Self-crosslinked and reducible fusogenic peptides for intracellular delivery of siRNA
Abstract
A novel self-crosslinked and reducible peptide was synthesized for stable formation of nanoscale complexes with an siRNA-PEG conjugate to enhance transfection efficiency in serum containing condition without compromising cytotoxicity. A fusogenic peptide, KALA, with two cysteine residues at both terminal ends was crosslinked via disulfide linkages under mild DMSO oxidation condition. The reducible crosslinked KALA (cl-KALA) was used to form nano-complexes with green fluorescent protein (GFP) siRNA. Size and morphology of various polyelectrolyte complexes formulated with KALA and cl-KALA were comparatively analyzed. cl-KALA exhibited more reduced cell cytotoxicity and formed more stable and compact polyelectrolyte complexes with siRNA, compared with naked KALA and polyethylenimine (PEI), probably because of its increased charge density. The extent of gene silencing was quantitatively evaluated using MDA-MB-435 cells. cl-KALA/siRNA complexes showed comparable gene silencing efficiency with those of cytotoxic PEI. In a serum containing medium, cl-KALA/siRNA-PEG conjugate complexes exhibited superior gene inhibition because of the shielding effect of PEG on the surface. The formulation based on the self-crosslinked fusogenic peptide could be used as a biocompatible and efficient nonviral carrier for siRNA delivery. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 881–888, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Synthetic small interfering RNAs (siRNA), composed of double strand 21–23 nucleotides, have been emerged as a new class of potent nucleic acid drugs for treating various genetic diseases.1-3 A number of studies demonstrated that siRNA induces specific degradation of a target mRNA with a cascade of RNA interference events in a posttranscriptional step. It was also shown that siRNA exhibited the efficient down-regulation of a target gene in a sequence-specific manner with a much lower dose, compared with antisense oligonucleotides and ribozymes.4-6 For therapeutic applications in vivo, however, various extracellular and intracellular delivery hurdles should be solved.7-9 Since siRNA has short half-life in the blood stream and exhibits poor and nonspecific cellular uptake, various kinds of cationic species such as polymers, lipids, and peptides, have been utilized to form nanosized polyelectrolyte complexes with siRNA. The resultant complexes showed enhanced intracellular delivery efficiency via an endocytic cellular uptake mechanism, and protected siRNA from degradation by nucleases.10-12 The siRNA polyelectrolyte complexes were further modified with poly(ethylene glycol) (PEG) for increasing colloidal stability with prolonged circulation in the blood stream. According to previous report, direct PEG conjugation to siRNA could form more stable polyelectrolyte complexes than those by PEG modified cationic polymers.8
Although various cationic polymers and lipids demonstrated good gene transfection efficiencies in vitro, most of them were highly cytotoxic and not directly applicable for in vivo uses.13, 14 For example, the most popularly used cationic polymer, high-molecular weight and branched polyethylenimine (HMWB-PEI), has been widely used for in vitro gene transfection agents, but its notorious cytotoxic property hampered clinical applications. To circumvent the cytotoxicity problems, low-molecular weight branched PEI (LMWB-PEI) was crosslinked via cleavable linkages, such as disulfide, hydrazone, and ester bonds, to increase charge density and molecular weight. The crosslinked LMWB-PEI with reducible or cleavable bonds exhibited comparable transfection efficiencies with HMWB-PEI, but demonstrated much lower cytotoxicities.15, 16
An amphipathic, cationic, and fusogenic peptide, KALA, is known to destabilize cellular membrane, which has been popularly exploited as an endosome breaking peptide for various plasmid DNA and siRNA polyplexes.10, 17-20 The synthetic KALA peptide consisting of 30 amino acids could form polyelectrolyte complexes with therapeutic nucleic acids by charge interactions, showing about 100-fold higher delivery efficiency of plasmid DNA than polylysine.18, 19 According to our previous study, KALA/siRNA complexes also showed noticeable gene silencing efficiency.10 However, it was observed that KALA/siRNA polyelectrolyte complexes did not show sufficient gene silencing extents in the presence of serum proteins. This was attributed to its low charge density (MW: 3132), resulting in unstable nanoparticulate complexes when ionically interacted with siRNA (MW: 13,494). Previously, we demonstrated that significantly more enhanced gene inhibition effect was attained by conjugating antisense oligonucleotides to the backbone of hyaluronic acid via di-sulfide linkages when complexed with protamine, compared with the complexes with unconjugated anti-sense oligonucletides.21 This suggests that the charge density of either cationic polymers or oligonucleotides plays a critical role in stabilizing the complex structure in the serum containing condition.
In this study, KALA peptides having terminal cysteine residues were self-crosslinked through reducible di-sulfide linkages to increase the charge density and the crosslinked KALA (cl-KALA) was used for delivery of siRNA. The colloidal stability and cell cytotoxicity of cl-KALA/siRNA polyelectrolyte complexes were determined in comparison with those of KALA/siRNA complexes. The extents of green fluorescent protein (GFP) gene inhibition by siRNA and siRNA-s-s-PEG conjugate, complexed with cl-KALA, KALA, and HMWB-PEI, were comparatively evaluated in a 10% serum containing medium using GFP over-expressing MDA-MB-435 cells.
MATERIALS AND METHODS
Materials
KALA peptide (WEAK LAKA LAKA LAKH LAKA LAKA LKAC EA) and cysteine-KALA-cysteine peptide (CWEAK LAKA LAKA LAKH LAKA LAKA LKAC) were purchased from Peptron Inc. (Daejeon, South Korea). GFP siRNA composed of sense (5′–AACUUCAGG GUCAGCUUGCdTdT–3′) and anti-sense (5′–GCAAGCUGACC CUGAAGUUdTdT–3′) strands were purchased from Qiagen Inc. (Valencia, CA). For preparing siRNA-s-s-PEG conjugate, the sense strand of the siRNA modified with a hexylamine group at its 3′ end was used for PEGylation.11 MDA-MB-435-GFP (human breast cancer cell) cells stably expressing GFP protein were kindly donated by Samyang Corp. (Daejeon, South Korea). Cell counting kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto, Japan). Fetal bovine serum, phosphate buffered saline (PBS) solution, and DMEM (Dulbecco's Modified Eagle Medium) were purchased from Invitrogen (Carlsbad, CA). Dimethylsulfoxide (DMSO), heparin (MW: 17,000–19,000), and N,N,N′,N′-tetramethylazodicarboxamide were products of Sigma (St. Louis, MO). All other chemicals were of analytical grade.
Methods
Preparation and Characterization of Crosslinked KALA.
KALA terminally modified with cysteine residues (5 mg, 1.7 μmol) was dissolved in 150 μl of PBS solution (pH 7.5), to which N,N,N ′,N ′-tetramethylazodicarboxamide (11 mg, 63.9 μmol) dissolved in 50 μl of DMSO was added. After incubating at room temperature for 4 days, the solution was dialyzed in deionized water using a dialysis membrane (MWCO 10,000). The synthesis of crosslinked KALA (cl-KALA) was confirmed by polyacrylamide gel (PAGE) electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF, Voyager DE-STR Perkin-Elmer PerSeptive Biosystem). Naked KALA and cl-KALA were loaded on a polyacrylamide gel (4–20% gradient gel, Invitrogen, Carlsbad, CA). After a gel electrophoresis was carried out at 120 V for 1 h, the gel was stained with 0.1% coomassie blue solution and then destained with an aqueous solution containing 10% methanol and 10% acetic acid. The fractional amount of each cl-KALA multimer (dimer, trimer, and etc.) was quantified by a densitometry using an Image J software (National Institute of Health, USA; http://rsb.info.nih.gov/ij/) based on the electrophoresis result.22 The synthesized cl-KALA was also analyzed by MALDI-TOF. The sample crystal was prepared with sinapinic acid as a matrix. The operation conditions were set as follows: mode of operation, linear; polarity, positive; an acceleration voltage, 25,000 V; delayed extraction time, 180 ns.
To show the cleavage of disulfide linkages under the reducing condition, cl-KALA was incubated in the PBS solution (pH 7.4) containing 10 mM dithiothreitol (DTT) for predetermined time (0, 10 min, 30 min, 1 h, and 3 h). After incubation, the samples were loaded on a polyacrylamide gel (17%) and stained with the coomassie blue solution.
Preparation and Characterization of siRNA/cl-KALA Complexes.
To determine the complex sizes, siRNA (4 μg) dissolved in 200 μl of water was mixed with various amounts of KALA or cl-KALA in 200 μl water at N/P ratios from 2.5 to 10. After incubation for 15 min at room temperature, 2.6 ml of water was added to the mixture. To determine the hydrodynamic diameter of the complexes, the resulting complex solutions were analyzed by a dynamic light scattering instrument (Zeta-Plus, Brookhaven, NY).
The morphological characters of the complexes formed from KALA or cl-KALA with siRNA at the N/P ratio of 3.75 were also observed by atomic force microscopy (AFM). Samples were loaded on a freshly cleaved-mica surface and dried in an air at room temperature. Samples were visualized by using a 50 × 50 μm scanner of PSIA XE-100 AFM system (Santa Clara, CA) in a noncontact mode. The scanned image was collected from a 3 × 3 μm area.
To evaluate the stability of the cl-KALA/siRNA complexes, a polyanion competition assay was done as previously reported.21, 23 After preparing KALA or cl-KALA (27 μg) polyelectrolyte complexes with siRNA (1 μg), different amounts of heparin (0, 1, 5, 10, and 50 μg) were added to the solution and further incubated for 15 min. The released amount of siRNA by heparin was analyzed by 2% agarose gel electrophoresis and visualized by staining with ethidium bromide.
Cytotoxicity Assay.
Cytotoxicities of KALA, cl-KALA, and branched PEI were evaluated by CCK-8 cell viability assay.21 HEK 293 cells were seeded in a 96-well plate at a density of 1 × 104 cells per well and incubated for 24 h. Next day, the three cationic carriers (KALA, cl-KALA, or branched PEI) were added to the culture media to give a final concentration of 0–200 μg/ml. After further incubation for 24 h, the amount of viable cells was measured by CCK 8 cell viability assay according to the manufacturer's protocol. The cytotoxic effects of siRNA complexes with cl-KALA, KALA, and HMWB-PEI (25 K) were also evaluated by quantifying total protein amounts from the transfected cells.24 MDA-MB-435-GFP cells were plated in a 12 well plate at a density of 2 × 105 cells/well and incubated at 37°C overnight. After transfecting the three GFP siRNA (1μg) complexes at various N/P ratios for 5 h in a 10% serum containing medium, the transfection medium was replaced with a fresh serum containing medium and incubated for 2 days. After cell lysis with 1% Triton X-100 in PBS solution, total protein amounts were determined using Micro-BCA protein assay (Pierce, Rockford, IL).
Cell Culture and Transfections.
GFP expressing MDA-MB-435-GFP cells were cultivated in 10% serum containing DMEM supplemented with penicillin (100 units/ml) and streptomycin (100 μg/ml) at 37°C in 5% CO2 humidified atmosphere. A siRNA-PEG conjugate linked via a di-sulfide linkage (PEG MW = 5000) was prepared according to the previous report.11 For GFP gene silencing experiment, MDA-MB-435-GFP cells were transfected with KALA/siRNA and KALA/siRNA-PEG complexes according to the previous method. After plating MDA-MB-435-GFP cells in a 12-well plate at a density of 2 × 105 cells/well and incubating for 24 h, the three GFP siRNA (1 μg) complexes at various N/P ratios were transfected for 5 h in a 10% serum containing medium. After incubation, the transfection medium was replaced with a fresh culture medium and cells were incubated for 2 days. To determine the extent of GFP protein expression, the transfected cells were harvested by treating with a cell lysis solution (1% Triton X-100 in PBS) and centrifuged to remove cell debris. The supernatant was analyzed by a spectrofluorophotometer (SLM-AMINCO 8100, SLM Instrument, Rochester, NY) with an excitation and an emission wavelength at 488 and 509 nm, respectively. HMWB-PEI (25 K) was used as a control cationic polymer. Relative GFP expression levels were then calculated based on the GFP expression percent of nontransfected MDA-MB-435-GFP cells as a 100% control.
RESULTS AND DISCUSSION
Preparation and Characterization of Crosslinked KALA
Cysteine modified KALA at both terminal ends had 29 amino acids without C-terminal glutamate and alanine residues originally present in the KALA peptide. Self-crosslinked KALA (cl-KALA) peptides were prepared under mild oxidation condition using both N,N,N ′,N ′-tetramethylazodicarboxamide and DMSO as oxidizing agents as shown in Figure 1. The two terminal cysteine residues were self-oxidized and crosslinked to form multimeric KALA peptides linked by di-sulfide linkages. It was postulated that the enhanced charge density of cationic peptides through reducible crosslinking could facilitate the formation of more stable and compact polyelectrolyte complexes with siRNA, thereby increasing the extent of cellular uptake. The self-crosslinking reaction was mediated by disulfide bonds, which are specifically cleavable in reductive endosome/lysosomes and cytosolic conditions. Previously, several groups reported the reductive cleavage of a disulfide bond during an endocytic pathway.25, 26 Moreover, it is known that the intracellular concentration of glutathione (GSH) ranges from 0.5 to 20 mM, making the cytosol environment more reductive than the extracellular region.21, 27 Thus, it is conceivable that the disulfide linkages in the cl-KALA would be readily cleaved in reductive endosome and cytosol compartments after the cellular internalization of cl-KALA/siRNA complexes. The self-degradation of cl-KALA might also accelerate the decomplexation of cl-KALA/siRNA complexes only in the cytoplasm with subsequently releasing free siRNA molecules for facile interactions with RNA-induced silencing complex (RISC) involved in the first step of RNAi events. In previous studies, several crosslinked cationic polymers with di-sulfide linkages were reported as cytosol-specific degradable gene carriers that exhibited less cytotoxic behaviors.28, 29 Additionally, various reducible polypeptides synthesized mainly from lysine and histidine amino acids were also reported for delivery of siRNA.30, 31 In this study, reducible and multimeric KALA polypeptides were synthesized not only to enhance the cationic charge density for effectively condensing siRNA into stable nanocomplexes, but also to endow a fusogenic activity with concomitantly reducing cytotoxicity.
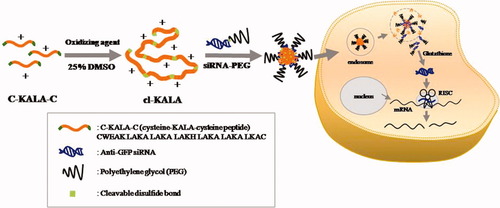
Schematic diagram of cl-KALA preparation and intracellular process of siRNA-PEG/cl-KALA nanocomplexes.
After the self-oxidation reaction of cyteine-KALA-cysteine, the resultant cl-KALA was analyzed by gel electrophoresis, as shown in Figure 2A. cl-KALA showed a retarded and ladder-like gel migration profile, compared with that of naked KALA, which indicates that cl-KALA was successfully synthesized. However, the resultant cl-KALA was a heterogeneous mixture of self-oxidized KALA multimers with showing up to self-crosslinked ∼10 monomer units. There was no monomeric KALA fraction after the self-oxidation. As shown in Figure 2B, the staining intensity for each band fraction of cl-KALA was quantitatively analyzed.22 The fractional amounts of each mutimeric species ranged from 5% to 10% with showing three major species: trimer (11.6 ± 3.0%), tetramer (13.6 ± 0.4%), and pentamer (11.5 ± 2.1%). The cl-KALA was also analyzed by MALDI-TOF analysis. The mass peaks of di-, tri-, tetra-, and pentameric species was clearly identified in Figure 2C, which correspond to the molecular weights of 6056, 9129, 12,205, and 15,220, respectively (the molecular weight of cysteine modified KALA was 3033). It appears that higher molecular weight cl-KALA multimer species above pentamer could not be detected in the MALDI-TOF spectra probably because of the sensitivity problem. From the gel electrophoresis and MALDI-TOF result, it can be deduced that cl-KALA had about 4–5 monomer units with an average molecular weight ranging 12,200–15,200.
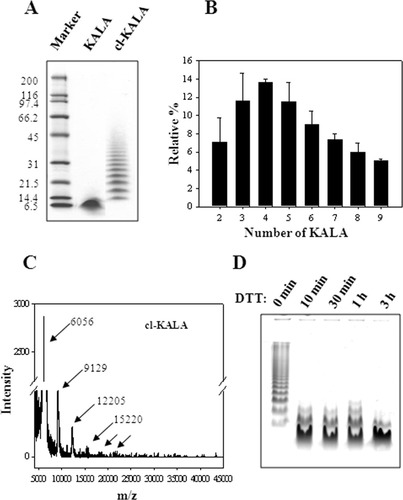
A: PAGE gel electrophoresis of KALA and cl-KALA. B: Quantification of relative fractional percentage of each cl-KALA multimer using an ImageJ software (NIH). C: MALDI-TOF analysis of cl-KALA. D: Cleavage of disulfide bonds of cl-KALA after incubated in 10 mM DTT solution.
The synthesized cl-KALA was treated with a reducing agent of 10 mM DTT solution to see whether monomeric KALA was regenerated by cleaving the di-sulfide linkages. Figure 2D shows that multimeric cl-KALA species were reduced to produce monomer, dimer, and trimer KALA peptides. Within 10 min, the fraction of monomer KALA was predominantly formed, indicating that cl-KALA was self-destroyed in a reductive condition like the cytosol environment.
Characterization of cl-KALA/siRNA Complexes
Dynamic light scattering (DLS) analysis was performed to confirm that cl-KALA could form more stable nanoscale complexes with GFP siRNA by electrostatic interactions, compared with KALA. The hydrodynamic sizes of siRNA polyelectrolyte complexes with KALA and cl-KALA were 1297.5 ± 43.2 nm and 143.8 ± 3.2 nm, respectively, at the N/P ratio of 3.75 (Figures 3A and 3B). The diameters of cl-KALA/siRNA complexes were near or below 200 nm over the entire N/P ratios, whereas those of siRNA/KALA complexes reached at 200 nm above the N/P ratio of 5. The morphologies of cl-KALA and KALA complexes with siRNA were visualized by AFM at the N/P ratio of 3.75. Although uniform and compact complexes about 100–200 nm could be seen for cl-KALA/siRNA complexes, uneven sized and aggregated complexes were observed for KALA/siRNA complexes (Figure 3C). These results clearly reveal that cl-KALA produced more compact complexes than KALA by increasing its charge density for condensing siRNA via electrostatic interactions with siRNA.
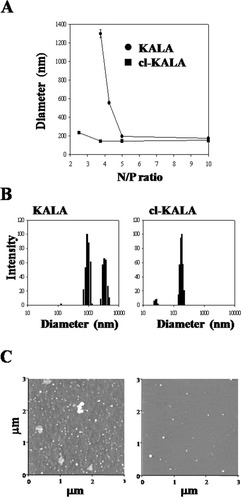
A: Effective diameters of KALA/siRNA and cl-KALA/siRNA complexes at various N/P ratios. B: Size distribution of KALA/siRNA and cl-KALA/siRNA complexes at N/P ratio of 3.75 measured by dynamic light scattering. C: Atomic force microscopic images of (left panel) KALA/siRNA and (right panel) cl-KALA/siRNA complexes.
To demonstrate that cl-KALA could form more stable complexes with siRNA, a heparin-induced decomplexation stability test for KALA/siRNA and cl-KALA/siRNA complexes was performed as shown in Figure 4. A competing polyanion, heparin, was added at various concentrations to induce the decomplexation of the condensed siRNA within the complexes. It can be seen that KALA/siRNA and cl-KALA/siRNA complexes began to be dissociated at heparin concentrations of 5 μg and 10 μg, respectively. About twofold higher heparin concentration was required to dissociate the cl-KALA/siRNA complexes than the KALA/siRNA complexes, suggesting that cl-KALA could form more stable complexes with siRNA than naked KALA because of the increased charge density.
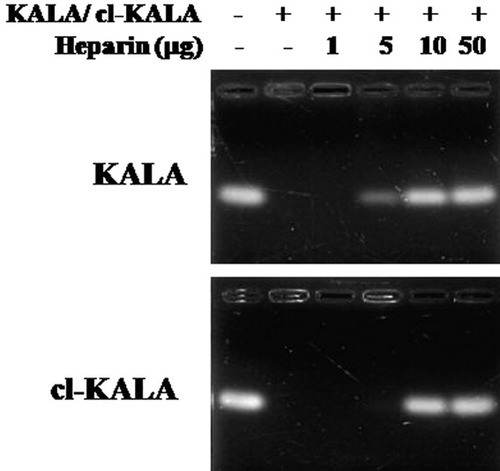
Stability assay of polyelectrolyte complexes in the presence of competing polyanion, heparin.
Cell Cytotoxicity of cl-KALA/siRNA Complexes
Cytotoxicities of cl-KALA, KALA, and HMWB-PEI (25 K) were determined using CCK8 assay, as shown in Figure 5A. cl-KALA showed significantly reduced cell toxicity, as compared with naked KALA and PEI. The percents of viable cells after incubating with PEI, KALA, and cl-KALA for 24 h at a polymer concentration of 100 μg/ml were (0.2 ± 0.3)%, (40.5 ± 3.2)%, and (74.9 ± 3.1)%, respectively. The relative percents of viable cells after 24 h incubation with the three cationic species were similar to those after 12 h incubation (data not shown), suggesting that the cell cytotoxicities observed in this study could be attributed to the destabilization of cell membrane, not to the mitochondria-mediated apoptosis.32 It was previously reported that KALA exhibits higher hemolytic activity at pH 7.5 than pH 5.0, causing nonspecific destabilization of cellular membrane with eliciting severe cytotoxicity in physiological conditions.20 Since an α-helical structure of KALA at pH 7.4 is mainly responsible for the destabilization of cellular membrane, the reduced cytotoxicity of cl-KALA could be attributed to significantly altered conformations different from the α-helical structure of KALA, as a result of self-crosslinking. In fact, the hemolytic activity of cl-KALA at pH 7.4 was twofold lower than that of naked KALA peptide (data not shown). Nevertheless, at acidic and reducible endosomal conditions, cl-KALA was cleaved to produce individually digested KALA peptides, which are likely to exhibit comparable fusogenic activities with that of naked KALA.
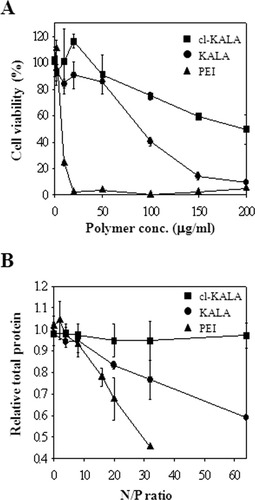
A: Comparative cytotoxicities after incubating HEK 293 cells with PEI, KALA, and cl-KALA for 24 h. B: Relative total protein amounts after transfecting siRNA complexes formulated with PEI, KALA, and cl-KALA for MDAMB-435 cells.
To show cytotoxic effects of siRNA complexes with cl-KALA, KALA, and HMWB-PEI (25 K), total cellular protein amounts were also measured after transfections.24 As shown in Figure 5B, relative total protein amounts were 0.95 ± 0.07, 0.83 ± 0.02, and 0.68 ± 0.10 (the total protein amount of untransfected cells: 1.0) for siRNA complexes with cl-KALA, KALA, and HMWB-PEI at the N/P ratio of 20, suggesting that both PEI and naked KALA were too cytotoxic to use above the N/P ratio of 8. However, cl-KALA/siRNA complexes showed negligible decrease in total protein amount up to the N/P ratio of 64, supporting the noncytotoxic nature of cl-KALA.
Gene Silencing Effect of cl-KALA/siRNA Complexes in 10% Serum Condition
To evaluate the extent of gene silencing by cl-KALA/siRNA complexes, GFP gene inhibition extents were quantitatively analyzed by measuring intracellular GFP fluorescence intensity after transfecting GFP siRNA complexes with cl-KALA in a 10% serum containing medium (see Figure 6). The extent of gene inhibition by cl-KALA/siRNA decreased gradually with increasing the N/P ratio. The marginally reduced gene silencing effect was partly because cl-KALA/siRNA polyelectrolyte complexes became unstable in the presence of serum proteins that mediated the intercomplex aggregation via nonspecific protein adsorption. To improve the stability of siRNA polyplexes and lipoplexes in the serum conditions, highly mobile and protein repellant PEG chains were introduced on their surface to maintain their colloidal stability. We previously demonstrated that siRNA-PEG conjugate could be used, as a novel and viable strategy, to form stable polyelectrolyte complexes with various cationic polymers, lipids, and peptides to confer superior PEGylated complex stability and to concomitantly enhance gene silencing in serum conditions.10, 11 Thus, siRNA-PEG conjugate linked via a di-sulfide linkage was used to produce stable cl-KALA/siRNA-PEG conjugate polyelectrolyte complexes. It was postulated that the siRNA part of the conjugate was ionically complexed with cl-KALA to form an inner core, whereas the PEG part was surface exposed to minimize nonspecific complex aggregation triggered by serum proteins. cl-KALA/siRNA and cl-KALA/PEG-siRNA conjugate complexes formulated at the N/P ratio of 64 showed (76.4 ± 1.9)% and (53.0 ± 3.5)% of GFP expression levels, respectively. On the other hand, PEI/siRNA-PEG conjugate complexes prepared at the N/P ratio of 8 showed a gene silencing extent of (77.5 ± 0.8)% which is statistically different from that by cl-KALA/siRNA-PEG conjugate complexes (P < 0.001). These data suggest that PEG conjugated siRNA showed more enhanced gene silencing effect in the presence of serum than naked siRNA due to the stealth effect of PEG.
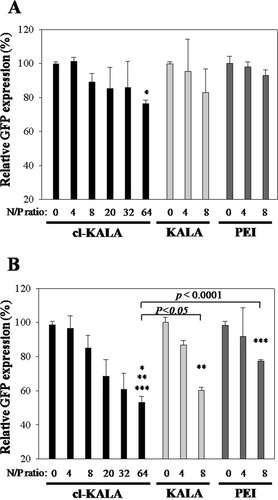
The extents of gene silencing by (A) cl-KALA/siRNA complexes and (B) cl-KALA/siRNA-PEG conjugate complexes for GFP over-expressing MDAMB-435 cells in a 10% serum containing medium. Naked KALA and HMWB PEI (25 K) were used as controls. *P < 0.001, **P < 0.05, and ***P < 0.0001.
Lastly, it should be noted that when functional cationic peptides such as KALA were used to form nanoscale complexes with siRNA for gene delivery, PEG can not be directly conjugated to the backbone of the peptides owing to the activity loss of the peptides, or can not be site-specifically conjugated to the N-terminal end due to the presence of lysine residues in the backbone. Therefore, cleavable siRNA-PEG conjugate can be used as an alternative approach to produce PEGylated siRNA nanocomplexes that are expected to be stable enough to prolong half-life in the blood stream.
CONCLUSIONS
KALA peptides were self-crosslinked to increase the charge density and to form stable and compact polyelectrolyte complexes with siRNA and siRNA-PEG conjugate. In the serum conditions, cl-KALA/siRNA-PEG conjugate exhibited far superior gene silencing efficiencies to PEI based siRNA and siRNA-PEG conjugate complexes. cl-KALA could be potentially used as a valuable noncytotoxic and fusogenic delivery carrier for nucleic acid based therapeutics including plasmid DNA, antisense ODN, and siRNA.




