New tools for the control of peptide conformation and supramolecular chemistry: Crown-carrier, Cα-methyl L-DOPA amino acids†
This article is dedicated to the memory of Dr. Arno F. Spatola, a dear friend and a pioneer in the field of backbone-modified peptides.
Abstract
The preferred conformation of five, terminally protected, model peptide series to the hexamer level, based on three novel crowned, Cα-methyl L-DOPA amino acids combined with either L-Ala/Aib or Gly/Aib, were assessed in structure supporting solvents using FT-IR absorption, 1H NMR, and CD techniques. The FT-IR absorption spectra strongly suggest that the contribution of the crowned Cα-tetrasubstituted residue to intramolecular H-bonding is equivalent to that of Aib and is much more significant than that of either L-Ala or Gly. In addition, the 1H NMR titrations and the CD patterns resemble those typically exhibited by (right-handed) 310-helical structures. © 2004 Wiley Periodicals, Inc. Biopolymers (Pept Sci), 2003
INTRODUCTION
In addition to the limited number of coded α-amino acids, a much more significant number of naturally occurring nonproteinogenic α-amino acids has been isolated and characterized. In spite of this abundant natural pool, the design and synthesis of artificial, enantiomerically pure, α-amino acids have recently emerged as a highly rewarding effort.1
Peptides are ideally suited to serve as spacers and templates in studies of probe–probe interactions and supramolecular chemistry because of the commercial availability and chirality of their amino acid building blocks, the potential control of their secondary structure, and the relative ease of their synthesis and purification/characterization procedures.2, 3 In this connection, synthetic, Cα-trisubstituted α-amino acids bearing crown ether side-chain moieties have been shown to be of relevant interest for the preparation of ion-selective molecular receptors and artificial ion channels.2, 4-12 Application of this concept to Cα-tetrasubstituted α-amino acids13-17 should result in more defined and predictable secondary structures, as these residues are known to be able to explore only a very limited amount of the ϕ, ψ conformational space available to Cα-trisubstituted α-amino acids.18-22 To date, only few and scattered data on conformation of peptides based on crown-carrier, Cα-tetrasubstituted, α-amino acids characterized by Cα↔Cα cyclization (side-chain derivatives of 1-aminocycloalkane-1-carboxylic acids) have been investigated.
In this article, we describe the infrared (IR) absorption, 1H nuclear magnetic resonance (NMR) and circular dichroism (CD) results of a conformational analysis in solution of five, terminally protected, peptide series to the hexamer level characterized by the novel family of crowned, Cα-methylated, α-amino acids. Following a route established by Voyer and coworkers,2 and by taking advantage of the catechol functionalities of the commercially available, optically pure, Cα-methyl L-DOPA (Mdp), some of us have recently prepared the three chiral, crown-carrier, Cα-tetrasubstituted α-amino acids shown in Figure 1.13 They differ by the size of the cyclic ether moiety and its hydrophilicity/hydrophobicity ratio. The amino acid derivatives and peptides examined in this study are listed in Figure 2. A preliminary report of part of this work has recently been submitted.23
Chemical structures of the three crown-carrier, Cα-methyl L-DOPA residues discussed in this work.

List of amino acid derivatives and peptides, based on the crown-carrier, Cα-methyl L-DOPA residues shown in Figure 1, discussed in this work.
MATERIALS AND METHODS
Synthesis of Amino Acids, Derivatives, and Peptides
The synthesis and characterization of three crown-carrier, Cα-methyl L-DOPA amino acids, their Boc and Boc/OMe derivatives, and selected di- and tripeptides have already been reported,13 while those of the other di- and tripeptides, and of all tetra-, penta- and hexapeptides discussed in this work will be reported in due course.24
IR Absorption
The Fourier transformed infrared (FT-IR) absorption spectra were recorded on a nitrogen-flushed Perkin-Elmer 1720X FT-IR spectrophotometer equipped with a sample-shuttle device at 2 cm−1 nominal resolution and an average of 100 scans. The solvent (baseline) spectra were obtained under the same conditions. Cells with path lengths of 0.1, 1.0, and 10 mm (with CaF2 windows) were used. Spectrograde deuterochloroform (99.8% D) was purchased from Fluka (Buchs, Switzerland).
Circular Dichroism
The CD spectra were obtained on a Jasco (Tokyo) J-715 spectropolarimeter. Cylindrical fused quartz cells (Hellma, Müllheim, Germany) of 1.0-mm path length were used. The values are expressed in terms of [θ]T, the total molar ellipticity (deg cm2 dmol−1). Spectrograde MeOH (Acros Organics, Geel, Belgium) was used as solvent.
NMR Spectrometry
The 1H NMR spectra were recorded with a Bruker model AM 400 (Karlsruhe, Germany) spectrometer. Measurements were carried out in deuterochloroform (99.96% D, Merck) and deuterated dimethylsulfoxide (DMSO, 99.96% D6; Acros Organics) with tetramethylsilane as the internal standard. The free radical 2,2,6,6-tetramethylpiperidin-1-yloxyl (TEMPO) was purchased from Sigma (St. Louis, MO).
RESULTS AND DISCUSSION
The preferred conformations adopted by the five N- and C-protected, crown L-Mdp-based peptide series were determined in secondary structure supporting solvents by FT-IR absorption, 1H NMR, and CD techniques as a function of concentration (over the range 1 × 10−2 to 1 × 10−4 M).
IR Absorption
Figure 3 shows the FT-IR absorption spectra (NH stretching region) of a representative series from the dipeptide to the hexapeptide in CDCl3 solution at 1 × 10−3 M concentration, while Figure 4 illustrates the spectra of the longest oligomers (hexapeptides) for each of the five series under the same experimental conditions. All the curves of the tri-, tetra-, penta-, and hexapeptides are characterized by two bands at about 3425 cm−1 (free, solvated NH groups) and in the 3370–3325 cm−1 spectral range (strongly H-bonded NH groups), respectively.25-27 The intensity of the low-frequency band relative to that of the high-frequency band increases as main-chain length increases. Concomitantly, the absorption maximum shifts markedly to lower wavenumbers. These combined effects are particularly evident when a crowned Mdp residue is incorporated in the sequence (e.g., from peptide 4c to 5c). From a detailed analysis of the inverted second derivative spectrum of peptide 6c (not shown), it is clear that a third band, located at 3398 cm−1 and assigned to the weakly H-bonded NH groups of the fully extended (C5) peptide conformation,28, 29 contributes to the absorption of this hexapeptide in this spectral region.
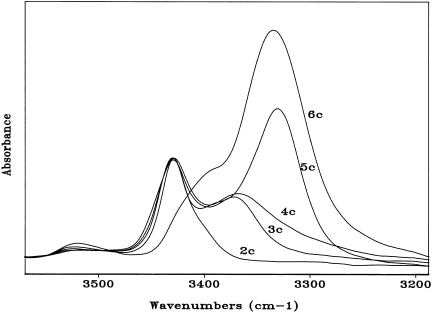
FT-IR absorption spectra in the 3550–3200 cm−1 region of Boc-(18-C-6)-L-Mdp-L-Ala-OMe (2c), Fmoc-L-Ala-(18-C-6)-L-Mdp-L-Ala-OMe (3c), Fmoc-L-Ala-L-Ala-(18-C-6)-L-Mdp-L-Ala-OMe (4c), Boc-(18-C-6)-L-Mdp-L-Ala-L-Ala-(18-C-6)-L-Mdp-L-Ala-OMe (5c), and Fmoc-[L-Ala-(18-C-6)-L-Mdp-L-Ala]2-OMe (6c) in CDCl3 solution (conc. 1 × 10−3 M).
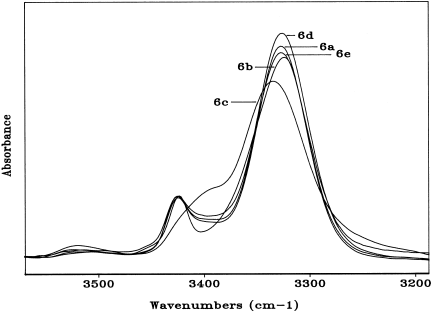
FT-IR absorption spectra in the 3550–3200 cm−1 region Boc-[Aib-(15-C-5)-L-Mdp-L-Ala]2-OMe (6a), Boc-[Aib-(15-C-5)-L-Mdp-Gly]2-OMe (6b), Fmoc-[L-Ala-(18-C-6)-L-Mdp-L-Ala]2-OMe (6c), Boc-[Aib-(18-C-6)-L-Mdp-L-Ala]2-OMe (6d), and Boc-[Aib-(benzo-24-C-8)-L-Mdp-L-Ala]2-OMe (6e) in CDCl3 solution (conc. 1 × 10−3 M).
The curves of the hexapeptides 6a, 6b, 6d, and 6e are all similar, in terms of both band positions and relative intensities. Interestingly, the only hexapeptide (6c) where a significant population of the fully extended conformation has been observed is also the only one with an N-terminal residue (preceding the first crowned Mdp in the sequence), L-Ala, much less helicogenic than Aib, which characterizes the N-terminus of the other four hexapeptides. We have also been able to demonstrate that in the concentration range examined (1 × 10−2–1 × 10−4 M) only marginal changes in the spectra of the various peptides take place (results not shown). Therefore, the observed band at 3370–3325 cm−1 should be interpreted as arising almost exclusively from intramolecular CO … HN H-bonding interactions. The present FT-IR absorption analysis has provided convincing evidence that main-chain length-dependent intramolecular H-bonding is a factor of paramount importance influencing the conformation of the terminally protected peptides based on the crown-carrier Mdp residue.
Our results also support the view that this Cα-tetrasubstituted α-amino acid, like Aib, is a stronger inducer of intramolecularly H-bonded, folded peptide structures than the Cα-trisubstituted protein amino acids L-Ala and Gly. The observation of the 3370–3325 cm−1 band in the highest oligomers, which is absent in the amino acid derivatives and dipeptide esters, seems to indicate that the crown-carrier Mdp-based peptides do not tend to fold into a γ-turn conformation,28, 30 and highlights the propensity of the tripeptide esters to adopt a β-turn conformation,28, 31, 32 which may evolve in a series of consecutive β-turns (310-helices33, 34) in the longer oligomers.
Nuclear Magnetic Resonance
To obtain more detailed information on the conformational preferences of the terminally protected, crowned Mdp peptides in CDCl3 solution, we carried out a 400-MHz 1H NMR investigation. The delineation of inaccessible (or intramolecularly H-bonded) NH groups was performed using: (a) solvent dependence of NH proton chemical shifts, by adding increasing amounts of the strong H-bonding acceptor solvent DMSO35, 36 to the CDCl3 solution, and (b) free radical (TEMPO)-induced line broadening of NH proton resonances.37 As typical examples, Figures 5 and 6 illustrate the behavior of the NH proton resonances of the two hexapeptide esters 6a and 6d upon addition of DMSO and TEMPO.
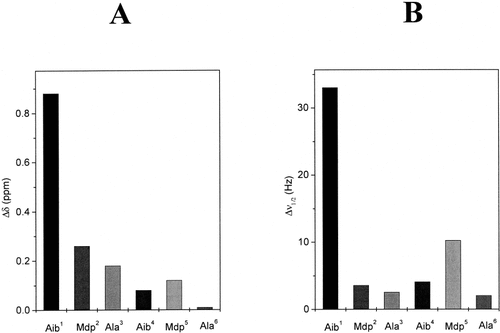
(A) Histogram of the variations of NH proton chemical shifts in the 1H NMR spectra of Boc-[Aib-(15-C-5)-L-Mdp-L-Ala]2-OMe (6a) upon addition of 7% DMSO (v/v) to the CDCl3 solution. (B) Histogram of the variations of bandwidths of the NH proton signals in the 1H NMR spectra of the same peptide upon addition of 0.2% TEMPO (w/v) to the CDCl3 solution (conc. 1 × 10−3 M).
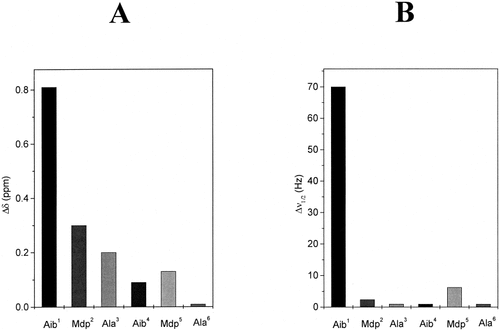
(A) Histogram of the variations of NH proton chemical shifts in the 1H NMR spectra of Boc-[Aib-(18-C-6)-L-Mdp-L-Ala]2-OMe (6d) upon addition of 7% DMSO (v/v) to the CDCl3 solution. (B) Histogram of the variations of bandwidths of the NH proton signals in the 1H NMR spectra of the same peptide upon addition of 0.2% TEMPO (w/v) to the CDCl3 solution (conc. 1 × 10−3 M).
The upfield resonance in CDCl3 solution is unequivocally attributed to the urethane N(1)H proton.26 All other proton resonances were assigned by virtue of their peak multiplicities and ROESY experiments. From an analysis of the spectra as a function of peptide concentration (from 1 × 10−2–1 × 10−3 M) in CDCl3 solution (not shown), it could be concluded that upon dilution only for the N(1)H proton resonance, a significant shift (0.12 ppm) takes place to higher fields, with the position of all other NH proton resonances remaining either unchanged or very modestly changed.
In the two crown-carrier Mdp hexapeptides studied in the CDCl3–DMSO solvent mixtures and in the presence of the paramagnetic perturbing agent TEMPO, two classes of NH protons were observed. Class (i) [N(1)H proton] includes a proton whose chemical shifts are markedly sensitive to the addition of DMSO and whose resonances broaden upon addition of TEMPO. Class (ii) [N(2)H to N(6)H protons] includes those that display a behavior more or less characteristic of shielded protons (limited sensitivity of chemical shifts to solvent composition and of line widths to the presence of TEMPO).
In summary, the 1H NMR results described in this study allow us to conclude that in CDCl3 solution at a concentration higher than 1 × 10−3 M, the longest, terminally protected, crowned Mdp peptides have some propensity to self-aggregate and that in this process the urethane N(1)H group plays the major role as an intermolecular H-bonding donor. At lower concentrations, the N(2)H to N(6)H protons of the tri-, tetra-, penta-, and hexapeptides are almost inaccessible. It is our view that the anomalously low sensitivity of the crowned L-Mdp N(2)H proton to perturbing agents should not necessarily be related to its involvement in intramolecularly H-bonding, but rather to a general inaccessibility due to the bulkiness and chemical characteristics of the amino acid side chain. Support for this contention is given by our observation (on peptides 2c and 5c) that even when the crowned L-Mdp residue is N-terminal, i.e., in a position where it cannot be involved in an intramolecularly H-bond of any folded conformation, the sensitivity to DMSO or TEMPO of its N(1)H proton is three to four times lower than that usually found for N(1)H protons (results not shown). On the basis of these findings and by analogy with the conformational tendency of other Cα-methylated α-amino acids18-22 it is reasonable to conclude that the most populated conformers adopted in CDCl3 solution by the terminally protected, crowned L-Mdp tri-, tetra-, and longer peptides are the β-turn, two consecutive β-turns (incipient 310-helix), and the 310-helix, respectively. These conclusions agree well with those extracted from the FT-IR absorption investigation discussed above.
Circular Dichroism
The CD spectra of the crowned L-Mdp hexapeptides 6a, 6b, 6d, and 6e in methanol solution in the region of absorption of the peptide chromophore are shown in Figure 7. We have not reported the dichroic curve of hexapeptide 6c, as it might be heavily influenced by contributions of electronic transitions of the fluorene chromophore of the Fmoc Nα-protecting moiety.38 Interestingly, the only source of chirality for hexapeptide 6b has to be found in the α-carbon atoms of the two crowned L-Mdp residue.
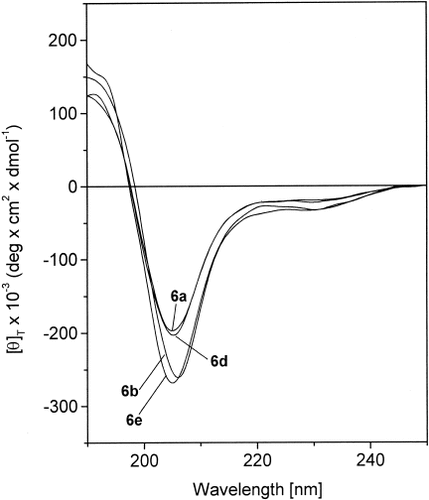
CD spectra of Boc-[Aib-(15-C-5)-L-Mdp-L-Ala]2-OMe (6a), Boc-[Aib-(15-C-5)-L-Mdp-Gly]2-OMe (6b), Boc-[Aib-(18-C-6)-L-Mdp-L-Ala]2-OMe (6d), and Boc-[Aib-(benzo-24-C-8)-L-Mdp-L-Ala]2-OMe (6e) in MeOH solution (conc. 1 × 10−3 M).
For all four hexapeptides, the CD pattern is characterized by a weak negative Cotton effect at 220–230 nm, followed by a very intense negative Cotton effect centered near 205 nm. The dichroism becomes positive below 198 nm. The ratio R = [θ]222/[θ]207 is in the range 0.2–0.3. Taken together, these CD properties resembles those typically exhibited by a right-handed 310-helical peptide structure.39-41 However, this conformational conclusion is not unambiguous, in view of the presence of one (or two) substituted phenyl moiety(ies) in the side chain of each crowned Mdp residue whose transitions are expected to overlap those of the peptide chromophores.42 Support for this cautionary note is also given by the abnormally high ellipticity values observed in the spectral range examined. It is evident that this tentative proposition should be confirmed by other chirospectroscopic techniques (e.g., vibrational CD) that are insensitive to the presence of aromatic chromophores in the peptide molecules.43
CONCLUSIONS
Overall, the FT-IR absorption, 1H NMR, and CD data collected in this work for peptides based on three different, crowned, Mdp residues are consistent with the contention that all these Cα-tetrasubstituted α-amino acids have a remarkable bias for β-turn and 310-helix structure formation, much more significant than that exhibited by protein (Ala and Gly) residues. This conclusion is in accordance with the known 3D-structural propensities of Cα-methylated α-amino acids.18-22
In addition, our preliminary CD results appear to justify the proposition that the preferred helical screw sense of these peptides (even of those lacking any other chiral residues, e.g., 6b) is right-handed. However, this conclusion should be corroborated by data from ancillary spectroscopies (e.g., VCD) in view of the observation that it is in contrast to the conformational preferences so far reported for other Cα-methylated α-amino acids with a Cγ-branched side chain.21, 22 In any case, neither the size of the cyclic ether moiety nor its hydrophilicity/hydrophobicity ratio seems to play any role in governing peptide conformation.
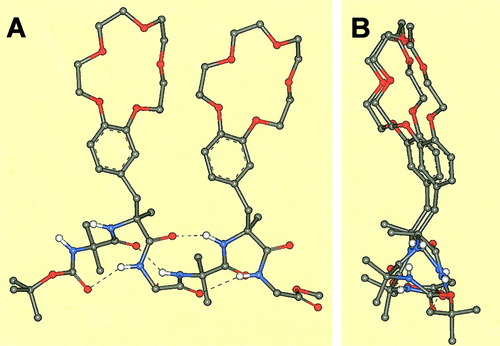
In summary, the crown-carrier Mdp residues discussed here add the remarkable property of a strong propensity for folded/helical structures to the interesting, crown-carrier, L-DOPA residues introduced by Voyer and coworkers.2, 4-12 This tendency, in turn, will allow formation of stable peptide helices as short as seven to eight residues, in contrast to helices based on Cα-trisubstituted α-amino acids only, which are known to require much longer sequences for a significant stabilization. It is worth pointing out that positioning of two crowned L-Mdp residues at i and i + 3 positions of the main chain of the ternary 310-helix, as in our penta- and hexapeptides, should allow a parallel orientation of their side-chain, crown-ether, receptors with opportunity for cooperative binding. Figure 8 presents two orthogonal views of a molecular model of hexapeptide 6b.
Computer model (WebLab ViewerPro 3.7) of the right-handed 310-helical structure of hexapeptide Boc-[Aib-(15-C-5)-L-Mdp-Gly]2OMe (6b): (A) side view, (B) top view. The χ1 and χ2 torsion angles for both crowned residues were taken as −60° and −90°, respectively, as these values represent the most probable side-chain conformation for a Phe residue in peptides.44 For clarity, of all hydrogen atoms, only those linked to a nitrogen are shown.




