Capturing the portrait of isolated individual natural cellulose molecules
Abstract
Natural cellulose molecules have a strong tendency of being aggregated into larger structures. Thus, the imaging of isolated individual cellulose molecules is hampered for a long time. In this work, we manage to observe, for the first time, the isolated individual natural cellulose chains on a sample surface by means of atomic force microscope. The advantage of the ionic liquid, in which natural cellulose can be molecularly dispersed, is considered to be the key point for the successful imaging. Moreover, we find that the surface charge can influence the morphology of the single cellulose chains upon adsorption. That is, on the positively charged surface, individual cellulose chains adopt an extended conformation; whereas on the negatively charged surface, a compact globule conformation is observed. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 1170–1173, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Natural cellulose is the most abundant natural macromolecules, which is mainly used in traditional industry at present. In the nature, plants are grown via bottom-up self-assembly of cellulose.1, 2 However, it seems that the fact has been neglected. As a result, the potential of wide application of cellulose in nanotechnology has not been recognized for many years. The advantages of cellulose, i.e., low cost, renewable, degradable, and biocompatible, will definitely contribute to the further development of nanotechnology.
It has been generally accepted that visualization will be very helpful for manipulating single macromolecules.3 However, because of the strong tendency of being aggregated into larger structures, the single-molecule imaging of cellulose, especially of the isolated individual chains, has not been reported yet.
Another obstacle for solving the above-mentioned problem is that cellulose can be hardly dissolved in common solvents. Recently, several kinds of ionic liquids (ILs) have been found to be nonderivatizing solvents for cellulose.4-6 The most outstanding advantage of ILs over other solvents of cellulose is that the ILs are monocomponent solvents, which simplifies the preparation of the cellulose solution. Other solvents of cellulose, such as cupriethylenediamine (CuEn)/water and N-methyl-morpholine-N-oxide (NMMO)/water systems, are multiple-component solvents, in which the solubility of cellulose is sensitive to the ratio between the components.7, 8 What is more interesting is that the ILs are true solvents, in which cellulose can be molecularly dispersed.5 With this feature, we expect that cellulose can be individually isolated on the solid surface. During the last two decades, atomic force microscope (AFM)9-11 has been developed into a powerful tool for imaging single molecules.12-19 In this work, we prepare samples by adsorption from a cellulose IL solution and then attempt to utilize AFM to observe the single cellulose molecules on the sample surface.
EXPERIMENTAL
Materials
The IL, 1-allyl-3-methylimidazolium chloride (AMIMCl), is synthesized following the literature.5 Natural cellulose (cotton linters) is purchased from Sigma-Aldrich (Product No. C6288). The viscosity-average degree of polymerization (DP) of the cellulose measured by using an Ubbelodhe viscometer in CuEn is 180. Muscovite mica is used as the substrate for AFM imaging. Deionized (DI) water is used in all experiments. 3-Aminopropyltriethoxysilane (APS) is chemical pure (Yaohua Chemical, Shanghai). Poly(sodium 4-styrene-sulfonate) (Mw = 200,000) is purchased from Sigma-Aldrich.
Sample Preparation
Natural cellulose is dissolved in AMIMCl by stirring the mixture at 75°C for ∼2 h to a concentration of 0.01 mg/mL. A freshly cleaved mica sheet is treated by an APS aqueous solution (V/V = 1:100) for 5 min, followed by thorough rinse with DI water, and then dried by air flow.17 This kind of amino groups-modified substrate is called amino-mica. A drop of the cellulose solution (∼ 10 μL) is deposited on the amino-mica (or unmodified mica) for 10 min, followed by thorough rinse with DI water, and then dried by air flow.
Sample Imaging
MultiMode scanning probe microscope (AFM, Veeco, CA) with a NanoScope IIIa controller operated in the tapping mode is used for the imaging of the cellulose sample. The scanning is performed in ambient condition with E-scanner and Si cantilever (Veeco, CA). The morphological data of the images are analyzed using the AFM-accessory software. All the images presented are unprocessed or treated only by flattening when necessary.
RESULTS AND DISCUSSION
The natural cellulose chains are weakly negatively charged because of the presence of hydroxyl groups.20 When cellulose solution is deposited on the amino group-modified mica (in brief, amino-mica) substrate, the cellulose chains tend to adsorb onto the positively charged surface. Interestingly, we observe long and thin randomly curved two-dimensional (2D) line on the amino-mica surface from the AFM height image, see Figure 1. Because cellulose is the only solute in the preparing procedure, the 2D structure is definitely attributed to be cellulose. We suggest that the observed curves in the images are isolated individual cellulose chains, not cellulose aggregates, for the following reasons: (1) The cross-section analysis showed that the curved materials have a height of ∼0.52 nm, (see Figure 1B) which is consistent with the diameter of a single cellulose chain.16 Cellulose aggregates are known to have a much larger value for this analysis. For instance, cellulose whiskers normally have a height of 10–15 nm.21 (2) The length–width ratio of the observed curve is very large (typically larger than 100), which differs a lot from that of cellulose aggregates, such as whiskers and fibrils.21, 22 (3) The IL is a true solvent for cellulose, in which cellulose chains can be molecularly dispersed. This situation should be held in our experiments since a dilute solution (0.01 mg/mL) of cellulose is used for deposition.4 The width of the observed line is almost a constant along the curve, which is an inherent characteristic for linear macromolecules that adopt extended conformation on the surface. (5) The curvature of the observed lines indicates that the material is flexible, which accordant well with the feature of a single linear macromolecules. Cellulose aggregates, such as whiskers and fibrils, are known to be much more rigid.21, 22
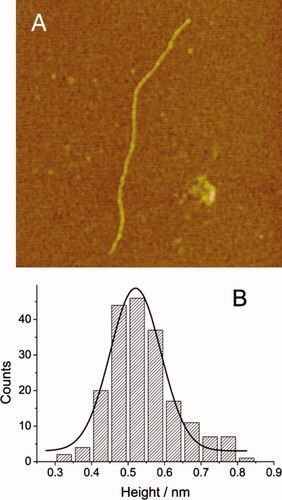
(A) AFM height image of an individual cellulose chain observed on the amino-mica. A 0.01 mg/mL cellulose AMIMCl solution is used for adsorption. Scan size: 1 μm × 1 μm. (B) The height distribution (n = 200) of the observed 2D curves (like that shown in Figure 1A) on the amino-mica surface. The Gaussian fitting indicates that the most probable height is 0.52 nm. The height data are obtained directly from the cross-section analysis by the AFM software.
The relative dielectric constant of imidazolium-based ILs is ∼10,23 which is only one-eighth of that of water. Although the electrostatic interactions between cellulose chain and the substrate in IL are weakened by the high salt concentration, the low dielectric constant environment will maintain the electrostatic attraction to a certain level. Besides electrostatic attraction, the hydrogen bonding between amino groups on the substrate and the hydroxyl groups in the cellulose chain may also contribute to the adsorption of cellulose onto the amino-mica. It is known that natural cellulose molecule has a large persistent length of ∼10 nm.24 If well solvated, for instance in the good solvent AMIMCl, the cellulose chains should adopt an extended conformation in a dilute solution. Upon adsorption, the 3D extended conformation in solution can be transformed into 2D extended conformation on the amino-mica surface (see Figure 1A).
As described earlier, we find that the single cellulose chain adopts an extended conformation on a positively charged, attractive surface. Differing from the amino-mica, the unmodified mica is negatively charged. An interesting question is that what conformation single cellulose chains will adopt on unmodified mica. To answer this question, we use the same cellulose solution to prepare the sample for AFM on a freshly cleaved mica. As shown in Figure 2, the cellulose chains adopt a compact globule conformation on the unmodified mica, which differs greatly from the result obtained from amino-mica (see also Figure 1). Each compact globule is isolated well, and is likely to correspond to an individual cellulose molecule, as the concentration of the cellulose solution is same to the case in Figure 1. A statistical analysis shows that the averaged size of the isolated globules in Figure 2 is 23.9 nm, with a SD of 5.0 nm (n = 22). The narrow size distribution of the nanodots supports that each dot is an individual cellulose chain.
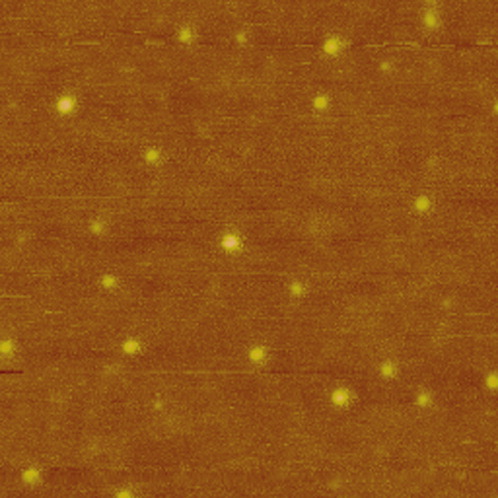
AFM height image of single chain cellulose nanodots obtained on mica. A 0.01 mg/mL cellulose AMIMCl solution is used for adsorption. Scan size: 1 μm × 1 μm.
Both of the unmodified mica and natural cellulose are negatively charged, which seems to prohibit the adsorption of natural cellulose onto the surface of unmodified mica. It is reported that the surface charge density of unmodified mica is in the range of 0.02–0.5 e−/nm2.25, 26 This relatively low value27 indicates that the negative charges are dispersed on the substrate, which may separate the surface into continuous negatively charged domains and isolated neutral domains. Each of the isolated neutral domains is attractive to the natural cellulose, possibly via hydrogen bonding. Because of the repulsion from the continuous negatively charged domains, each of the cellulose molecules has to adopt a compact globule conformation upon adsorption on the unmodified mica surface, leading to a dotted feature in Figure 2.
Strong negatively charged polyelectrolyte, such as poly(sodium 4-styrene-sulfonate) (PSS), adopts extended conformation in solution because of the repulsion between its repeating units. However, we find that PSS also adopts a compact nanoglobular conformation on the unmodified mica surface upon adsorption (data not shown). This result suggests that the surface charge density of the unmodified mica is low enough for a negatively charged polymer to adsorb, at the same time however, high enough to isolate individual polymer chains. In this way, the negatively charged polymer molecules are isolated as compact globules on the surface of the unmodified mica. Our observation provides another example that, for a fixed combination of solute and solvent, the electrostatic property of the surface can regulate the conformation of the adsorbed macromolecule.3
One may argue that the nanodots can also be attributed to aggregates of cellulose chains. To clarify this, we prepare a sample by a much higher concentration of cellulose (1.0 mg/mL), where aggregation of cellulose molecules is more likely to occur upon adsorption. As shown in Figure 3, the observed structures are similar to the cellulose whiskers and fibrils,21 suggesting aggregation of cellulose is occurred at higher concentration upon adsorption. When solution concentration is low (the cases of Figures 1 and 2), the aggregation can be avoided upon adsorption and individual cellulose molecules can be observed.
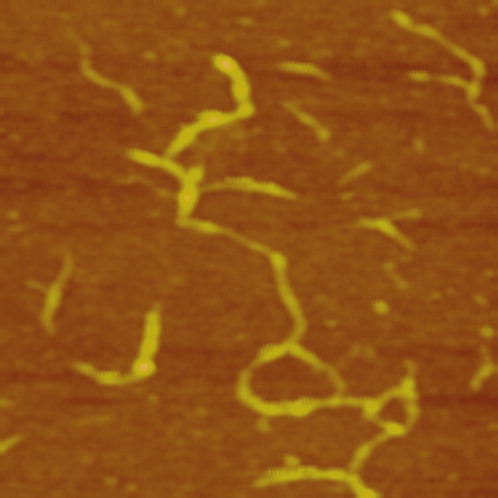
AFM height image of cellulose aggregates. A 1.0 mg/mL cellulose AMIMCl solution is used for adsorption. Scan size: 1 μm × 1 μm.
Go back to Figure 1A, we notice that the observed cellulose chain is unexpectedly long. A statistical analysis shows that the contour lengths of the observed cellulose chains range from 782 to 2995 nm, with an average value of 1952 nm (n = 13), which is much larger than that calculated from the viscosity-average DP (∼ 90 nm).28 This exceptional deviation may result from the following two reasons: (1) The cellulose sample has a wide molecular weight distribution, which is not reflected by the viscosity-average DP. A small fraction of cellulose molecules may have a much larger DP than the averaged value. (2) The density of the positive charge on the amino-mica is relatively low.29 Thus, the shorter cellulose chains will have fewer anchor points upon adsorption, which can be removed easily by water rising during the sample preparation. Considering that the adsorption density of cellulose chains on amino-mica (Figure 1A) is much lower than that on unmodified mica (see Figure 2), these two suppositions are reasonable.
The single-molecule observations obtained in this study may benefit from the IL solvent. There is an equilibrium between solubilize and aggregate as well as between adsorption and desorption. In this study, the equilibriums are governed by the interactions among cellulose molecules, the solvent, and the substrate. The fact that the IL is a monocomponent solvent indicates that IL is a more powerful solvent for cellulose than others. The strong interactions between IL and cellulose greatly weakened both aggregation among cellulose molecules and adsorption onto substrate, which is helpful for the single-molecule observation of cellulose.
CONCLUSIONS
Because of the strong tendency of being aggregated into larger structures, the imaging of isolated individual natural cellulose molecules has not been reported previously. In this work, we manage to visualize, for the first time, the isolated individual natural cellulose chains on a sample surface by means of AFM. The advantage of the IL, in which natural cellulose can be molecularly dispersed, is considered to be the key point for the successful imaging. By changing the charge of the surface, we find that individual cellulose molecules can adopt different conformations. That is, on the positively charged surface (amino-mica), individual cellulose chains adopt an extended conformation; whereas on the negatively charged surface (unmodified mica), a compact globule conformation is observed. Our findings lay a good foundation for manipulating single cellulose molecules on the surface in the future.
Acknowledgements
The authors thank Prof. S. Minko at Clarkson University for kindly providing the “2D Single Molecules” software.




