A conserved stable core structure in the passenger domain β-helix of autotransporter virulence proteins†
This paper is dedicated to the late Elkan R. Blout, an extraordinary chemist whose pioneering studies on pepdide conformation produced a permanent imprint on protein science, via both the results and the scientists his lab produced.
Abstract
In Gram-negative bacteria, a wide variety of virulence factors are secreted via the autotransporter (AT) pathway. Intriguingly, there is no significant concentration of ATP in the periplasm, nor a proton gradient across the OM, so the energetic origin of efficient secretion of AT proteins is unknown. More than 97% of AT proteins are predicted to contain right-handed parallel β-helical structure, and the three crystal structures available for AT passenger domains each contain a long right-handed parallel β-helix. Previous studies have shown that pertactin, an AT from Bordetella pertussis, exhibits three-state folding and has a C-terminal stable core structure. Here, we show that Pet, an unrelated AT from Escherichia coli, also exhibits three-state unfolding and also has a stable core structure. Deletion mutants, mass spectrometry, and N-terminal sequencing demonstrate that the Pet stable core is also located near the C-terminus of the passenger domain. Moreover, sequence analysis suggests that three-state folding and a C-terminal stable core structure could be important general features of the biogenesis of AT proteins in vivo. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 420–427, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Previously, we reported the equilibrium unfolding/refolding behavior of pertactin, a single-domain right-handed parallel β-helix protein (Figure 1b).1 Despite having a native structure that comprises only a single structural domain, pertactin unfolds and refolds via two distinct transitions at equilibrium. At intermediate denaturant concentrations, pertactin adopts a partially folded state in which the C-terminal half of the protein is stably folded, but the N-terminus is disordered. Three-state folding is not, however, a characteristic feature of all right-handed parallel β-helix proteins: the functionally unrelated proteins P22 tailspike and pectate lyase C are also composed primarily of right-handed parallel β-helix structure, but both unfold at equilibrium in a single largely cooperative transition.2, 3 These results raise questions regarding the origin of pertactin's three-state folding behavior and a possible biological role for a partially folded structure.
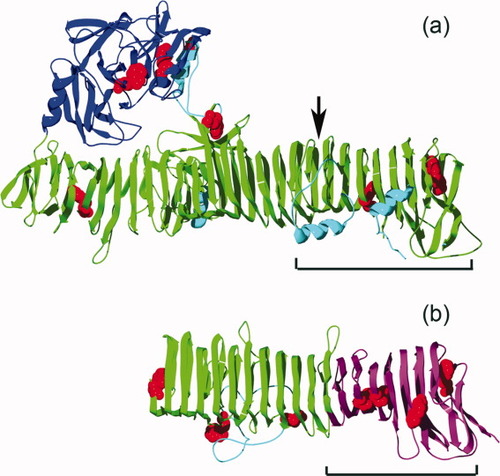
Ribbon diagrams representing the crystal structures of (a) Hbp (PDB ID: 1WXR) and (b) pertactin (1DAB). Pet is a homolog of Hbp (26% identical and 43% similar). The pertactin stable core is shown in purple; other β-helical structure is shown in green. The Hbp N-terminal protease domain is shown in blue; other non-β-helical structure is shown in teal. There are seven tryptophan residues in Pet (equivalent locations in Hbp are shown in red). The mature Pet protease domain is 108 kDa (33 kDa protease domain and a 75 kDa β-helix domain) vs. pertactin, which comprises a single domain of 55 kDa. The black brackets represent the residues aligned by PSI-BLAST (292 to 537 of pertactin and 688 to 940 of Pet; see Figure 9 and text for more details). The arrow indicates the position of G730.
Pertactin is an autotransporter (AT) protein. AT proteins are found across many species of pathogenic Gram-negative bacteria, including Escherichia coli, Bordetella pertussis, Yersinia pestis, Helicobacter pylori, Pseudomonas aeruginosa, and Neissera, and are often associated with virulence.4 Each AT protein sequence consist of three parts: an N-terminal signal sequence, a central heterologous passenger domain that represents the mature, functional virulence protein, and a C-terminal porin domain.5 The signal sequence facilitates translocation across the inner membrane. The C-terminal porin domain folds into a β-barrel across the outer membrane, where it is thought to act as a pore for the transport of the passenger domain across the OM.5 The crystal structure of the porin domain from the AT NalP revealed a pore diameter that is too small (1–2 nm) to allow passage of a folded passenger domain, suggesting the passenger domain might pass through the porin unfolded, then fold extracellularly.6 The crystal structures of two AT passenger domains have been solved: pertactin7 and hemoglobin protease8 (Figure 1), as well as a portion of the VacA passenger domain.9 All three structures include a long right-handed parallel β-helical structure, and pertactin and Hbp are the two longest β-helical structures solved to date. Intriguingly, >97% of all known AT proteins are predicted to include β-helical structure.1
There is no significant concentration of ATP in the periplasm, nor a proton gradient across the OM,5 leaving the energetic driving force for efficient OM secretion of the AT passenger unknown. We have hypothesized that the free energy released on folding of the passenger β-helix could actively contribute to efficient secretion, or alternatively passively prevent sliding of the passenger back into the periplasm during OM secretion.1 Several labs have hypothesized that OM secretion of the passenger proceeds from C→N terminus.10-12 In this scenario, extra stability at the C-terminus of the passenger might produce a folding template or nucleus that could promote the folding of the remainder of the β-helix during OM secretion.
The serine protease autotransporters of the Enterobacteriaceae (SPATEs) are a growing family of AT proteins from E. coli and Shigella that contain a serine protease motif and are associated with pathogenesis.5 Two well-studied examples are hemoglobin protease (Hbp), a serine protease involved in iron scavenging for the human pathogen E. coli EB1,13 and plasmid-encoded toxin (Pet) from E. coli 042, a pathogenic strain that causes diarrhea.14 The crystal structure of the Pet passenger domain has not been solved, but Pet and Hbp passenger domains share 26% sequence identity plus 43% sequence similarity. Here, we report the folding and stability of Pet in vitro. Pet, like pertactin, unfolds via two transitions, and populates a partially folded stable core structure at intermediate GdnHCl concentrations. The sequence comprising this stable core is located near the C-terminus of the passenger domain. These results suggest that three-state folding could represent a general feature of bacterial virulence proteins secreted via the AT mechanism.
RESULTS
Pet Can Be Purified from Cell Culture Supernatant and is Rich in β–Sheet Structure
HB101 cells expressing the plasmid pCEFN1 secrete Pet into the culture media.15 Pet was purified to ∼95% purity using ammonium sulfate precipitation and gel filtration chromatography (Figure 2a).16 The far-UV CD spectrum of folded Pet is characteristic of a structure rich in β-sheet,17 including a minimum at 218 nm and a maximum at 200 nm (Figure 3). On heating to 100°C, the spectrum retained some negative ellipticity at 218 nm, but changed to a single minimum at 200 nm, indicative of an unfolded conformation.
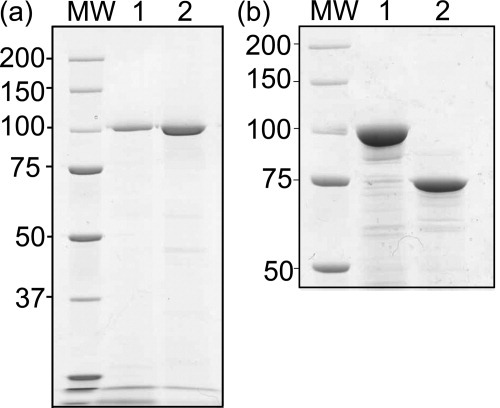
(a) Purification of Pet. Lane 1: concentrated cell culture supernatant before gel filtration chromatography. Lane 2: pooled and concentrated fractions from the size exclusion column. (b) Secretion of PetΔprotease. Concentrated cell culture supernatant of HB101 cells expressing pCEFN1 (lane 1) and pCEFN1Δprotease (lane 2). Both gels were stained with Coomassie.
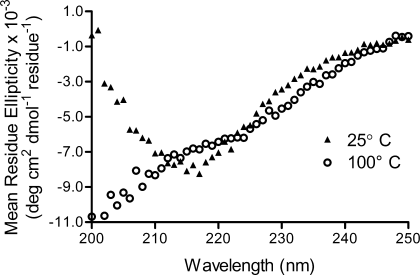
Far-UV CD wavelength scan of 2 μM Pet at 25°C and 90°C, showing the loss of β-sheet structure upon thermal denaturation.
We also used the β-helix prediction program BetaWrap18 to identify β-helix content in the Pet passenger domain sequence. The Pet passenger domain produced a BetaWrap score of −21.93 (P-value of 0.0048). In comparison, the scores for Hbp and pertactin are −21.27 and −18.2 (P-values of 1 × 10−5 and 0.0021), respectively. These statistically significant P-values (http://groups.csail.mit.edu/cb/betawrap/betawrap.html) suggest high β-helical content.
Pet, Like Pertactin, Unfolds in Two Transitions
The Pet passenger domain contains seven tryptophan residues, located throughout the sequence (Figure 1). The change in Trp fluorescence emission intensity was used to monitor Pet denaturation in increasing concentrations of GdnHCl. The wavelength of maximum fluorescence emission for Pet in 0M GdnHCl was 325 nm (Figure 4a), and this wavelength was used to monitor fluorescence intensity changes as a function of denaturant concentration (Figure 4b). This analysis revealed that Pet unfolds in two distinct transitions, with the midpoint of first transition at 0.9M GdnHCl and the second at 1.4M GdnHCl.
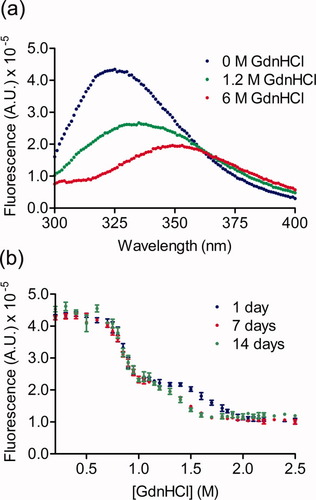
(a) Steady state fluorescence emission of Pet monitored in 0M (blue), 1.2M (green), and 2.5M (red) GdnHCl. (b) Steady state fluorescence emission of Pet in varying concentrations of GdnHCl monitored at 325 nm. Samples were incubated for 1, 7, or 14 days before data acquisition.
Limited Proteolysis Reveals a Partially Folded State
Limited proteolysis was used to investigate the structure of the partially folded state of Pet, using proteinase K, a non-specific protease. Typically, unfolded proteins are degraded much faster than folded proteins,19 and hence limited exposure to proteinase K can be used to reveal residual folded structure. Native Pet was relatively resistant to digestion by proteinase K: the band at 100 kDa, corresponding to full length Pet, persisted for several hours (Figure 5a). However, when a similar digestion was performed on Pet equilibrated in 1.2M GdnHCl, the band corresponding to full length Pet rapidly disappeared, and was undetectable after 10 min. Intriguingly, a band corresponding to a fragment of ∼25 kDa rapidly appeared (Figure 5b) and persisted for several hours. Several other bands were also present at shorter digestion times, including t = 0 min; these bands are believed to arise from gradual autoproteolysis of Pet after purification.
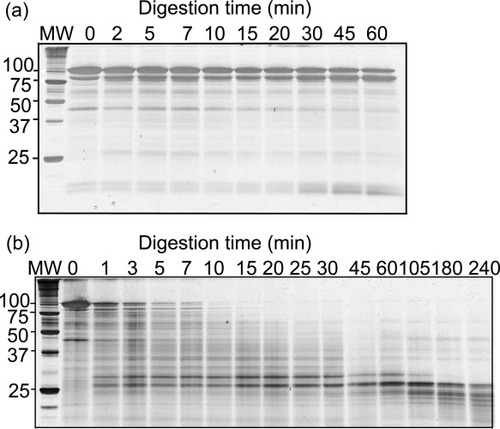
Limited proteolysis with a molar ratio of 1:10,000 proteinase K to Pet. Products were separated by 16% SDS-PAGE and silver stained. (a) Pet in 0M GdnHCl and (b) Pet incubated in 1.2M GdnHCl for 24 h. Digestion of Pet in 1.2M GdnHCl revealed a 25 kDa fragment that was resistant to digestion.
PetΔprotease Unfolds in Two Transitions and Forms a Partially Folded State
To determine the effect of the protease domain on Pet folding and stability, including the partially folded state, a construct was created that lacks the N-terminal protease domain (PetΔprotease). When PetΔprotease was expressed in E. coli HB101, a 75 kDa protein accumulated in the spent culture media (Figure 2b). PetΔprotease was purified in a manner similar to wild type Pet (see Materials and Methods for details). Like wild type Pet, the maximum fluorescence emission for PetΔprotease in 0M GdnHCl was at 325 nm (Figure 6a), and PetΔprotease also unfolded in two transitions (Figure 6b), although this process was slower for PetΔprotease and the first transition is not as distinct. Moreover, native PetΔprotease was resistant to proteinase K digestion, while PetΔprotease incubated in 1.2M GdnHCl was quickly digested to a 25 kDa fragment (Figure 7), very similar to the results obtained for wild type Pet.

(a) Steady state fluorescence emission of 0.25 μM PetΔprotease monitored from 300 to 400 nm in 0M (blue), 1.2M (green), and 2.5M GdnHCl (red). (b) Steady state fluorescence emission monitored at 325 nm. PetΔprotease (0.25 μM) in varying concentrations of GdnHCl were incubated for 1, 7, or 14 days before data acquisition.

Limited proteolysis with a molar ratio of 1:10,000 proteinase K to PetΔprotease. Products were separated by 16% SDS-PAGE and silver stained. (a) PetΔprotease in 0 M GdnHCl and (b) PetΔprotease incubated in 1.2M GdnHCl for 24 h. Digestion of PetΔprotease in 1.2M GdnHCl revealed a 25 kDa fragment that was resistant to digestion.
The Pet Stable Core is Located in the C-Terminus of the β-Helix
The mass of the Pet protease-resistant stable core was determined to be 24.3 kDa by MALD-TOF mass spectrometry (Figure 8a). The N-terminus of the stable core was identified via Edman degradation, which produced the sequence GGYELTEDGANFTA. This sequence corresponds exactly to residues 730 to 743 of the Pet passenger domain. When the stable core was digested with trypsin and the resulting fragments analyzed by MALDI-TOF mass spectrometry, four tryptic fragments were identified, all from the C-terminal portion of the Pet passenger (Figures 8b and 8c).
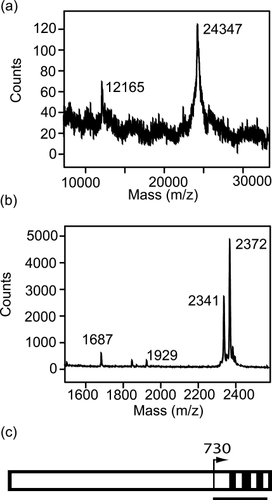
MALDI-TOF mass spectrum of (a) the intact stable core (24.3 kDa; the peak at 12,165 mass units corresponds to a doubly charged ion) and (b) tryptic fragments of the stable core. (c) Schematic diagram of the Pet passenger domain showing the location of the tryptic fragments (black bars). The peaks at 2341, 2372, and 1687 mass units were identified as residues 775–795, 833–853, and 868–882, respectively. The peak at 1929 mass units corresponds to residues 866–882, i.e., one missed cleavage at K867. The black line represents the mass of the intact stable core, indicating it extends near the end of the Pet passenger domain.
Pet and Pertactin Passenger Domain Sequences Show Weak Similarity at Their C-Termini
The sequences of the Pet and hemoglobin protease passenger domains were compared using BLAST, with the BLOSUM62 matrix, a gap opening penalty of 11, and gap extension penalty of 1. The BLAST search aligned Pet and hemoglobin protease with an E-value of 5 × 10−87. This detected alignment has 26% sequence identity and 43% similarity. The passenger domains of Pet and pertactin were aligned using the same parameters. No significant BLAST alignment (no E-value less than 0.01)20 was obtained. The Pet passenger domain was then subjected to a PSI-BLAST search. The second iteration of the search produced an alignment between Pet and the pertactin passenger sequence with an E-value of 1 × 10−7. The detected alignment is between residues 292 to 537 of pertactin and 688 to 940 of Pet (Figures 1 and 9) and has 19% sequence identity and 35% similarity.

Residues 292 to 537 of pertactin and 688 to 940 of Pet were aligned using PSI-BLAST. Identical residues are shaded with dark gray and similar residues are light gray. Gaps in the alignment are represented by a dash. Aligned residues are also indicated by the black brackets in Figure 1.
DISCUSSION
The AT secretion pathway is the most common secretion pathway used by pathogenic Gram-negative bacteria, and is responsible for the secretion of proteins with a wide variety of sizes and functions.5 The mechanism of AT secretion is not well understood, nor is it known if a result obtained for an individual AT protein represents a general feature of the AT mechanism, or a protein-specific trait.
The denaturant-induced unfolding of the Pet AT passenger reported here is strikingly similar to the equilibrium unfolding behavior of the pertactin AT passenger.1 Pet, like pertactin, unfolds in two transitions and adopts a partially folded state at intermediate concentrations of denaturant. Also like pertactin, the Pet partially folded state consists of a protease-resistant core structure, although the Pet stable core is slightly larger (24 kDa) than the pertactin stable core (21 kDa).1
The construction and analysis of a Pet mutant lacking the N-terminal protease domain (PetΔprotease) clarified which of the two unfolding transition corresponds to the unfolding of this globular domain, versus the long β-helix domain. Deletion of the protease domain results in the deletion of two (of seven) tryptophan residues, and the fluorescence emission intensity of native PetΔprotease is ∼60% that of wild type Pet (Figure 6). Like wild type Pet, PetΔprotease also unfolds in two transitions, yet there are two significant differences. First, the relative amplitude of the fluorescence emission intensity change for the first transition is smaller for PetΔprotease than for wild type Pet, suggesting the globular protease domain unfolds in this first transition. Second, for PetΔprotease the midpoint of the first unfolding transition is shifted to slightly lower concentrations of denaturant (0.7M vs. 0.9M GdnHCl for wild type Pet), also suggesting that the protease domain contributes to the stability of the native structure, rather than the partially folded state. This interpretation is also supported by the proteinase K digestion of the PetΔprotease partially folded state (Figure 7), which produces a protease-resistant fragment with a size indistinguishable from the size of the corresponding digestion fragment for wild type Pet. Taken together, these results indicate that the unfolding of the N-terminal protease domain contributes to, but does not represent the entirety of, the first unfolding transition of the Pet passenger. In contrast, the second unfolding transition, from the partially folded state to a completely unfolded protein, is largely unaltered for PetΔprotease, suggesting it exclusively represents the unfolding of some portion of the β-helical portion of the Pet passenger.
Mass spectral analysis and N-terminal sequencing of the stable core were used to identify the location and boundaries of the Pet stable core. Edman degradation identified the N-terminus of the stable core as G730 within the Pet passenger domain. The theoretical mass of a Pet fragment that begins at G730 and extends to the end of the Pet passenger sequence has a calculated size of 25.5 kDa, which is slightly larger than the mass of the intact stable core (24.3 kDa). The Hbp passenger domain crystal structure has an α-helix at the extreme C-terminus, which might be more easily digested by proteinase K than β-helical structure. The calculated mass of a Pet C-terminal fragment lacking the sequence corresponding to this α-helix is 24.6 kDa, closer to the experimental value of the stable core. The identification of four tryptic fragments from the stable core, each corresponding to Pet passenger sequences that are C-terminal to G730, supports the assignment of the stable core to the C-terminal portion of the Pet β-helix.
When the Pet passenger domain was subjected to a PSI-BLAST search, an alignment was detected between the passenger C-termini of Pet and pertactin (Figure 9). Since a majority of the proteins identified in the first iteration of the PSI-BLAST search are ATs (not shown), the substitution matrix used for the second iteration is therefore likely optimized to identify AT proteins.21 More than 97% of AT proteins are predicted to be β-helical, and hence the second iteration of PSI-BLAST might also be optimized to identify residues that have a propensity to form β-helical structure. Nevertheless, it is striking that such a relatively weak alignment score (E-value of 1 × 10−7) gives rise to such similar folding behavior. This alignment might suggest sequence elements that distinguish AT β-helical proteins from non-AT β-helical proteins, and result in three-state folding.
Our results demonstrate that the stable core structures of both Pet and pertactin are located at or near the C-terminus of the β-helix domain, suggesting the stable core might be a common feature of AT passenger domains. Could a stable core have relevance for secretion in vivo? Recent studies have provided conflicting results: a study with the BrkA AT showed that a C-terminal portion of its passenger domain is necessary for correct folding and secretion of BrkA,22 suggesting that a C-terminal stable core may be important for secretion. However, studies with other ATs have replaced the entire passenger domain with a non-β-helical heterologous protein, and not abolished secretion.23-25 These studies did not, however, provide quantitative measurements of AT secretion efficiency, so it is unclear what effect, if any, AT passenger replacement has on secretion efficiency.
The absence of an obvious external energy source leaves the energetic driving force for efficient secretion unknown. The correct folding of a stable C-terminal passenger structure could create a scaffold that would allow folding of the more N-terminal portions of the passenger, or more passively, prevent the passenger domain from sliding back into the periplasm. Extra stability at the C-terminus of the passenger could also reduce degradation of AT passengers during OM secretion.
In conclusion, we have shown that three-state unfolding behavior and a stable core structure are found in at least two AT passenger domains. Since three-state folding is not observed for all right-handed parallel β-helix proteins, the stable core may be important for secretion of AT passenger domains via the AT pathway. As additional AT passenger domains are subjected to biophysical analysis, it will be interesting to determine if the stable core is a universal feature, or restricted to some particular subset of ATs, as well as its exact role in the secretion mechanism.
MATERIALS AND METHODS
Pet Purification
Pet was expressed from plasmid pCEFN1. Typically, 1 L of LB media supplemented with 100 μg mL−1 ampicillin was inoculated with 20 mL of an overnight culture of E. coli HB101 transformed with pCEFN1, and grown for 18 h at 37°C. Cells were pelleted by centrifugation and the spent culture media was precipitated with 70% ammonium sulfate. The ammonium sulfate pellet was resuspended in 10 mL of 10 mM Tris pH 8.0 and concentrated using a centrifugal concentrator (Millipore) to a final volume of ∼2 mL, and treated with 2 mM PMSF to inhibit protease activity. Two hundred and fifty microliter of this solution was loaded onto a Superdex 200 HR 10/30 gel filtration column (GE Healthcare) equilibrated in 10 mM Tris pH 8.0, 300 mM NaCl. Fractions were eluted using the same buffer at a flow rate of 0.5 mL min−1, and analyzed by SDS-PAGE using 10% polyacrylamide gels. Fractions containing Pet were pooled, concentrated, and desalted using a centrifugal concentrator (Millipore). The concentration of purified Pet was determined using an extinction coefficient of 81,960 M−1 cm−1, calculated according to published procedures.26 PetΔprotease was purified using the same procedure, except a HiLoad 16/60 Superdex 75 gel filtration column (GE Healthcare) was used for the chromatographic step.
Construction of pCEFN1/Δprotease, Expressing PetΔprotease
Site-directed mutagenesis was used to introduce two NheI restriction sites in the Pet gene. The first NheI site was introduced after the signal sequence at Ala53, using the following oligonucleotides (mutations are underlined): 5′-AAT ATA ATA TAT GCC GCT AGC ATG GAT ATA TCT AAA GCA TGG GCC-3′ and 5′-GGC CCA TGC TTT AGA TAT ATC CAT GCT AGC GGC ATA TAT TAT ATT-3′. The second NheI site was introduced after the protease domain using the following oligonucleotides: 5′-GAT AAC AAT ACT GCT AGC ATT GGT GGT GGT AAG-3′ and 5′-CTT ACC ACC ACC AAT GCT AGC AGT ATT GTT ATC-3′. This double mutant construct was digested overnight with NheI (New England Biolabs), and the larger band was gel purified (Promega), and ligated back together using T4 DNA ligase (New England Biolabs). The final PetΔprotease construct contains the wild type signal sequence followed by an Ala-Ser linker and the remainder of the wild type Pet sequence, starting at Ile322.
Fluorescence Spectroscopy
A QM-6 fluorimeter (PTI, Birmingham, NJ) was used for all fluorescence measurements. Samples were measured at 25°C, with a Pet concentration of 0.25 μM in 10 mM Tris pH 8.0 in a 10 mm cuvette. All data were collected using an excitation wavelength of 280 nm, with an integration time of 1 s and a slit width of 1.25 mm.
Circular Dichroism Spectroscopy
Far-UV circular dichroism spectra were collected using an Aviv model 62DS spectropolarimeter (Lakewood, NJ). Protein samples (2 μM, in 10 mM phosphate pH 8.0) were measured at 25°C in a 2 mm cuvette. Data was collected from 200 to 250 nm with 1 nm steps, 1 s average time, and a 1 nm bandwidth.
Limited Proteolytic Digestion
Pet was digested with proteinase K (1:10,000 molar ratio, proteinase K:Pet) at room temperature in 10 mM Tris pH 8.0, 7.5 mM CaCl2, with or without 1.2M GdnHCl. Proteolysis was stopped by boiling samples for 10 min. Digestion fragments were resolved by SDS-PAGE using 16% polyacrylamide gels and silver stained.
Identification of the Pet Stable Core
MALDI-TOF mass spectral analysis was performed as described previously.1 Edman degradation was performed at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University.
Acknowledgements
pCEFN1 was a generous gift from James Nataro (U. Maryland Medical School). We thank Mirco Junker for many helpful discussions, William Boggess and Michelle Joyce for help with mass spectral experiments, Holly Goodson for helpful discussions on sequence alignments, and the members of the Clark laboratory for valuable input.




