review. YX, CYMC, LL, and KYT contributed to data collection as well as the preparation of figures and tables. YX, CYMC, LL, and HPHC finished manuscript writing and manuscript review. DFEK, SHC, CM, and ZZ offered essential insights and edited the manuscript. All authors endorsed the final version.
ACKNOWLEDGMENTS
This work was supported by Research Grants Council of Hong Kong SAR (14121121, DMW; 14118620, DMW; 24201720, DFEK), National Natural Science Foundation of China/Research Grants Council Joint Research Scheme (N_CUHK409/23, DMW), The Innovation and Technology Commission, Hong Kong SAR (Theme-based Midstream: ITS-020-23MX, DMW). This study was supported in part by the InnoHK initiative of the Innovation and Technology Commission of the Hong Kong Special Administrative Region Government (Health@InnoHK CNRM, DFEK, DMW).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies

Yang Xu obtained her Bachelor and Master of Rehabilitation Medicine in 2023 from the Sichuan University. Yang XU is a first-year PhD student in the School of Biomedical Sciences at the Chinese University of Hong Kong. Her research interest focuses on the application of stem cell-derived extracellular vesicle (EV) therapy for tendon regeneration.

Chung Yin Matthew Cheung obtained his Bachelor of Biomedical Engineering in 2022 from the CUHK. He subsequently joined the Institute of Tissue Engineering and Regenerative Medicine and the Division of Neurology at CUHK. His research interests focus on material development for the regulation of stem cell differentiation to facilitate tendon healing and regeneration, and the investigation of novel medication therapies to potentially relieve the symptoms of Alzheimer's disease.

Zhiyong Zhang joined Guangzhou Medical University in 2017 as a leading medical talent to engage in the transformation of regenerative medicine in Guangzhou. His research focuses on the clinical translational research of stem cell and biomaterial-based approaches for tissue repair and regeneration in various medical fields, including orthopedics, plastic surgery, dermatology, respiratory medicine, obstetrics, and gynecology. In addition to his research work, Professor Zhang serves as an external audit expert for several international scientific research funds, such as Reumafonds in the Netherlands, SingHealth in Singapore, and HMRF in Hong Kong. He also has expertise in evaluating proposals for China's national Key Research Program and the National Natural Science Foundation.

Dan Michelle Wang assumed the position of Assistant Professor at CUHK with appointments in the School of Biomedical Sciences (SBS) and Institute for Tissue Engineering and Regenerative Medicine (iTERM) in 2022. Professor Wang's research focuses on elucidating the synergistical contributions of cell microenvironmental features, such as extracellular matrix (ECM), substrate stiffness, mechanical loading, during tendon development, regeneration, and the pathogenesis of diseases. This knowledge will be applied towards the development of innovative therapeutics and the engineering of novel biomaterials for musculoskeletal tissue regeneration.




