Design, synthesis, conformational and membrane ion transport studies of proline-adamantane hybrid cyclic depsipeptides†‡
This article is a US Government work and, as such, is in the public domain in the United States of America.
In memory of Elkan Blout, a man and a scientist for all occasions. His presence and activities will be sorely missed.
Abstract
The design, synthesis, conformational, crystallographic, and ion transport studies of 30-membered, proline containing depsipeptides that incorporate the rigid low molecular weight lipophilic adamantane (Adm) building blocks are reported. The adamantyl groups provide the desired membrane permeability and conformational constraint for efficient transport in lipid membranes. The novel cyclic depsipeptides are: c[AdmC(O)Pro OCH2 CHRNHC(O)ProC(O) AdmC(O)ProC(O)NHCHRCH2 OProC(O)] where RH for A and RCONHAdm for B. Crystal structure analysis of A established that the two peptide segments are identical in formula and in conformation and that the peptides are bonded to the interleaving Adm at the 1 and 3 positions. However, the complete ring is highly asymmetric in shape since bonds for both Peptide-Adm-Peptide segments have the syn-anti motif. Torsional angles for the connecting bonds to Adm are −162°, +71° and −169°, −48°. The irregular clamshell shape of the molecule has three internal CO moieties directed in a manner that could provide three Na+O ligands. While A exhibited negligible transport of Na+ ions across membranes, peptide B endowed with two additional adamantanes in the periphery did transport Na+ ions from outside to inside. © 2007 Wiley Periodicals, Inc. Biopolymers 89: 471–478, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Design and synthesis of novel macrocyclic molecules capable of performing specific functions in biological systems continues to attract the attention of synthetic chemists. Conformationally constrained cyclopeptides are particularly attractive as designer targets in view of their demonstrated potential to act as regulators of membrane ion-transport, scaffolds in the de novo design of artificial proteins and rigid β-turn templates for the construction of simple mimics of biologically active peptides.1-5
Among the aminoacids, which are present in cyclopeptides, proline plays an important role. It is found frequently in naturally occurring biologically active peptides6-8 including peptide hormones such as oxytocin, angiotensin, peptide antibiotics such as gramicidin S and the peptide antitoxin antamanide. In addition, the importance of proline has been established as a conformational determinant in structural proteins such as collagen9 and as a frequent participant in the reversal of direction of peptide chains in globular proteins.10, 11 Peptide chains which contain proline have higher probability of cyclising than chains which contain other aminoacids.12
Transport of metal ions in biological systems is a function essential to life. One method of transporting a metal ion through a lipophilic system is to encapsulate the metal ion with a cyclic peptide molecule that has lipophilic side chains. For the metal ion-peptide complex to be active in a lipophilic membrane, the outer surface of the cyclic peptide needs to be lipophilic, hence the peptide residues should have nonpolar side chains that will extend outward and cover the complex.
The proline residue allows a cis-trans isomerisation of the peptide bond,13 which has been considered to be favorable for complex formation. Although a large number of proline containing cyclic peptides has been reported14-18 to form complexes with metal ions, the de novo design of membrane ion-transporting macrocyclic peptides is still scarce.19, 20
In addition to proline, ion-transporting peptides harbor amino acids having lipophilic side chains. Indeed the present design is based on antamanide,21, 22 a naturally occurring cyclic decapeptide having a 30 atom ring essentially consisting of Pro (4) Phe (4) Ala (1) Val (1). Antamanide forms complexes with a variety of metal ions. Our extensive studies have shown that adamantane (Adm), disubstituted at the 1, 3 positions has been used to constrain the conformation of several flexible molecules in order to limit the number of conformational possibilities.23 In the area of peptides, interest had developed to impose constraints by inserting adamantane into cyclic backbones that could create a shape conducive to selected ion transport by appropriate ion complexation.24, 25
RESULTS AND DISCUSSION
Design of Structures
In the present work the combination of proline residues to favor conformations that would complex with ions and adamantane that provides the hydrophobic surface was tested by synthesis of 30 membered macrocyclic peptides A and B. It may be noted that while peptide A contains four prolines and two adamantanes, peptide B is endowed with four adamantanes.
Suitable crystals for X-ray diffraction of peptide A were obtained by crystallization from a mixture of EtOAc and methanol. X-ray diffraction analysis of single crystals of A showed no internal symmetry despite the repetitive sequence in the two sides of molecule A (see diagram 1 for the numbering of atoms). The construction of the macrocycle consists of two depsipeptide segments that are almost identical in conformation. Each contains a pseudo-beta turn with a satisfactory NH…OC hydrogen bond having 10 atoms in the loop, as shown in Figure 1 and listed in Table I. These two linear segments are covalently bonded at each terminus to the 1 and 3 positions of two adamantyl groups, Figure 2. Each pair of attachments have the syn-anti motif with torsional angles N2C1'C1C35 = 162° and N25C26C27C35 = 71° at one adamantane and N7C8'C9C14 = 169° and N20C19C13C14 = −48° at the other adamantane. The only significant difference in torsional angles between the two halves of the molecule occurs at the attachments to the adamantane groups, Table II. The asymmetric conformation of the molecule results in a collapsed inner space, as seen in the stereodiagram, Figure 3. The syn-anti arrangement of the bisamide NHCO groups on either side of the 1,3-adamantyl spacers is a common occurrence, as for example in the complexes of dicarboxylic acids with 1,3-bis[(pyrid-2-ylamino)carbonyl]adamantane.23 1.
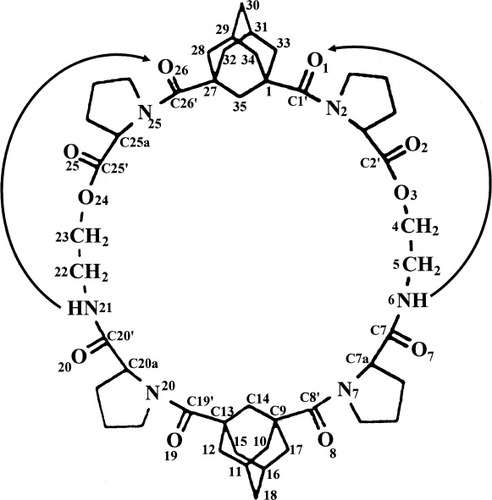
DIAGRAM 1
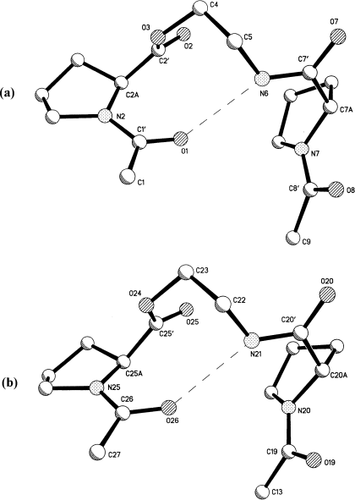
Comparison of conformation of the two linear depsipeptide chains in the molecule. Dashed lines indicate the NH…OC hydrogen bonds in a pseudo beta bend. (a) right chain; (b) left chain.
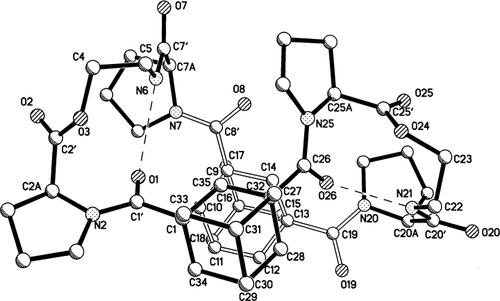
View of the complete macrocyclic molecule with two adamantane spacers and two depsipeptide chains. The rear part of the molecule is illustrated with the open bonds.
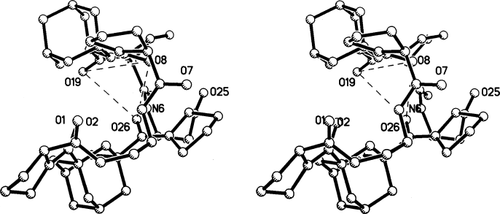
Stereodiagram viewed along the long dimension of the molecule that shows the clam-shell shape. Three closest O…O approaches, between O8, O19, and O26, are indicated by dotted lines.
| Donor | Acceptor | DA (Å) | DHaA (Å) | <DHA (°) |
|---|---|---|---|---|
| N6H | O1 | 2.926 | 2.11 | 150 |
| N21H | O26 | 2.824 | 2.05 | 143 |
| W1 | O20 | 2.855 | ||
| W1 | W2b | 2.936 | ||
| W2 | O25 | 2.826 |
- a Hydrogen atoms were placed in idealized positions with NH = 0.90 Å.
- b Acceptor at symmetry equivalent 2 − x, ½ + y, −z.
| Labela | Left Side Angle | Right Side Angle | ||
|---|---|---|---|---|
| ψ | C35C1C1'N2 | −161.7(4) | C35C27C26N25 | 70.9(5) |
| ω | C1C1'N2C2A | 169.9(4) | C27C26N25C25A | −171.8(4) |
| ϕ | C1'N2C2AC2' | −52.0(5) | C26N25C25AC25' | −59.4(6) |
| ψb | N2C2AC2'O3 | −28.4(6) | N25C25AC25'O24 | −19.9(6) |
| ωb | C2AC2'O3C4 | 170.7(4) | C25AC25'O24C23 | 165.7(4) |
| ϕb | C2'O3C4C5 | −108.4(5) | C25'O24C23C22 | −93.8(5) |
| ψbc | O3C4C5N6 | 73.5(6) | O24C23C22N21 | 68.3(6) |
| ϕ | C4C5N6C7' | 103.5(6) | C23C22N21C20' | 105.5(5) |
| ω | C5N6C7'C7A | −174.9(4) | C22N21C20'C20A | 178.8(4) |
| ψ | N6C7'C7AN7 | −13.0(7) | N21C20'C20AN20 | 4.6(6) |
| ϕ | C7'C7AN7C8' | −88.0(5) | C20'C20AN20C19 | −96.5(4) |
| ω | C7AN7C8'C9 | −164.9(4) | C20AN20C19C13 | −169.9(4) |
| ψ | N7C8'C9C14 | −168.8(4) | N20C19C13C14 | −47.7(5) |
- a Label for torsional angles in true peptides.26
- b Depsipeptide residue.
- c Reversal of backbone direction; retro peptide segment.
The front portion of the cyclic molecule is displayed in Figure 2 with bold bonds while the rear portion that attaches directly to the front portion is shown with open bonds. The pair of pseudo beta-like turns resemble Type I turns, Table I. In Type I, Φ and ψ angles have values near −60°, −30°; −90°, 0° compared to the angles in the left side of A (−52°, −28°; −108°,+73°) and the angles in the right side of A (−59°, −20°; −94°, +68°). Differences between type I hydrogen bonds and those in molecule A occur only in the final ψ values. They are caused by chain reversals and methylene insertion at C5 and C22. A curious feature is the close approaches of N6-N7 at 2.83 Å and N20-N21 at 2.77 Å, but the N6H-N distance is 2.36 Å and the N21H-N20 distance is 2.43 Å, values longer than those that occur for N6H-O1 (2.11 Å) and N21H-O26 (2.05 Å) in the hydrogen bond, Table I. Pucker in the proline rings is quite normal, as shown by the torsion angles in Table III.
| i = 2 | i = 7 | i = 20 | i = 25 | |
|---|---|---|---|---|
| NiCiACiBCiG | −30 | +29 | +37 | −26 |
| CiACiBCiGCiD | +41 | −32 | −37 | +38 |
| CiBCiGCiDNi | −36 | +22 | +23 | −35 |
| CiGCiDNiCiA | +18 | −3 | 0 | +19 |
| CiDNiCiACiB | +7 | −16 | −23 | +5 |
- a E.s.d.'s 0.4° to 0.6°.
An additional view of the macrocycle is displayed in Figure 4 that shows the length of the cavity in the “half-shell” construction. Hydrogen bonds N6H…O1 and N21H…O26 are indicated by dashes. The closest interatomic approaches across the cavity are O8-O26 at 4.01 Å, O19-O28 at 4.05 Å and O8-O19 at 6.11 Å, indicated by dotted lines. Atoms O8, O19 and O26 form a triangle where distances O8-O19, O19-O26, and O26-O8 are compatible with three of six distances in a face of an octahedron that could surround a Na+ ion. The geometric arrangement of a single molecule of A suggests that a 1:2 complex with a Na+ ion could be formed with two peptide molecules enclosing a Na+ ion by providing six O atoms from six CO moieties to form six Na+O ligands in an approximately octahedral fashion. Examples of 1:2 sandwich complexes do exist for other metal ion: 2(cyclic peptides) systems, such as Mg++: 2[c-(GlyProPro)2].27
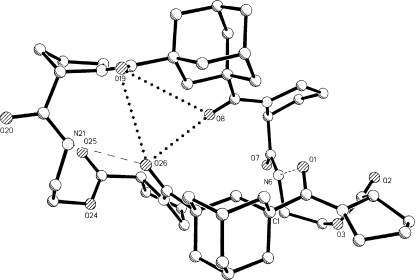
Figure 3 rotated by 90° around the vertical direction.
A serious drawback to the suggested model of Na+ complex is the nature of the preformed peptide geometry in the uncomplexed peptide A. Several of the carbonyl oxygens, namely O2, O7, O20, and O25 protrude out of the outer surface of the folded peptide and break the hydrophobic covering. A packing diagram, viewed down the a axis in Figure 5, shows a single layer of molecules that extends over four cells. The molecules are related by vertical twofold screw axes between columns. The carbonyl oxygens O7, O20, and O25 and their equivalents in neighboring molecules can be seen clearly on the surface of the folded molecules. Moreover, two of the carbonyls make hydrogen bonds with cocrystallized water molecules: O20W1W2O25 (Table I). Such a condition is not conducive for transport through lipophilic membranes. A model for complexation would involve a significant conformational change in the peptide. Large conformational changes upon complexation have been documented in a number of cyclic systems, including antamanide (extracted from the poisonous Amanita phalliodes mushroom)21, 22 and valinomycin.28 In all the examples contained in Ref.28, only one CO group on the uncomplexed peptides extends to the outside of the cyclic backbone and appears to be the contact point for attracting a M+ and drawing it into the interior of the molecule. In the present molecule, there are four CO groups that would have to be folded into the interior. The lack of a crystal of a complex between Na+ and peptide A prevents a more definitive resolution of the structure of the complex.
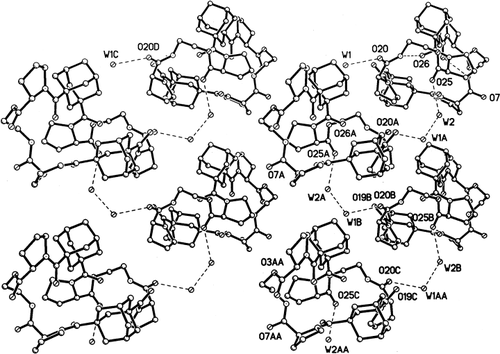
Packing of peptide A. Cocrystallized water molecules W1 and W2 join the peptide molecules to form a continuous spiral around the screw-axis (vertical axis b) by groups of three hydrogen bonds such as O25W2W1AO20A.
The suggested mechanism for Na+ ion transport finds support in the ion transport studies carried out with peptide B that has two additional adamantyl units in the periphery. Valinomycin is added to KCl loaded palmitoyl oleoyl phosphatidyl choline (POPC) vesicles suspended in NaCl solution, that facilitate the transport of K+ ions from inside the vesicles, which results in decrease in fluorescence. Addition of the 30-membered cyclic depsipeptide B, with two adamantane units placed symmetrically on the exterior, increases the fluorescence, indicating the transport of Na+ ions from outside to inside vesicles (Figure 6); the other cyclic peptides have showed negligible transport of Na+ ions. It is clearly evident from these results that enhanced hydrophobicity in the cyclic peptide design promotes ion transport.
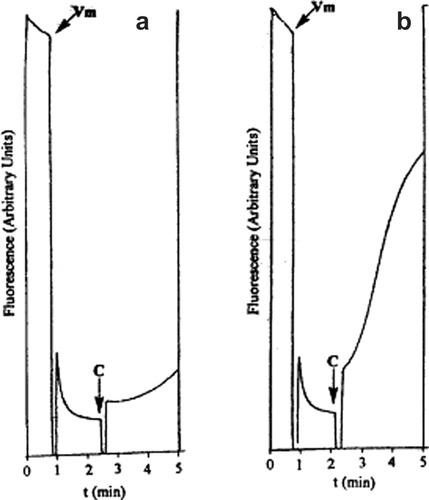
Effect of the addition of cyclic depsipeptide B on the diffusion potential set up by addition of valinomycin (lipid: Vm ratio 1000: 1) as monitored by the fluorescence of cyanine dye. Vm and C respectively denotes the points at which valinomycin and peptide B was added. The concentrations of peptide B in (a) 4 μM: (b) 8 μM. The spikes seen on profile at each point (Vm and C) results from the opening of the chamber door of the fluorescence spectrophotometer.
Ion-transport across membranes can take place either by carriers or by channel formers. The two mechanisms can be easily distinguished by monitoring the release of carboxyflorescein (CF) trapped in lipid vesicles by fluorescence spectroscopy. Since a channel former leads to the formation of large pores in membranes, the trapped CF leaks out of the vesicles into the bulk medium, that causes a sudden enhancement of its fluorescent intensity due to dilution.29 Carrier molecules, on the other hand, diffuse through the hydrocarbon region of the membrane and do not cause the escape of CF into the medium, and hence no change in fluorescence is seen.
In the present case, the macrocycle B did not cause the release of entrapped carboxyflorescein indicating that the transport of ions across the lipid bilayer was not due to the formation of large pore or detergent-like action. The ion flux may thus be ascribed to a carrier type of ion-transport mechanism like that of valinomycin.30, 31
CONCLUSIONS
The dominant features of peptide A are the constraints provided by the presence of two adamantane moieties in the peptide ring, the pseudo-beta-bends containing NH…OC hydrogen bonds, the attachment of two chemically identical segments with very similar conformations but with different conformational angles to the adamantanes and the resulting irregular and collapsed macrocycle. The surface of uncomplexed peptide A lacks the appropriate hydrophobicity for ion transport through lipid membranes.
MATERIALS AND METHODS
Synthesis
Cyclic peptide-A
The condensation of Boc-pro-OH with ethanolamine followed by esterification with Z-pro-OH afforded the linker 3 (Scheme 1). Attachment of two units of 3 was accomplished by boc-deprotection followed by reaction with 1,3-adamantane dicarbonyl dichloride to provide the open system which on Z-deprotection followed by linking of second unit of 1,3-adamantane dicarbonyl dichloride gave peptide-A.
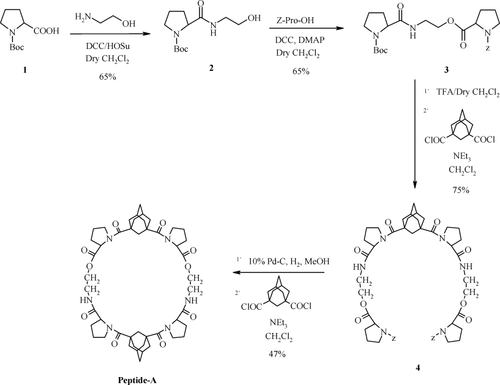
Synthesis of peptide-A.
Selected experimental data: Peptide A: M.p.: 245–248°C, [α]D25 = −25.18 (c, 0.25, CHCl3), IR (KBr): 3491, 3353, 2928, 1740, 1676, 1603, 1528, 1440, 1384, 1168 cm−1, 1H NMR (500 MHz, DMSO-d6): δ 1.59–2.11 (m, 44H, Adm H + Pro CγH2 + Pro CβH2), 3.18 (m, 2H, NH CH2), 3.25 (m, 2H, NH CH2), 3.71 (m, 8H, Pro CδH2), 3.94 (m, 4H, COOCH2), 4.25 (brs, 4H, Pro CαH), 7.62 (br, 2H, Amide NH); FAB MS m/z (%): 887 (M + H+) (100), 909 (M + Na)+ (34).
Cyclic peptide-B
Condensation of Z-ser-OH with 2-adamantyl amine afforded 5 which on coupling with Boc-Pro-OH followed by Z-deprotection and condensation with Z-Pro-OH afforded linker 7 (Scheme 2). This was attached to 1,3-adamantane dicarbonyl dichloride after Boc-deprotection to give 8 which after Z-deprotection was linked to another unit of 1,3-adamantane dicarbonyl dichloride to give peptide-B.
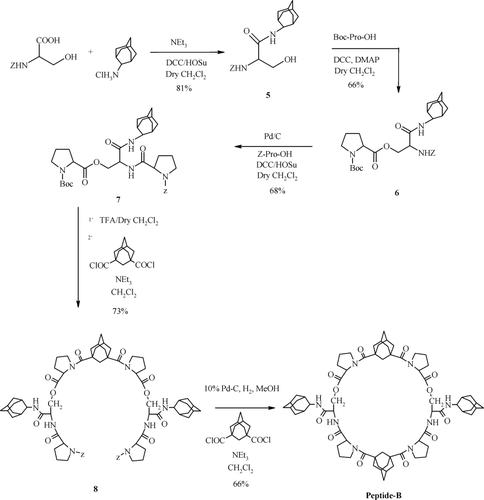
Synthesis of peptide-B.
Peptide B: M.p.: 234–236°C, [α]D25= −45.96 (c, 0.25, CHCl3), IR (KBr): 3433, 3336, 2900, 1736, 1657, 1600, 1529, 1440, 1382, 1168 cm−1. 1H NMR (500 MHz, DMSO-d6): δ 1.46 (m, 4H, Adm H), 1.59–2.10 (m, 70H, Adm H + Pro CγH2 + Pro CβH2), 3.73 (m, 6H, Pro CδH2), 3.82 (m, 2H, Pro CδH2), 4.00 (m, 2H, Pro CαH), 4.21 (m, 4H, Ser CβH2), 4.36 (brs, 2H, Pro CαH), 4.48 (m, 2H, Ser CαH), 7.73 (brs, 2H, Adm NH), 7.83 (brs, 2H, Ser NH). ES MS m/z (%): 1243 (M + H+) (4), 1265 (M + Na)+ (23).
The structural assignments for all the compounds presented in Scheme 1 and Scheme 2 are fully supported by spectral and analytical data.
Ion transport studies
The membrane ion-transport capability of cyclic peptides was measured by using the cyanine dye method.32-38 Valinomycin was used to create a diffusion potential. The lipophilic fluorescent cyanine dye is very sensitive to potential changes across the membrane and changes in fluorescence are observed according to change in membrane potential due to ion flux. The hyperpolarization (inside of the vesicle becomes negative) results in uptake of dye molecules by the vesicles that results in decrease of fluorescence. Depolarization (inside of the vesicle becomes positive) results in the release of dye molecules which results in increase of fluorescence.
X-Ray Diffraction
Crystals of A were obtained by slow evaporation from MeOH solution. X-ray data were collected at room temperature with CuKα radiation (λ = 1.54178 Å) on a Bruker P4 four-circle diffractometer with a θ/2θ scan mode, a scan-width of 2.0° +2θ(α1–α2). 15°/min scan speed and 2θmax = 125° (resolution 0.87 Å). A total of 6514 independent data were measured of which 5447 reflections were observed with intensities >2σ(I). Three check reflections remained constant during the data collection. The crystal was wedge-shaped with dimensions 0.60 × 0.40 × (0.23–0.05) mm.
The structure was solved by direct phase determination and refined by full-matrix least-squares on F2 values. The crystal data are: C48H66N6O10.2H2O, formula weight 919:07, space group P21, a = 11.208(2) Å, b = 11.625(2) Å, c = 18.259(4) Å, β = 92.77(2)°, V = 2376.2(8) Å3, Z = 2, dcalc = 1.285 mg/mm3. The final least-squares with anisotropic thermal parameters and 66 H atoms placed in idealized positions and riding on the C or N atoms to which they are bonded resulted in R1 = 0.0592 for I > 2σ(I), R1 = 0.0740 for all the data and wR2 = 0.1711 for all the data. The programs used were those in the SHELXTL package.39 Tables of coordinates, bond lengths and angles, anisotropic thermal factors, and hydrogen coordinates are deposited with the Cambridge Crystallographic Data Center, CCDC#659904. Copies of the data can be obtained free of charge on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ (Fax: +44 1223 336 033; e-mail: [email protected]). The chemical connectivity and numbering scheme are shown in diagram 1.
Acknowledgements
The authors are extremely grateful to Prof. S. Ranganathan for making available the notes of Darshan Ranganathan (his wife) after her passing, arranging her plans and ideas for this project and the synthesis of the cyclic peptide. We are most grateful to Mr. Y B.R.D. Rajesh for his valuable help in the preparation of the manuscript.




