The effect of methylmercury(II) binding on the conformation of poly(L-glutamic acid) and poly(L-lysine): A raman spectroscopic study
Abstract
The binding of the methylmercury cation CH3Hg+ by poly(L-glutamic acid) (PGA) and by poly(L-lysine) (PLL) has been investigated by Raman spectroscopy. Coordination on the side-chain COO− and NH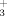 groups of these polypeptides gave characteristic ligand–Hg stretching modes at ca. 505 and 450 cm−1, respectively. Precipitation generally occurred upon formation of the complexes and changes of conformation were common. The solid complex obtained from PGA at pH 4.6 was found to have a mostly disordered conformation, which differed from the respective α-helical and β-sheet structures of the dissolved and precipitated uncomplexed polypeptide in the same conditions. An α-helical structure was generally adopted by the complex formed with PLL, even in pH and temperature conditions where the free polypeptide normally exists in another conformation. The addition of a stronger complexing agent, glutathione, to the PLL/CH3Hg+ complex caused a migration of the bound cations and a restoration of the polypeptide to its original state.
groups of these polypeptides gave characteristic ligand–Hg stretching modes at ca. 505 and 450 cm−1, respectively. Precipitation generally occurred upon formation of the complexes and changes of conformation were common. The solid complex obtained from PGA at pH 4.6 was found to have a mostly disordered conformation, which differed from the respective α-helical and β-sheet structures of the dissolved and precipitated uncomplexed polypeptide in the same conditions. An α-helical structure was generally adopted by the complex formed with PLL, even in pH and temperature conditions where the free polypeptide normally exists in another conformation. The addition of a stronger complexing agent, glutathione, to the PLL/CH3Hg+ complex caused a migration of the bound cations and a restoration of the polypeptide to its original state.




