High-resolution structures of collagen-like peptides [(Pro-Pro-Gly)4-Xaa-Yaa-Gly-(Pro-Pro-Gly)4]: Implications for triple-helix hydration and Hyp(X) puckering
Abstract
Structures of (Pro-Pro-Gly)4-Xaa-Yaa-Gly-(Pro-Pro-Gly)4 (ppg9-XYG) where (Xaa, Yaa) = (Pro, Hyp), (Hyp, Pro) or (Hyp, Hyp) were analyzed at high resolution using synchrotron radiation. Molecular and crystal structures of these peptides are very similar to those of the (Pro-Pro-Gly)9 peptide. The results obtained in this study, together with those obtained from related compounds, indicated the puckering propensity of the Hyp in the X position: (1) Hyp(X) residues involved in the Hyp(X):Pro(Y) stacking pairs prefer the down-puckering conformation, as in ppg9-OPG, and ppg9-OOG; (2) Hyp(X) residues involved in the Hyp(X):Hyp(Y) stacking pairs prefer the up-puckering conformation if there is no specific reason to adopt the down-puckering conformation. Water molecules in these peptide crystals are classified into two groups, the 1st and 2nd hydration waters. Water molecules in the 1st hydration group have direct hydrogen bonds with peptide oxygen atoms, whereas those in the 2nd hydration group do not. Compared with globular proteins, the number of water molecules in the 2nd hydration shell of the ppg9-XYG peptides is very large, likely due to the unique rod-like molecular structure of collagen model peptides. In the collagen helix, the amino acid residues in the X and Y positions must protrude outside of the triple helix, which forces even the hydrophobic side chains, such as Pro, to be exposed to the surrounding water molecules. Therefore, most of the waters in the 2nd hydration shell are covering hydrophobic Pro side chains by forming clathrate structures. © 2009 Wiley Periodicals, Inc. Biopolymers 91: 361–372, 2009.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Collagen is the most abundant protein, as well as the major structural protein, in animals. More than 27 different types of collagens are found in humans1; the most abundant of these are fiber-forming collagens (types I, II and III), which form the structural foundations of skin, tendon, bone, and cartilage. Based on X-ray fiber diffraction data and model building, together with early amino acid composition and sequence data, the molecular conformation of type I collagen was proposed to be a triple-helical structure.2 Collagen has a characteristic amino acid sequence in which a glycine (Gly and G for three- and one-letter abbreviation, respectively) residue appears every third position, forming the triplet repeat Gly-Xaa-Yaa, and a high imino acid content (∼20%). The proposed triple-helical structure nicely explains this characteristic collagen amino acid sequence, that is, a Gly residue is required every third position because the collagen helix places every third residue near the central common axis, where space is very limited. Furthermore, the allowed ϕ and ψ values of imino acid residues fit well with those of the Xaa and Yaa residues in the Gly-Xaa-Yaa sequence. Here, imino acid residues include proline (Pro, P) and hydroxyproline (Hyp, O). For convenience, we will use “Hyp, O” to indicate (2S, 4R)-4-hydroxyproline and “alloHyp, aO” to indicate (2S, 4S)-4-hydroxyproline.
In the triple-helical structure described above,2 each peptide strand has 10 Gly-Xaa-Yaa triplets and one helical turn with a pitch length of 85.8 Å. This conformation can be designated as a 10/1-helix. Three such strands assemble around the common axis and make a triple helix. The resultant triple helix has been designated as a 10/3-helix because we can consider this triple helix to be a hypothetical, single 10/3-helix.3 Because this helix is left-handed, it should properly be called a “left-handed” 10/3-helix. Historically, however, the collagen helix has been designated simply the “10/3-helix,” and we will follow this convention in this manuscript. Because the first triple-helical structure2 had some short contacts between adjacent chains, and the amino acid sequence, Gly-Xaa-Pro, was not compatible with Gly-Pro-Hyp, the more current knowledge at that time, Rich and Crick modified this model while holding the same 10/3-helical symmetry and a fiber period of 28.6 Å.4, 5 This model was called the “Rich and Crick model”; until recently, it was believed to be the correct molecular structure of collagen.
On the other hand, the X-ray structure analysis for the single crystal of a collagen model peptide, (Pro-Pro-Gly)10 (hereafter, PPG10), revealed that the conformation of this peptide is a triple-helical structure with 7/2-helical symmetry and a 20 Å fiber period.6, 7 Because this model could explain the fiber diffraction pattern of native collagen, it was proposed as a new structural model for collagen.8 Recently, refinement calculations using molecular transforms showed that both 7/2- and 10/3-helical models could explain the diffraction data from native collagen (R = 0.23, 0.26 for the 7/2- and the 10/3-helical models, respectively).9 Furthermore, many single crystal structures of collagen model peptides analyzed at high resolution supported the 7/2-helical model, whereas no such support was found for the 10/3-helical model.10 Of course, we have observed relaxed conformations whose helical twists are closer to the 10/3-helix (36°) rather than the 7/2-helix (51.4°) in the several host-guest peptides, such as IBP,11, 12 Gly→Ala13 and T3-785 peptides,14 together with the human type III collagen G991-G1032 cystine knot containing peptide.15 In these cases, the 10/3-helical conformation exists with the support of the stable 7/2-helical conformation. So far, we have never observed a stable, solo 10/3-helical structure. Therefore, we think it is quite reasonable to adopt the 7/2-helical model as the reference structure of native collagen.3
Structural information based on the single crystal structures of collagen model peptides has contributed a great deal to our understanding of the mechanisms of stabilization and destabilization by Hyp, as well as the molecular structure of native collagen. Hydroxylation of Pro residues in the Y position is essential for the formation of the stable triple helix, and therefore for the formation of regular fibers. A dietary deficiency of ascorbic acid (Vitamin C) causes the disease “scurvy,” because ascorbic acid is required as a reducing agent in the reaction catalyzed by collagen hydroxylase. Collagen synthesized in the absence of ascorbic acid cannot properly form fibers; therefore, vitamin C deficiency results in skin lesions and blood vessel fragility. The helix-coil transition temperature (Tm) of (Pro-Hyp-Gly)10 (POG10) is higher than that of PPG10 by 30°C,16 whereas (Hyp-Pro-Gly)10 (OPG10) cannot form a triple-helical conformation under the same aqueous conditions.17 Over the last three decades, a great deal of effort has been expended to explain these experimental data.18-23 The latest explanation was based on the propensity-based hypothesis,23 which can explain both the stabilizing and destabilizing effects of Hyp observed in POG10 and OPG10 peptides. This hypothesis is based on the intrinsic preference of Hyp for up-puckering, and the preference of imino acids in the collagen helix for down- and up-puckering when located in the X and Y positions, respectively (positional preference).23 However, this hypothesis cannot provide an explanation for the stabilizing effects of Hyp in the X position of Ac-(Gly- Hyp-Thr)10-NH2,24 Ac-Gly-(Pro-Hyp-Gly)3-Hyp-Hyp-Gly-(Pro-Hyp-Gly)4-Gly-NH2,25 (Hyp-Hyp-Gly)10 (OOG10)26 and Ac-(Gly-Hyp-Hyp)10-NH2.27 Structural analyses of (Gly-Hyp-Hyp)9 (GOO9)28 and OOG1029 revealed that Hyp residues in the X position adopt the up-puckering conformation, according to the intrinsic preference of Hyp but in conflict with this residue's positional preference in the X position. According to the quantum chemistry analysis of the interactions between Hyp residues in the X and Y positions of adjacent strands in the same triple helix, significant van der Waals and dipole-dipole interactions play important roles in triple-helix stabilization.20
In this study, we report single crystal structures of newly synthesized host-guest peptides, (Pro-Pro-Gly)4-Pro-Hyp-Gly-(Pro-Pro-Gly)4, (Pro-Pro-Gly)4-Hyp-Pro-Gly-(Pro-Pro-Gly)4 and (Pro-Pro-Gly)4-Hyp-Hyp-Gly-(Pro-Pro-Gly)4 (ppg9-POG, ppg9-OPG, and ppg9-OOG, respectively). We studied the puckering propensity of Hyp in the X position in terms of imino acid(X):imino acid(Y) stacking pairs and interaction with surrounding molecules, and we also discuss hydration states of triple-helical structures in relation to the content of Pro in the X and Y positions. Although puckering conformations of Hyp in ppg9-POG and ppg9-OPG molecules were briefly cited elsewhere,30 we elucidated further insight in this study by comparing with the structure of ppg9-OOG.
MATERIALS AND METHODS
Peptide Synthesis
The peptide ppg9-OOG was synthesized by standard Fmoc protocol with stepwise coupling on a polystyrene resin (Fmoc-PAL-PEG-PS resin; Applied Biosystems) with an Applied Biosystems Peptide Synthesizer, Model 433A with HATU as coupling reagent. After construction, the peptide resin was treated with Reagent R (trifluoroacetic acid/thioanisole/1,2-ethanedithiol/anisole (90:5:3:2) at room temperature for 3 h. The mixture was poured into cold diethyl ether at 4°C to precipitate the crude compound, which was dissolved in 0.1% trifluoracetic acid (TFA). Purification was performed by preparative HPLC (Vydac® C18, 5 μm, 300Å, 218TP101550 50 × 250 mm2, W.R. Grace & Co., molecular dynamics (MD), USA). Peptides were characterized by electrospray/quadrupole/time-of flight mass spectrometry and amino acid analysis.
The peptide ppg9-POG was prepared by Fmoc method with segment condensation.31 Fmoc-Gly-Wang-PEG resin was used as a starting material. After removal of Fmoc group, Fmoc-Gly-Pro-Pro-OH was coupled four times. Subsequently, Fmoc-Hyp(tBu)-OH, Fmoc-Pro-OH (for ppg9-POG) and Fmoc-Gly-OH were introduced. Then, condensation of Fmoc-Gly-Pro-Pro-OH three times and Boc-Pro-Pro-OH were followed to construct the required sequence. The peptide resin was treated with TFA/H2O (95:5) for 2 h in an ice bath. The mixture was poured into cold diethyl ether to precipitate the objective compound, which was extracted with 10% acetic acid and lyophilized. The crude material was purified by Sephadex G-50 (1.8 × 80 cm2) with 40% acetic acid. Corresponding fractions confirmed by RP-HPLC (YMC-Pack C8) were collected and lyophilized. The peptide was characterized by matrix assisted laser desorption ionization-time of flight-mass spectroscopy (MARDI-TOF-MS) analysis on Voyager DE with 2-cyano-4-hydroxycinnamic acid as a matrix. Peptide ppg9-OPG was also prepared by segment condensation method as mentioned above.
Crystallization and Data Collection
Three peptides were crystallized at 4°C using the hanging drop vapor diffusion method. For crystallization, 3 μl of the peptide solution was mixed with 3 μl of the reservoir solution, which produced rectangular single crystals. Details of crystallization conditions are summarized in Table I. Single crystals were dipped into a mixture of cryoprotectant, 2-methyl-2,4-pentanediol (MPD), and the same volume of the reservoir solution for several seconds before X-ray measurement. Details of data collection are shown in Table II, together with those of PPG9 (ppg9-PPG)32 and ppg9-PaOG.31 All of these have the same space group and very similar unit cell parameters; hence, they have the same number of peptide molecules in an asymmetric unit and very similar pseudo-tetragonal packing arrangements.
| ppg9-POG | ppg9-OPG | ppg9-OOG | |
|---|---|---|---|
| Peptide solution | 10.4 mg/ml peptide | 8.0 mg/ml peptide, 10% (v/v) acetic acid | 4.0 mg/ml peptide |
| Reservoir solution | 22.5%(w/v) PEG400 | 30%(w/v)PEG400 | 32.5%(w/v) PEG1000, 0.1M acetate buffer |
| Cryoprotectant | 30%(w/v) MPD | 30%(w/v) MPD | 10%(w/v) MPD |
| Crystallization term | 2–3 weeks | 1–2 weeks | 1–2 weeks |
- MPD stands for 2-methyl-2,4-pentanediol.
| Peptide | ppg9-PPG | ppg9-POG | ppg9-OPG | ppg9-PaOG | ppg9-OOG |
|---|---|---|---|---|---|
| A. Data collection | |||||
| Facility | SPring-8 BL40B2 | PF BL6A | SPring-8 BL40B2 | SPring-8 BL40B2 | PF BL-6A |
| Data collection device | ADSC Quantum 4R | ADSC Quantum 4 | ADSC Quantum 4R | ADSC Quantum 4R | ADSC Quantum 4 |
| Data collection temperature (K) | 100 | 100 | 100 | 100 | 95 |
| Wave length (Å) | 1.0 | 0.978 | 1.0 | 0.8 | 0.978 |
| Resolution (Å) | 8.0–1.33 | 8.0–1.26 | 8.0–1.36 | 30.0–1.10 | 26.6–1.22 |
| (last shell) | 1.38–1.33 | 1.31–1.26 | 1.41–1.36 | 1.14–1.10 | 1.26–1.22 |
| Number of unique reflections | 24,336 | 26,765 | 23,170 | 43,437 | 32,577 |
| Overall completeness (%) | 97.7 | 89.3 | 96.9 | 99.7 | 98.7 |
| (last shell) | 99.8 | 93.7 | 98.0 | 99.9 | 99.8 |
| Rmerge (last shell) | 0.07 (0.27) | 0.09 (0.33) | 0.08 (0.29) | 0.07 (0.22) | 0.08 (0.29) |
| Redundancy (last shell) | 3.2 (3.3) | 6.9 (7.4) | – | – | 3.5 (3.8) |
| 〈Io/σ(Io)〉 | 4.8 | – | – | 2.4 | 4.5 |
| Space group | P21 | P21 | P21 | P21 | P21 |
| Unit cell dimensions | |||||
| a/Å | 25.95 | 26.08 | 26.01 | 26.45 | 25.99 |
| b/Å | 26.56 | 26.53 | 26.54 | 26.04 | 26.67 |
| c/Å | 80.14 | 79.84 | 80.15 | 80.31 | 79.84 |
| γ/° | 90.0 | 89.97 | 89.9 | 90.0 | 90.03 |
| B. Structure Refinement | |||||
| Resolution range (Å) | 8.0–1.33 | 8.0–1.26 | 8.0–1.36 | 8.0–1.1 | 8.0–1.22 |
| Data cutoff for refinement (σ|Fo|) | 1.0 | 4.0 | 1.0 | 1.0 | 1.0 |
| No. of refls. for refinement | 23,020 | 21,318 | 21,767 | 41,288 | 30,417 |
| No. of refls. for Rfree | 1185 | 1122 | 1071 | 2149 | 1627 |
| R | 0.182 | 0.183 | 0.180 | 0.173 | 0.193 |
| Rfree | 0.229 | 0.235 | 0.257 | 0.234 | 0.255 |
| No. of refined parameters | 9953 | 9908 | 9897 | 11,488 | 10,425 |
| Max/Min peaks in D-maps (e Å3) | 0.31/−0.29 | 0.59/−0.32 | 0.45/−0.31 | 0.60/−0.35 | 0.41/−0.36 |
| No. of peptide non-hydrogen atom | 958 | 968 | 962 | 992 | 895 |
| No. of water sites | 332 | 304 | 326 | 284 | 363 |
| RMS deviations from standard geometry | |||||
| Bonds (Å) | 0.009 | 0.008 | 0.009 | 0.013 | 0.010 |
| Angles (Å) | 0.023 | 0.022 | 0.024 | 0.027 | 0.024 |
| Planes (Å) | 0.027 | 0.028 | 0.027 | 0.039 | 0.028 |
| Atomic displacement parameters (Å2) | |||||
| Peptide atoms | 17.0 | 18.9 | 17.5 | 14.4 | 16.6 |
| Water oxygen atoms | 31.3 | 26.9 | 29.0 | 23.8 | 26.4 |
| PDB code | 2CUO | 2D3F | – | 1X1K | 2D3H |
Structure Determination and Refinement
Three structures were solved by molecular replacement using the X-PLOR33 program and the molecular structure of [(Pro-Pro-Gly)8]3 obtained from PPG932 (PDB code 2CUO) as a probe. The two triple-helices in an asymmetric unit were searched their positions and orientations by X-PLOR with rigid-body refinement cycles in a resolution range of 10 to 2 Å. Structure refinements were carried out using SHELX-L34 by gradually adding reflection data at higher resolutions. When the reflection data were expanded to ∼1.8 Å resolution, water molecules were introduced based on the Fo-Fc electron density map, hydrogen bonding geometry, and distance from other water oxygen atoms. In each case, the location of Hyp in the guest triplet was confirmed by a positive electron density in the Fo-Fc map, corresponding to the hydroxyl oxygen atom. In the last stage of analysis, an anisotropic treatment was applied for atomic displacement parameters of nonhydrogen atoms of the peptide. During each refinement, 5% of the reflections were used for R-free monitoring. The refinement statistics are summarized in Table II. Atomic coordinates have been deposited in the Protein Data Bank (PDB) with entry codes 2D3F (ppg9-POG) and 2D3H (ppg9-OOG).
CD Measurements
CD spectra were recorded on an Aviv 202 spectropolarimeter (AVIV Biomedical, Lakewood, NJ) using a Peltier thermostatted cell holder and a 1-mm path length rectangular quartz cell (Starna Cells, Atascadero, CA). Peptide concentrations were determined by amino acid analysis (L-8800A, Hitachi High Technologies America, San Jose, CA). The temperature scanning experiments were run at 0.1 K/min. The ellipticity at 225 nm was monitored as a function of time. Apparent helix-coil transition temperature (Tm) was calculated from the two-state model. Fraction folded, F is calculated as below. Tm is the temperature where F = 0.5. F = (θobs− θDN)/(θN−θDN), where θobs is the observed ellipticity of the sample, θN is the ellipticity of the triple-helical structure determined at low temperature range, and θDN is the ellipticity of the denatured monomeric peptide at high temperature range.
RESULTS AND DISCUSSION
Thermal Stability of ppg9-XYG in the Aqueous Solution
The transition temperatures (Tm) of all peptides except ppg9-OOG were obtained from the literature,30 while that of ppg9-OOG was measured in this study. The Tm of PPG9 was also measured as a standard to compare Tm of ppg9-OOG with those of other four peptides (Table III). Although the Tm of PPG9 (19.0°C) obtained in this study is different from the previous value (17.7°C),30 the stabilizing (ppg9-POG and ppg9-OOG) and destabilizing (ppg9-OPG and ppg9-PaOG) tendencies can be observed as observed in repetitive peptides, (XYG)1017, 25, 35, 36 (Table III).
Peptide Main Chain Conformation
The main chain conformations of ppg9-POG, ppg9-OPG, and ppg9-OOG were essentially the same as that of ppg9-PPG (PPG9),32 which adopts a triple-helical structure very close to the ideal 7/2-helical model for collagen.8, 9, 37 These peptide conformations allow interchain hydrogen bonds between the Gly NH of one chain and the carbonyl oxygen in the Xaa residue of the adjacent chain. Average hydrogen bond lengths (distance between N and O atoms) were 2.93 Å in ppg9-POG, ppg9-OPG, and ppg9-OOG peptides; this value is the same as that found in PPG9.32 One of the characteristic features of collagen helices is the presence of long hydrogen bonds between NH and CO. In these structures, two independent molecules exist in an asymmetric unit. The root-mean-squares deviations (RMSDs) of these two molecules were 0.24 (ppg9-POG), 0.37 (ppg9-OPG), and 0.17 Å2 (ppg9-OOG), showing that the conformations of the two independent molecules are very similar. This was also observed in PPG9 (0.30 Å2). Stereoscopic molecular structures of the central 13 triplets for one of two independent molecules are superposed onto that of PPG9 (Figure 1). The root mean square deviation (RMSD) of the main chain atoms in the central 13 triplets of PPG9 and ppg9-XYG was 0.24 (ppg9-POG), 0.08 (ppg9-OPG), and 0.24 Å2 (ppg9-OOG), showing that the molecular conformations of these peptides are very similar.
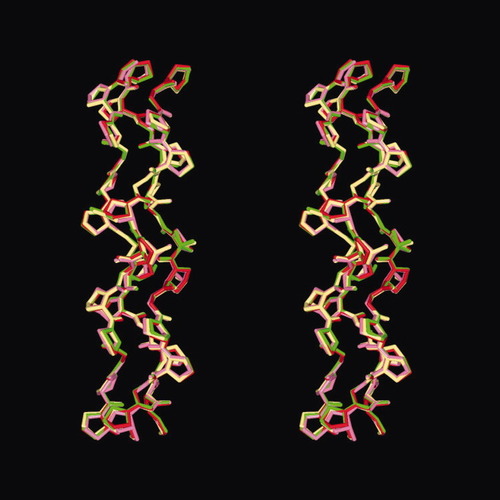
Stereoscopic molecular structures of three ppg9-XYG peptides superposed on the ppg9-PPG (PPG9). The central 13 triplets of these molecules are shown with different colors (ppg9-PPG, dark red; ppg9-OOG, yellow; ppg9-OPG, green; and ppg9-POG, pink).
Proline Ring Puckering
As observed in many protein single crystals and computational chemistry,23 the Pro residue adopts both up- and down-puckering, whereas the Hyp residue prefers up-puckering. This type of preference accompanied by Pro and Hyp residues is called “intrinsic preference.” Here, the up- and down-puckering are defined by the negative and positive values, respectively, of the dihedral angle, χ1(N-Cα-Cβ-Cγ). In the collagen helix, the X position prefers down-puckering and the Y position prefers up-puckering.23 This type of preference for residues in the X and Y positions of the Gly-Xaa-Yaa sequence is called “positional preference.” The propensity-based hypothesis, which correlates the intrinsic preference of the individual imino acids with the positional preference in the X and Y positions, has been able to explain many of the experimental data23 concerning the triple-helix stabilization and destabilization mechanism induced by Hyp. For example, this hypothesis can explain why POG10 is more stable than PPG10, while OPG10 does not form a triple-helical structure in aqueous solution. However, we found some opposing puckering of Pro in the POG10 and POG11 structures.38 Furthermore, the Hyp-Hyp-Gly and Hyp-Thr-Gly sequences with Hyp in the X position are known to be stable.27-29, 39 This is very peculiar, because the intrinsic preference of Hyp is up-puckering while the positional preference of the X is down. Single crystal analyses revealed that in the X position of GOO9 and OOG10, Hyp assumes the up-puckering conformation28, 29 against its positional preference.
In this study, we analyzed three ppg9-XYG peptides with Hyp in the X and/or Y positions. In the Y position of ppg9-POG, Hyp is in the up-conformation, the same as in POG10 and POG1138; in contrast, in the X position of ppg9-OPG, all six Hyp residues are down-puckered, following the positional preference rather than the intrinsic preference (Table IV). The latter situation was also observed for the puckering of alloHyp in ppg9-PaOG,31 where alloHyp adopts up-puckering following the positional preference rather than the intrinsic alloHyp preference (Table IV). In the crystal structure of ppg9-OPG, the Hyp13B and Hyp13E residues involved in the strong intermolecular hydrogen bond between hydroxyl oxygen atoms (O- - -O distance, 2.49 Å). In the crystal structure of ppg9-PaOG,31 out of six, the hydroxyl oxygen atoms of alloHyp14C (abbreviated as hyp14C in Table IV) and alloHyp14D residues involved in the similar strong hydrogen bond (O- - -O distance, 2.48 Å) between two independent molecules. Furthermore, those of alloHyp14B and alloHyp14F residues also form a hydrogen bond with longer hydrogen bond length of 2.91 Å. The rest of the residues, alloHyp14A and alloHyp14E have some contacts with adjacent molecules (for example, Cγ of alloHyp14A- - -Cγ of Pro16C (symmetry related), 3.53 Å; Oδ of alloHyp14E- - -Cγ of Pro13D (symmetry related), 3.50 Å). If all these residues adopted the down-conformation according to the intrinsic preference of alloHyp, hydrogen bonds of the former four residues would be broken, whereas the latter two residues, alloHyp14A and alloHyp14E, would not have any steric hindrance with surrounding molecules. At present, the driving force to determine the puckering conformation is not clear.
| Xaa | χ1 (°) | Yaa | χ1 (°) | Contact Between Xaa (or Yaa) and Adjacent Molecules | |
|---|---|---|---|---|---|
| ppg9-PaOG | Pro13A | 26.8 | alloHyp14C | −27.7 | Oδ hyp14C---Oδ hyp14D*, 2.48 Å |
| Pro13B | 25.7 | alloHyp14A | −22.9 | Oδ hyp14A---Cγ Pro16C*, 3.53 Å | |
| Pro16C | 31.1 | alloHyp14B | −23.8 | Oδ hyp14B---Oδ hyp14F*, 2.91 Å | |
| Pro13D | 34.2 | alloHyp14F | −21.9 | Oδ hyp14F---Oδ hyp14B*, 2.91 Å | |
| Pro13E | 22.6 | alloHyp14D | −26.4 | Oδ hyp14D---Oδ hyp14C*, 2.48 Å | |
| Pro16F | 34.1 | alloHyp14E | −22.3 | Oδ hyp14E---Cγ Pro13D*, 3.50 Å | |
| ppg9-OPG | Hyp13A | 27.5 | Pro14C | −22.3 | Oδ Hyp13A---Cγ Pro17F*, 2.81 Åa |
| Hyp13B | 26.1 | Pro14A | −25.9 | Oδ Hyp13B---Oδ Hyp13E, 2.49 Å | |
| Hyp13C | 31.1 | Pro11B | −23.0 | Oδ Hyp13C---Cβ Pro16E*, 3.68 Å | |
| Hyp13D | 27.3 | Pro14F | −23.7 | No contact less than 4.5 Å | |
| Hyp13E | 35.7 | Pro14D | −24.8 | Oδ Hyp13E---Oδ Hyp13B, 2.49 Å | |
| Hyp13F | 32.4 | Pro11E | −22.3 | Oδ Hyp13F---Cδ Pro17A*, 3.30 Åa | |
| ppg9-OOG | Hyp13A | 26.3 | Hyp14C | −20.4 | Oδ Hyp13A---Cδ Hyp14E*, 3.29 Åa |
| Hyp13B | −9.9 | Hyp14A | −24.2 | No contact less than 5 Å | |
| Hyp13C | 31.2 | Pro11B | −26.6 | Oδ Hyp13C---Cβ Pro16F, 3.50 Å | |
| Hyp13D | 21.9 | Hyp14F | −12.5 | Oδ Hyp13D---Cδ Hyp14B*, 3.35 Åa | |
| Hyp13E | −16.7 | Hyp14D | −27.7 | No contact less than 5 Å | |
| Hyp13F | 29.4 | Pro11E | −21.2 | Oδ Hyp13F---Cβ Pro7B, 3.41 Å |
- * Denotes the symmetry related residue.
- a The distance would become closer if Xaa residue adopted up-puckering.
In the ppg9-OOG peptide, all six Hyp(Y) residues of two independent molecules adopt up-puckering following both intrinsic and positional preferences, whereas two Hyp(X) residues adopt up-puckering (Hyp13B and Hyp13E), and the other four Hyp(X) residues adopt down-puckering (Table IV). In the triple-helical structures of polypeptides with Pro-Pro-Gly, Pro-Hyp-Gly, and Hyp-Hyp-Gly sequences, imino acid side chains in the X position stack with those in the Y position of the adjacent strand. This provides a stacking pair of proline ring (X) and proline ring (Y) in the adjacent chain. In the ppg9-OOG peptide, because of the one-residue-stagger between adjacent strands, Hyp(X) residues are classified into two types of stacking pairs, Hyp(X):Hyp(Y) and Hyp(X):Pro(Y) (Figure 2). More precisely, Hyp13A(X): Hyp14C(Y), Hyp13B(X):Hyp14A(Y), Hyp13D(X):Hyp14F (Y), and Hyp13E(X):Hyp14D(Y) belong to the first type, and Hyp13C(X):Pro11B(Y) and Hyp13F(X):Pro11E(Y) belong to the second (Figure 2).
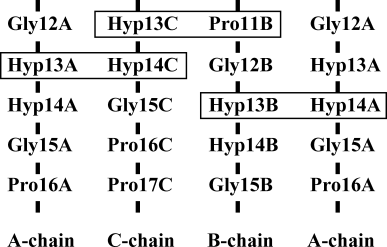
Hyp(X):Hyp(Y) and Hyp(X):Pro(Y) stacking pairs of one of two ppg9-OOG molecules in an asymmetric unit. One of them, Hyp13B(X):Hyp14A(Y) stacking pair, is visualized in Figure 3. Stacking pairs of the other molecule are obtained by changing A, B, and C in the above to D, E, and F, respectively.
Among the first ones, Oδ atoms of Hyp13B(X) and Hyp13E(X) have no contact at distances of less than 5 Å with surrounding peptide molecules. These two Hyp residues in the X position adopt the up-conformation (Figure 3). On the other hand, the other two residues in the X position, Hyp13A(X) and Hyp13D(X) adopt down-conformation and have contacts with surrounding molecules, Oδ of Hyp13A- - -Cδ of Hyp14E (symmetry related); 3.29 Å, and Oδ of Hyp13D- - -Cδ of Hyp14B (symmetry related); 3.35 Å. If Hyp13A(X) and Hyp13D(X) adopted the up-conformation, some atomic contacts between Hyp13A(X) and symmetry related Hyp14E, and those between Hyp13D(X) and symmetry related Hyp14B would become closer, and hence serious steric hindrance would occur. Therefore, these two residues must adopt the down-conformation as we observed. In both stacking pairs, Hyp13A(X):Hyp14C(Y) and Hyp13D (X):Hyp14F(Y), there were hydrogen bonds between hydroxyl oxygen atoms, Oδ of Hyp13A- - -Oδ of Hyp14C, 3.07 Å and Oδ of Hyp13D- - -Oδ of Hyp14F, 3.27 Å. The former hydrogen bond is shown in the central region of ppg9-OOG by the green dotted line (Figure 3). This type of hydrogen bond was predicted by molecular modeling of the GOO10 peptide,26 but was not observed in the real single crystal structures of GOO928 and OOG10.29 Judging from rather long hydrogen bond distances, these hydrogen bonds do not seem to contribute to the stabilization of triple-helical conformation. In the second type of stacking pairs, Hyp13C(X) and Hyp13F(X) adopt the down-conformation against their intrinsic preference. If these residues adopted the up-conformation according to their intrinsic preference, there would be no steric hindrance between these residues and surrounding molecules. The reason why they adopt the down-conformation is not clear, and we observed the similar circumstances in the ppg9-PaOG crystal as cited above.
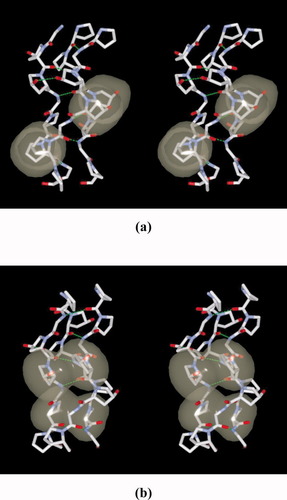
Stereoscopic structures of Hyp13B(X):Hyp14A(Y) and Pro16A(X):Pro17C(Y) stacking pairs of ppg9-OOG. Atoms in the proline rings of these pairs are shown by van der Waals spheres, showing interactions within each stacking pair. Except for Pro16A(X), other three proline rings assume the up-conformation. Green dotted lines indicate hydrogen bonds between NH of Gly in one chain and OC of Xaa residue in the adjacent chain, together with one hydrogen bond between hydroxyl oxygen atoms of Hyp13A(X) and Hyp14C(Y). The molecular orientations in (a) and (b) are related with 90° rotation around the axis.
From this experimental evidence, we concluded that (1) Hyp(X) residues involved in the Hyp(X):Pro(Y) stacking pair prefer down-puckering, as we saw in the ppg9-OPG and ppg9-OOG; and (2) those involved in the Hyp(X):Hyp(Y) stacking pair prefer the up-conformation if there is no specific reason to adopt down-conformation, such as steric hindrance of surrounding molecules if it is in the up-conformation, or an attractive interaction if it is in the down-conformation. The GOO9 peptide showed an example of the first reason, where out of 53 Hyp(X) residues of two independent molecules, 50 residues adopt up-puckering while three residues adopt down-puckering.28 The three down-puckered residues have contacts with adjacent molecules, and if they adopted the up-conformation, the contact distance would become shorter, and hence serious steric hindrance would occur. On the other hand, the OOG10 peptide showed an example of the second reason, where 24 out of 27 Hyp(X) residues adopt up-puckering, while three residues adopt down-puckering. In this case, the hydroxyl groups of these three down-puckered residues form hydrogen bonds with hydroxyl groups of Hyp(X) in the adjacent molecules.29 In the aqueous solution, all of these residues in the down-conformation will adopt the up-conformation, because there would be no such intermolecular repulsive or attractive interactions. For imino acid rich sequences, the interaction between proline ring (X) and proline ring (Y) seems to be important for the stabilization of the triple helix.40 The above two conclusions from the experimental evidence supported the recent estimation of the electrostatic interaction energies of stacking pairs with Pro(X):Hyp(Y), Hyp(X):Pro(Y) and Hyp(X):Hyp(Y).20
1st and 2nd Hydration Waters Around the Triple Helix
Similar to the fiber specimens of native collagen, single crystals of collagen-like peptides contain considerable numbers of water molecules. Many of their locations were observed by high-resolution analyses. We studied the distributions of water molecules around the triple helix formed by different peptide sequences. Figure 4 shows distributions of water molecules from the nearest atom of ppg9-XYG peptides, together with other related peptides. In all of these distributions, there are two distinct peaks centered at 2.75 and 3.55 Å, which correspond to the 1st and 2nd hydration shells, respectively. Water molecules in the 1st hydration shell directly link to the peptide atoms by hydrogen bond, while those in the 2nd hydration shell have no direct hydrogen bond with peptide atoms. As seen in Table V, the numbers of water molecules in the 2nd hydration shell of PPG9 and PPG10 are larger than those in the 1st hydration shell of the corresponding peptides. The ratio between the number of water molecules in the 1st shell and that in the 2nd shell for PPG9 (0.78) is quite similar to that for PPG10 (0.77). In this study, we reported three crystal structures of host-guest peptides, ppg9-POG, ppg9-OPG, and ppg9-OOG. The former two of these, as well as the ppg9-PaOG peptide, have one Hyp or alloHyp in their sequences. These three peptides showed similar ratios (0.93–0.99), which are significantly larger than those of PPG9 and PPG10. The ratio was further increased in the ppg9-OOG (1.27) where two Pro were replaced by Hyp in the guest triplet. Five ppg9-XYG peptides have very similar lattice constants (Table II) and the same space group, P21, and showed very similar pseudo-tetragonal packing arrangements. Therefore, the above findings suggested that the introduction of Hyp in the Pro-Pro-Gly sequence caused an increase in the ratio between the numbers of water molecules in the 1st and 2nd hydration shells. Because the number of water molecules in the 1st hydration shell is in the range of 70–76, the increase of the ratio was attributed to the decrease of the number of water molecules in the 2nd hydration shell (Table V).
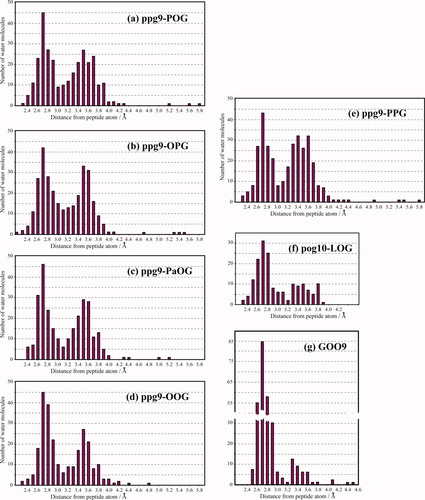
Distribution of water molecules around the triple helix as a function of their distance from the nearest peptide atom. (a) ppg9-POG, (b) ppg9-OPG, (c) ppg9-PaOG, (d) ppg9-OOG, (e) PPG9 (ppg9-PPG), (f) pog10-LOG, and (g) GOO9. It should be noted that one asymmetric unit contains one molecule in the pog10-LOG crystal, whereas it contains two molecules in the other crystals.
| Peptide | Resolution (Å) | No. of Water Moleculesa | Ratio | Packing | Refs. | |
|---|---|---|---|---|---|---|
| 1st Shell | 2nd Shell | |||||
| (Pro-Pro-Gly) sequence | ||||||
| PPG10 | 1.3 | 147 (66.2) | 192 (86.4) | 0.77 | T | 43 |
| PPG9 | 1.33 | 142 (71) | 182 (91) | 0.78 | T | 32 |
| ppg9-POG | 1.26 | 143 (71.5) | 154 (77) | 0.93 | T | – |
| ppg9-OPG | 1.36 | 151 (75.5) | 153 (76.5) | 0.99 | T | – |
| ppg9-PaOG | 1.1 | 139 (69.5) | 140 (70) | 0.99 | T | 31 |
| ppg9-OOG | 1.22 | 144 (72) | 113 (56.5) | 1.27 | T | – |
| (Pro-Hyp-Gly) sequence | ||||||
| POG11b | 1.26 | 30 (115.7) | 20 (77.1) | 1.50 | H | 38 |
| pog10-EKG | 1.75 | 96 (86.4) | 53 (47.7) | 1.81 | H | 41 |
| pog10-POA | 1.85 | 103 (92.7) | 59 (53.1) | 1.75 | H | 13 |
| pog10-LOG | 1.6 | 110 (99) | 60 (54) | 1.83 | H | 42 |
| pog8-PRG | 1.45 | 87 (97.9) | 39 (42.8) | 2.23 | T | – |
| (Hyp-Hyp-Gly) sequence | ||||||
| GOO9 | 1.55 | 241 (120.5) | 39 (19.5) | 6.17 | H | 28 |
| OOG10 | 1.5 | 135 (121.5) | 37 (33.3) | 3.65 | T | 29 |
- T, pseudo-tetragonal packing; H, pseudo-hexagonal packing.
- a The number of water molecules in the 1st and 2nd hydration shells was obtained by summing up the number of water molecules less than 3.1 Å and within the range of 3.1–4.1 Å, respectively. Values in parentheses were for one triple helix with nine triplet for each strand.
- b Sub-cell structure with c = 20 Å, Number of water molecules are for 7 triplets.
To confirm the above findings, we examined hydration states in the peptides with Pro-Hyp-Gly or Hyp-Hyp-Gly sequence repetition. Because no single crystal analysis for the full-cell structure of the former peptide has succeeded, the corresponding values of four host-guest peptides with Pro-Hyp-Gly as a host peptide were also included in Table V. These peptides are (Pro-Hyp-Gly)4-Glu-Lys-Gly-(Pro-Hyp-Gly)5 (pog10-EKG),41 (Pro-Hyp-Gly)4-Pro-Hyp-Ala-(Pro-Hyp-Gly)5 (pog10-POA),13 (Pro-Hyp-Gly)4-Leu-Hyp-Gly-(Pro-Hyp-Gly)5 (pog10-LOG),42 (Pro-Hyp-Gly)3-Pro-Arg-Gly-(Pro-Hyp-Gly)4 (pog8-PRG). On the replacement of Pro by Hyp, a significant increase in the ratio of the number of water molecules was observed (1.50–2.23). Although the POG11 peptide is ideal as the peptide with Pro-Hyp-Gly sequence, its crystal structure was determined only for the sub-cell structure with c = 20 Å. Therefore, only the average molecular conformation of seven triplets is available, instead of the full-cell structure.38 This might be the reason for the rather small ratio (1.50) of POG11 compared with other host-guest peptides. For the peptides with Hyp-Hyp-Gly sequence repetition, data from single crystals of the GOO928 and OOG1029 peptides are listed. The increase of the ratio of water molecules in the 1st and 2nd hydration shells is further advanced in these peptides (GOO9; 6.17 and OOG10; 3.65) because of the obvious increase of the number of water molecules in the 1st hydration shell and the obvious decrease of that in the 2nd hydration shell compared with those for Pro-Hyp-Gly sequence. This is clearly shown in Figure 5, where the numbers of water molecules in the 1st and 2nd hydration shells of Pro-Pro-Gly, Pro-Hyp-Gly, and Hyp-Hyp-Gly sequences are plotted.
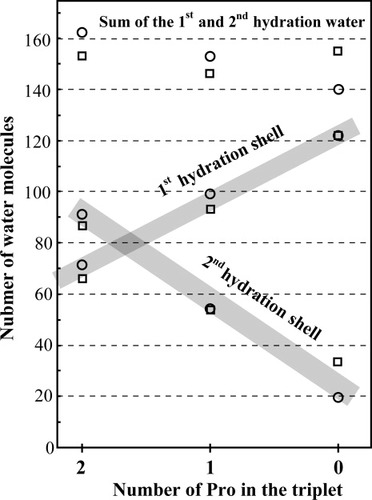
Number of water molecules in the 1st and 2nd hydration shells for the Pro-Pro-Gly, Pro-Hyp-Gly, and Hyp-Hyp-Gly sequence peptides. The numbers of water molecules of PPG9 and PPG10, pog10-LOG and pog10-POA, and GOO9 and OOG10 are plotted as the first, second, and third groups of peptides, respectively.
All the water molecules in the 1st hydration shell link to the peptide atoms by hydrogen bond. The carbonyl oxygen atoms of Gly and Yaa residues protrude outside of the triple helix, which enables water molecules to access these atoms. On the other hand, the carbonyl oxygen atom of the Xaa residue is directed to the inside of the triple helix and makes a hydrogen bond with NH of Gly in the adjacent chain. This blocks access of water molecules to this oxygen atom. Therefore, the numbers of water molecules hydrogen-bonded to the carbonyl oxygen atoms of the Xaa, Yaa and Gly residues are usually 0, 2, and 1, respectively, because the second hydration site of the Gly carbonyl oxygen is usually hindered by the adjacent chain. Hydroxyl oxygen atoms of the Hyp in the X and Y positions are located outside of the rod-like molecule, which usually adds two more water molecules linked to this oxygen. As a result, the numbers of water molecules for Pro-Pro-Gly, (Pro-Hyp-Gly or Hyp-Pro-Gly), and Hyp-Hyp-Gly sequences are, on average, usually 3, 5, and 7 per triplet, respectively. Therefore, in the case of PPG9, for example, the expected number of water molecules in the 1st hydration shell is 81 (= 3/triplet ×9 triplets/strand ×3 strands). The difference between the observed and expected numbers is attributed mainly to the disorder in the N- and C-terminal regions. These water molecules bound to the peptide oxygen atoms may act as anchoring points for hydration networks within the triple helix and also between triple helices.43 If so, it is very reasonable to expect that the number of water molecules in the 2nd hydration shell increases with the increase of the number of water in the 1st hydration shell. However, what we saw in Table V and Figures 4 and 5 is quite opposite. That is, even though the number of water molecules in the 1st hydration shell increases with Hyp content, that in the 2nd hydration shell decreases drastically. This indicates that the number of water molecules in the 2nd hydration shell depends inversely on the number of water molecules in the 1st hydration shell. In other words, it depends directly on the content of Pro in the triplet.
Clathrate Structure Enclosing Pro Side Chain
In globular proteins, hydrophobic amino acid residues fold inside of the proteins to avoid exposure to the surrounding water molecules. However, in the rod-like collagen molecule, amino acid residues in the X and Y positions protrude outside of the triple-helix, which forces even the hydrophobic side chains to be exposed to solvent water molecules. Introduction of Hyp in the X and Y positions of (Pro-Pro-Gly)n decreases the amount of hydrophobic surface area. Distribution diagrams of water molecules for GOO9 and OOG10 are similar to those observed in globular proteins (for example, Refs.44 and45), which suggests a strong relation between the hydrophobic surface area of the molecule and the number of water molecules in the 2nd hydration shell.
To improve our understanding, we examined the hydration networks involving each water molecule in the 2nd hydration shell of PPG9 and GOO9. When the 2nd hydration water links to a peptide atom via one of the 1st hydration water molecules, we shall call this the 2W network (Figure 6). When the 2nd hydration water links to the peptide atoms via two water molecules in the 1st hydration shell, we call this the 3W network. Similarly, as shown in Figure 6, the 4W and 5W networks were also considered. Although 2nd hydration waters in the 4W network seem to be very stable, the number of molecules in this network is very limited in the real structure because of geometrical constraints. Outside the 4W network, the 2nd hydration waters in the 3W network are the most stable. Although the number of water molecules (39) in the 2nd shell of GOO9 is small, most of the water molecules (30) were involved in the 3W network. On the other hand, out of 182 water molecules in the 2nd hydration shell of PPG9, only 73 water molecules were involved with this network, and most of them (109) were involved with much weaker networks such as the 2W and 5W networks. Therefore, most of the water molecules in the 2nd hydration shell of PPG9 are not strongly linked to the peptide atoms. As we saw, the number of water molecules in the 2nd hydration shell does not depend on the number of water molecules in the 1st hydration shell, but depends on the hydrophobic surface area of the peptide molecule. Therefore, we concluded that most of the water molecules in the 2nd shell of ppg9-XYG are covering hydrophobic Pro side chains by making clathrate structures via hydrogen-bond networks. In fact, in the PPG9 structure, almost all the nearest polypeptide atoms from the 2nd hydration water molecules are Cα, Cβ, Cγ and Cδ atoms of the Pro residues. Contrary to this, in the GOO9 peptide, one half of the nearest polypeptide atoms are peptide oxygen atoms.
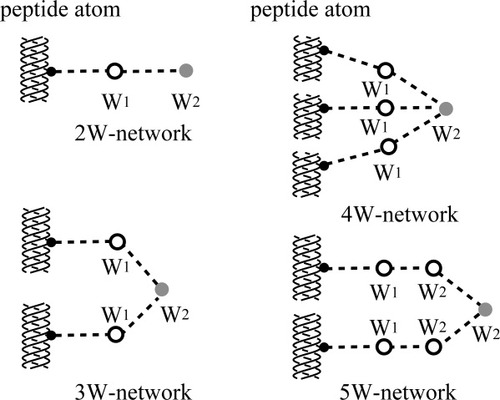
Schematic illustration of several types of hydration networks, which link the 2nd hydration water (filled gray circle) and peptide atoms (filled black circles in triple helices). Broken line stand for hydrogen bonds, and open circles stand for the 1st or 2nd hydration waters involved in the network.
Two typical packing arrangements of collagen model peptides have been observed depending mainly on their amino acid sequences. One is pseudo-hexagonal packing, which is usually observed for polypeptides with Pro-Hyp-Gly rich sequences, such as POG11,38 pog10-LOG,42 and pog10-POA peptide.13 Pseudo-hexagonal packing is very common for rod-like molecules. The other arrangement is pseudo-tetragonal packing which is usually observed for polypeptides with Pro-Pro-Gly rich sequence, such as PPG9,32 PPG10,43 and ppg9-XYG discussed in this study. The former packing arrangement can be considered to be made up of one triangular clustering column,42 whereas the latter can be considered to be made up of two different clusters, one triangular and one square column.32 In the case of peptides with Hyp-Hyp-Gly sequence repetition, both packing arrangements have been observed, for example, pseudo-hexagonal packing for GOO928 and pseudo-tetragonal packing for OOG10.29 Despite their different arrangements, the distribution diagrams of the GOO9 and OOG10 peptides are very similar, which shows no direct relation between packing arrangements and distribution diagrams of water molecules around the triple helix.
Comparison with Triple-Helix Hydration in Aqueous Solution
In our previous study46 of hydration behavior of collagen model peptides in an aqueous solution, the partial specific volumes of peptides around the transition temperature (Tm) indicated that the hydration number for a peptide with Gly-Pro-Hyp sequence increases with triple-helix formation, whereas the hydration number for a peptide with Gly-Hyp-Hyp sequence decreases. Above Tm, carbonyl and hydroxyl oxygen atoms, and a hydrophilic amide group of Gly protrude outside peptide molecules with both types of sequences, and hydrophobic Pro side chains of the peptide with Gly-Pro-Hyp sequence are folded to avoid access of water molecules. Below Tm, the hydration number for a peptide with Gly-Hyp-Hyp sequence decreases, because NH of Gly and one of CO move to the inside of the triple helix to make regular hydrogen bonds. For the peptide with Gly-Pro-Hyp sequence, in addition to this decrease of hydration number, there is an increase of hydration because of the formation of clathrate structures to cover the Pro side chains protruding outside the triple helix, as we saw in this study. Because the number of water molecules involved in the clathrate structure is more than those involved in hydrogen bonds with NH of Gly and CO of Xaa residue, we observed an increase in hydration number for a peptide with Gly-Pro-Hyp sequence. If this holds true, this tendency should also be observed for a peptide with Gly-Pro-Pro sequence. In the previous study,46 however, because of the lower-than-expected Tm of (Gly-Pro-Pro)9 peptide, this peptide had a partially formed triple helix at 4°C.
Acknowledgements
The synchrotron radiation experiments were performed at the SPring-8 BL40B2 and BL44XU, and at the Photon Factory BL-6A.




