Research highlights
One Polymerase, Two Jobs
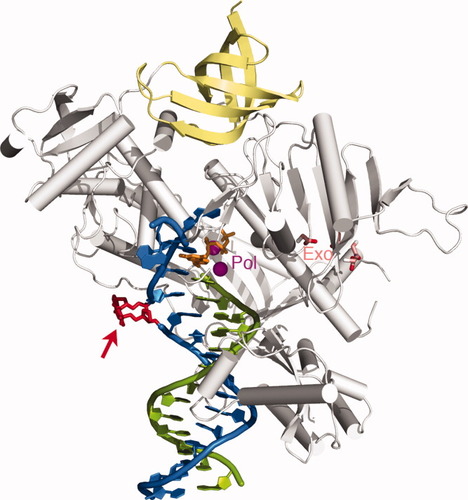
Escherichia coli DNA polymerase II (Pol II) is something of an oddity. It is a member of the B family of DNA polymerases, generally known for their involvement in high-fidelity DNA replication, with an exonuclease domain for error correction. However, it also shares functional characteristics with members of the Y family that participate in gap and mismatch repair, and it remains unclear how Pol II achieves such multitasking capabilities. To resolve this riddle, Wang and Yang generated high-resolution crystal structures that catch Pol II in the act of DNA synthesis; unfortunately, as described in their recent article from Cell, these revealed no striking differences between the catalytic sites of Pol II and those of purely replicative polymerases such as bacteriophage-derived gp43. Nevertheless, functional assays confirmed that Pol II exhibits the capacity to efficiently extend DNA synthesis across abasic lesions (AP) or nucleotide mismatches in the template strand, which gp43 effectively lacks. When the researchers produced a second set of Pol II crystal structures that attempt to reenact this gap-jumping process, they observed an enlarged template-binding pocket that can accommodate a 2- or 3-nucleotide out-looping of the DNA strand in such situations. Such looping mechanisms apparently enable the polymerase to effectively deal with a variety of genomic lesions that might block the progress of replicative polymerases, andthis process is further facilitated by structural elements in Pol II that effectively segregate the primer-extension site from the exonuclease domain, thwarting proofreading and enabling the continuation of synthesis beyond genomic lesions. In addition to highlighting an unusual mechanism for dual polymerase functionality, the authors conclude that these findings may illustrate an evolutionary process by which proteins can acquire useful functional attributes via multiple changes outside of the active site—where dramatic changes could easily prove lethal. – Michael Eisenstein
Wang, F. & Yang, W. Cell, 2009, 139, 1279–1289.
Finding Proteins that Cause 'the Bends'
Although a diverse array of methods exist for the screening of interactions between proteins and DNA, these generally do not enable characterization of structural changes induced by protein binding, and the tools available for such analyses are highly limited in throughput. To remedy this situation, Spuhler et al. have developed a novel platform comprised of fluorophore-tagged oligonucleotides anchored to a gold electrode surface in the presence of an electric field. Application of a negative potential repels the DNA, which assumes a rigid conformation perpendicular to the surface and fluoresces brightly; on the other hand, when a positive current is applied, the DNA is attracted to the surface and fluorescence is quenched by energy transfer to gold surface plasmons. This behavior can vary due to conformational changes induced by protein binding, however, as demonstrated in a recent article from Proceedings of the National Academy of Sciences, USA, in which the authors test their platform with Escherichia coli integration host factor (IHF) —a protein known to introduce kinks in DNA that enable other regulatory factors to bind. Depending on where the IHF binding site was positioned within the probe—distally, centrally, or proximally—the authors were able to observe different effects via their sensor's fluorescent readout, confirming the capacity of IHF to introduce DNA bends and accurately recapitulating previously-determined binding kinetics for this protein. The authors also demonstrate the ability to manipulate the sensor to characterize effects of probe density on binding activity—a potentially useful means for determining the influence of steric factors on protein-DNA interaction—and suggest that this platform could ultimately enable high-throughput screening via integration into a microarray format. – Michael Eisenstein
Jayasinghe, I.D. et al. Biophys. J., 2009, 97, 2664–2673.
Ribosome Repair by Protein Exchange
The synthesis of new ribosomes is an energy-intensive process, often consuming half or more of the metabolic resources in a bacterial cell. Thus, it makes sense for bacteria and other microorganisms to repair inactivated ribosomes by exchanging damaged ribosomal proteins for new ones. A recent report by Jaanus Remme and colleagues in Molecular Microbiology shows that this is indeed what happens. Using radioistotopic labels (35S) or mass labels (15N), the authors demonstrated that at least ten of the proteins in purified 70S ribosomes can exchange with unbound ribosomal proteins in vitro. Many of the exchangeable proteins are located on the surface of the subunit, explaining why exchange occurs under milder conditions than subunit reconstitution. More importantly, they found that ribosomes previously inactivated by N-ethylmaleimide could regain function when incubated with undamaged ribosomal proteins. In a final set of experiments, cells were allowed to enter stationary phase, in which ribosome biosynthesis grinds to a halt and new ribosomal proteins are infrequently translated. They then labeled newly synthesized ribosomal proteins with 35S-methionine and cysteine, and found that at least 18 proteins in 70S ribosomes became labeled, showing that protein exchange can also occur in cells under stress conditions. An accompanying perspective by J. Cate discusses how chaperone complexes may enable the exchange of ribosomal proteins, thus preserving the translational capacity of the cell in the face of chemical damage. – Sarah Woodson
Pulk A, Liiv A, Peil L, Maiväli U, Nierhaus K, Remme J. Mol Microbiol 2009 Dec 4. Doi: 10.1111/j.1365-2958. 2009.07002.x
Cate J. H. Mol Microbiol. 2009 Dec 16. Doi: 10.1111/j.1365-2958.2009.07017.x
Searching for an Angle on RNA Tertiary Structure
RNA double helices are locally stiff, so intricate tertiary structures are achieved by bending and twisting the more flexible junctions between double helices. Since the conformation of a junction determines the orientation of the adjoining helices, predicting the range of motion and preferred conformations of helix junctions can take us a long way toward predicting the overall structure of an RNA. In a recent report, Bailor et al. tackled this problem by measuring the angles between two helices in the HIV TAR stem-loop. The number of nucleotides in the junction was varied systematically. Bend angles and rotation angles measured by NMR chemical shift perturbation and from order parameters derived from residual dipolar coupling measurements. These were compared to helix angles computed from analogous helix junctions in the Protein Data Bank (pdb). The experimentally determined interhelical angles fell within an unexpectedly narrow envelope of sterically allowed orientations, also calculated by the authors. This suggests that RNA tertiary interactions stabilize particular conformations among a distribution of preferred helix geometries defined by the secondary structure of the RNA. Bailor et al. further show that aminoglycoside antibiotics can lock the HIV TAR stem-loop in particular conformations within the allow ensemble of helix bend angles. The magnitude of the bend can be tuned by varying the size of the antibiotic. This holds promise for the rational design of inducible three-dimensional structures in RNA. – Sarah Woodson
Bailor, M. H., Sun, X., Al-Hashimi, H. M. Science 2010 Jan 8, 327(5962), 202–6.