Endocrine controls of keratin expression
Abstract
Keratins are a family of intermediate filaments that serve various crucial roles in skin physiology. For mammalian skin to function properly, and to produce epidermal and hair keratins that are optimally adapted for their environment, it is critical that keratin gene and protein expression are stringently controlled. Given that the skin is not only targeted by multiple hormones, but also constitutes a veritable peripheral endocrine organ, it is not surprizing that intracutaneous keratin expression is underlined by tight endocrine controls. These controls encompass thyroid hormones, steroid hormones such as glucocorticoids (GCs), retinoic acid (RA) and vitamin D, and several neuroendocrine mediators. Here, we review why a better understanding of the endocrine controls of keratin expression is not only required for an improved insight into normal human skin and hair function, but may also open new therapeutic avenues in a wide range of skin and hair diseases.
Abbreviations:
ACTH, adrenocorticotropic hormone; α-MSH, alpha-melanocyte-stimulating hormone; D3, 1,25(OH)2D3; EBS, epidermolysis bullosa simplex; GC, glucocorticoid; HF, hair follicle; GR, glucocorticoid receptor; KAP, keratin-associated protein; KRE, keratin-response element; ORS, outer root sheath; POMC, propiomelanocortin; PRL, prolactin; PTHrP, parathyroid hormone-related protein; RA, retinoic acid; T3, triiodothyronine; TH, thyroid hormone; TR, thyroid receptor; TRE, thyroid responsive element; VDR, vitamin D receptor; VDRE, vitamin D responsive element.
Introduction
Human skin represents a target tissue that is exposed to and depends on the regulation of complex chemical messengers, including a variety of different hormones.1, 2 The importance of hormonal regulation in the skin is illustrated by the existence of numerous endocrine abnormalities that are associated with disorders of the epidermal barrier, skin pigmentation, and the growth, color, shaft structure, and development of hairs. Thyroid disorders, for example, lead to changes in hair quality and growth, androgen excess causes sebaceous hyperplasia and alopecia, and adrenocorticotropic hormone (ACTH) overproduction leads to hyperpigmentation and hypertrichosis.3-5 In addition, in monilethrix, a keratin-related hair disorder characterized by beading and fragility of the hair shaft, there is a substantial, although temporary, improvement in hair growth during pregnancy (Fig. 1).6 Keratinization, i.e., the organized process of terminal differentiation of epidermal keratinocytes from stratum basale to stratum corneum, is greatly influenced by hormones.7
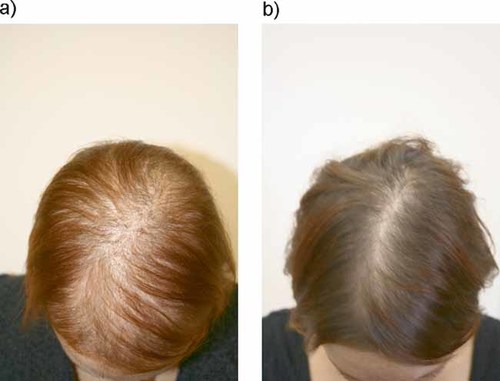
Hair growth improvement in a monilethrix patient during pregnancy. a: Non-pregnant state. b: Same patient at 32nd week of gestation.
In this context, it is important to recognize that the skin is not only a prominent hormone target, but is also a veritable endocrine organ by itself, which serves as a potent source of synthesis and metabolism of numerous endocrine signals that exert major impact on skin physiology and pathology.2, 8 For example, in vitro and in vivo, various human skin cell populations can synthesize and metabolize steroid hormones like vitamin D, androgens, estrogens, and even cortisol from cholesterol.1 In addition, they can produce peptide hormones like prolactin (PRL), growth hormone, corticotropin-releasing hormone, and propiomelanocortin (POMC) derivatives such as ACTH and alpha-melanocyte-stimulating hormone (α-MSH), catecholamines, acetylcholine, and glycoprotein hormones like erythropoietin.1, 9-11 Cells in the hair follicle (HF) epithelial stem cell compartment, called the HF bulge, locally synthesize eicosanoids from fatty acids, controlling, for example, injury-induced HF epithelial cell proliferation.1, 10, 11 Peripheral equivalents of the hypothalamic-pituitary-adrenal axis and complex pathways for steroidogenesis and steroid metabolism all exist in the skin and thus enable it to function as an autonomous endocrine organ.2, 12
It is on this background that the endocrine controls of keratins are to be examined. Keratins are a family of intermediate filaments that serve crucial roles in normal skin physiology.13 They comprise about 30% of the protein of the epidermis, and more than 90% of the hair shaft protein. Therefore, as expected, the proper function of mammalian skin and its appendages critically depend on stringent controls of keratin gene and protein expression so as to guarantee the generation of environmentally optimally adapted epidermal and hair keratins. Here, we review these—as yet incompletely characterized—hormonal controls, and explain why they deserve scrutiny not only in the context of normal skin biology, but also for the development of novel therapeutic strategies in a wide range of skin and hair diseases.
The keratin family
The family of keratin genes contains 54 functional genes that have a very distinct mode of expression in different epithelial layers and in different epithelial organs, representing the physiological and pathological states of the epithelial cells and the epidermis.14 The keratins assemble into intermediate filaments located in the cytosol, between the nucleus envelope and the cell surface membrane, and serve as the foundations that allow epithelial cells to sustain mechanical and non-mechanical stresses.15
The recent consensus nomenclature for mammalian keratins14 divides the keratin family into three categories: epithelial keratins, HF-specific epithelial keratins, and hair keratins (Fig. 2). Each of these categories is further divided into type I and type II keratins. Several epithelial keratins are constitutively expressed in the HF. For example, K17 is highly expressed in the suprabasal cell layers of the follicular outer root sheath (ORS).16 K15, whose expression is mainly restricted to the outermost ORS cells of HFs, appears to identify epithelial progenitor cells, including HF bulge epithelial stem cells.17 The cells of the bulge region display the greatest in vitro growth capacity and clonogenicity compared to cells from other regions of the HF and epidermis.18 The promoter of the KRT15 gene is up-regulated by thyroid hormones (THs) and the cytokine interferon-γ, while glucocorticoids (GCs) down-regulate the KRT15 promoter in isolated normal human foreskin keratinocytes.19 This represents strong evidence for stem and/or progenitor cell activation or maintenance by hormonal regulations of different keratin expression patterns (e.g., KRT15 and KRT19).1
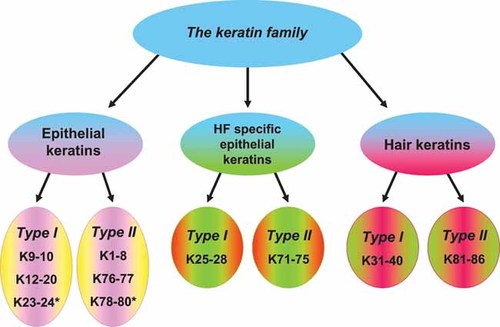
The keratin family, according to the new human keratin nomenclature (modified from Schweizer et al.14). Asterisks indicate that only gene data are available, and expression pattern is still unknown.
Mutations in 17 epithelial keratins (out of 28) have been found to be responsible for various genodermatoses. Mutations in KRT5 or KRT14 genes lead to epidermolysis bullosa simplex (EBS); those in KRT1, KRT2, or KRT10 give rise to epidermolytic hyperkeratosis; and mutations in KRT6a, KRT16, or KRT17 give rise to pachyonychia congenita. In addition, mutations in the KRT2 gene are associated with a mild disruption in the epidermis known as ichthyosis bullosa of Siemens.20 Only 5 out of 26 HF-specific keratins have so far been reported to cause genothrichoses.21 Mutations in the genes encoding human hair keratins KRT81, KRT83, and KRT86 cause monilethrix (Fig. 1).21, 22 Defective KRT85 is involved in ectodermal dysplasia of the hair and nail type, and sequence variants in KRT75 are implicated as risk factors in the loose-anagen syndrome and pseudofolliculitis barbae.21 It is likely that keratin genes play a part in other hair disorders and in normal variations in hair characteristics.23
Moreover, it has recently become evident that at least some keratins serve a much wider spectrum of functions than that of structural proteins traditionally attributed to them. For example, K17 regulates the cyclic transformation of murine HFs, which is mediated at least in part via the modulation of tumor necrosis factor-α secretion.24 K17, which is rapidly induced in wounded stratified epithelia, is now considered to directly impact cell growth and protein synthesis, e.g., through binding to, and serum-dependent relocalization of, the adaptor protein 14-3-3σ (stratifin) from the nucleus to the cytoplasm.25 These recently recognized regulatory functions of K17 render it even more pertinent to investigate endocrine controls of keratin gene and protein expression, since selected keratins may well play a much wider role in skin biology than that of mere structural proteins.
Glucocorticoids
Hypercortisolism is a hormonal state characterized by marked clinical effects on the skin, including epidermal and subcutaneous atrophy, telangiectasia, striae distensae, seborrhea, facial acne, hirsutism and hyperpigmentation.4 GCs are among the most extensively used therapeutic agents in dermatology, where they are employed as an anti-inflammatory, anti-proliferative, and immunosuppressive agents, reproducing all of the recognized cutaneous signs of hypercortisolism.26 Thus, skin is a major target tissue for endogenous GCs action,26 and it is both biologically and clinically important to clarify any regulatory effects of GCs on keratin expression/synthesis. GCs are also key ligands for the mineralocorticoid receptor in mammalian skin, whose importance in epidermal and hair biology has only recently become fully appreciated.27
As summarized in Table 1, GCs appear to preferentially inhibit those keratin genes whose expression tends to be up-regulated during inflammatory skin responses and epidermal regeneration. This has profound clinical implications.
| Keratin regulation | Comments | Clinical effect | References |
|---|---|---|---|
| KRT5 ↓ | K5 and K14 serve as markers for the highly proliferating, basal layer epidermal and ORS keratinocytes. Their suppression is associated with thinning of the epidermis seen after prolonged treatment with topical GCs. | Thinning of epidermis | |
| KRT14 ↓ | |||
| KRT15 ↓ | |||
| KRT6 ↓ | KRT6, KRT16, and KRT17 genes are robustly induced after acute injury to epidermis and other compound epithelia. Suppression of these genes by GCs correlates with their anti-inflammatory and growth-inhibition effects, which lead to wound healing inhibition. | Wound healing inhibition | |
| KRT16 ↓ | |||
| KRT17 ↓ | |||
| KRT16 ↓ | Treatment of psoriatic skin with GCs revealed decreased expression of K16, with correlating clinical reduction in epidermal thickening. | Treatment of psoriasis |
- 1.
Keratin-response elements (KREs): These highly conserved gene promoters are the first group of native negative regulators identified in a gene family.28 Binding of four monomers of the ligand-bound GC receptor (GR) to the KREs leads to suppression of transcription.28 KREs may also simultaneously bind multiple receptors, thus providing fine-tuning of transcriptional regulation.29
- 2.
Transcription factor AP-1: Binding of the AP-1 transcription factor to recognition motifs in the regulatory regions of target genes up-regulates their transcription. GCs block induction of AP-1 by forming an inactive complex, thus indirectly controlling keratin gene expression.26
The epidermal effects of GCs also extend to skin appendages. HFs are profoundly affected by GCs, which arrest murine HFs in telogen (resting phase of the HF) and induce premature catagen (transition stage at the end of anagen, the active growth phase of HFs) development,30, 31 while topical application of GC analogues can induce HF dysplasia.32 The latter is consistent with the phenotype seen in transgenic mice overexpressing GR in basal layer keratinocytes, which show 50% decrease in the number of HFs, dysplastic HFs, and ectopic, hyperplastic sebaceous glands.33 Interestingly, GR stimulation up-regulates a large number of hair keratins,32 as well as many different hair keratin-associated protein (KAPs) genes.32 KAPs are important components of the hair fiber, and are crucial for the formation of a strong hair shaft through cross-linking with abundant cysteine residues of hair keratins, forming disulfide bonds and/or hydrophobic connections.34 Moreover, several hox genes are down-regulated by GR stimulation.32 Thus, GR stimulation is likely to play important roles in HF morphogenesis, growth, and cycling through the coordinated regulation of the genes for hair-specific keratins, KAPs, and keratin expression-regulating hox genes.
Retinoic acid
The cutaneous effects of vitamin A, a precursor of retinoic acid (RA), were first observed in 1922.35 Hypervitaminosis A inhibits keratinization, causes hyperplasia, and obstructs terminal keratinocyte differentiation. Hypovitaminosis A results in opposite effects: causing epidermal hyperkeratosis and keratinization of non-keratinized epithelia, such as the cornea and conjunctiva.36 Retinoids are widely used as a treatment modality, having vast effects on disorders of keratinization, sebaceous gland function, and even epithelial malignancies. They are used in a large number of epidermal disorders ranging from ichthyoses to wrinkles.37 However, systemic treatment with retinoids may lead to diffuse hair loss.38 The retinoid all-trans-RA leads to decreased hair shaft elongation and a premature catagen-like phase.38
Several of these effects are mediated through the actions of RA on keratin synthesis. There are likely to be at least two distinct effects on keratin synthesis in the epidermis:39 (1) qualitatively, by controlling the expression of secondary regulators of epidermal keratinocyte differentiation,40 and (2) quantitatively, by direct action of its receptors on gene regulatory sites – nuclear receptors interact with co-regulators and the transcriptional machinery to regulate transcription.41 By this mechanism, RA suppresses the expression of specific, disease-associated keratin genes, which are known to have KREs in their promoter regions.
The effects of RA on epidermal keratins may be applied in clinical practice, as RA up-regulation of K4 was suggested to be utilized as a sensitive test for retinoid bioactivity in epidermis in vivo.42 The direct and indirect effects of RA on keratin expression and their implications, in skin and other tissues, are summarized in Table 2.
| Tissue | Keratins up-regulated | Keratins down-regulated | Comments | References |
|---|---|---|---|---|
| Epidermis – direct regulation | K5, K6, K14, K15, K16, K17 | Direct regulation by binding to KREs | ||
| Epidermis – indirect regulation | K4, K13, K19 | K2 | Indirect unknown mechanisms. K4 up-regulation was suggested to serve as a sensitive test for retinoid bioactivity in epidermis in vivo. Down-regulation of K2, which serves as a substitute to K1, might explain unresponsiveness to RA treatment in epidermolytic hyperkeratosis patients with K1 mutations. | |
| Embryonic stem cells | K14, K18 | RA was found to play an important role in epithelial differentiation of embryonic stem cells. This process has been used to generate keratinocyte cultures capable of terminally differentiating and forming coherent epithelial sheets. | ||
| T47D (human breast cancer cells) | K8, K18, K19 | Retinoid inhibition of T47D cell growth was accompanied by increases in mRNA levels of K8, K18 and K19, which serve as markers of luminal differentiation. | ||
| Oral gingival epithelia | K8, K18, K19 | K8, K18 and K19, normally distinguishing junctional from oral gingival epithelia, were up-regulated in oral gingival epithelia. | ||
| Airway epithelial cells | K7, K8, K10, K13, K15, K18, K19 | K5, K6, K13, K14, K16, K17 | Phenotipically, retinol treatment induces mucous cell differentiation in cultured airway epithelial cells. The inhibition of the squamous differentiation markers K5 and K13 was suggested as a potential treatment for metaplastic lesions. | |
| Mesothelial cells | K7, K8, K18, K19 | Positive regulation of K7, K8, K18 and K19 is accompanied with regulation of cell differentiation and morphology. |
Thyroid hormone
The skin and hair are well recognized as targets for THs.43, 44 In hyperthyroidism, skin changes include erythema, palmoplantar hyperhidrosis, acropathy, and infiltrative dermopathy; also, Graves' disease may be associated with generalized pruritus, chronic urticaria, alopecia areata, vitiligo, and diffuse skin pigmentation. In hypothyroidism, the skin is cool, dry with a pasty appearance. The epidermis is thin and hyperkeratotic, alopecia may develop, and there is diffuse myxedema.
Keratin genes are known to be affected by triiodothyronine (T3), and this regulation is critical during development. This is already evident in lower vertebrates: During amphibian metamorphosis, the skin is transformed from a bilayered non-keratinized epidermis with a thin dermis into a stratified, keratinized epithelium. This transformation is controlled by T3 and correlates with the appearance of adult keratins and the loss of embryonic keratins.45 T3 is also important in wound healing, and hypothyroid states result in poorer wound healing.46 This is mediated in part by TH-induced up-regulation of the keratins involved in keratinocyte proliferation, both on the mRNA and the protein levels. If they are as effective in human as they appear to be in mouse skin, topical T3 may be an inexpensive wound healing-promoting agent.47 Another conceivable therapeutic use of TH is to utilize its recognized activation of KRT15 promoter in vitro to treat the genetic keratin disorder EBS.19 Similar to RA and GCs, THs exert their effects by direct binding of their receptor (TR) to thyroid responsive elements (TREs), inducing a suppression signal to the keratin genes.29 However, K15, which has a TRE in its promoter was found to be induced by T3, suggesting an indirect induction of keratin-gene stimulating pathways or as-yet-unidentified positive TRE for the keratin genes.19, 46
The hair is a prominent target for TH control, and patients with thyroid disease demonstrate a variety of hair abnormalities. Recently, human HFs were found to be a direct target for TH, which prolongs anagen and stimulates matrix keratinocytes proliferation and melanin synthesis. Not surprisingly, TH effects were also seen on keratin proteins in the HF, demonstrating regulation of the recognized TH-responsive keratins in the ORS of the HF.44 Table 3 summarizes the recognized regulation of keratin gene expression by TH.
| Keratin regulation | Description | Clinical effect | References |
|---|---|---|---|
| sseKer1 ↓ | The transformation of amphibian skin to stratified epithelium is mediated by TH, with down-regulation of amphibians' specific keratins. Several of these keratins bear resemblance to human keratins, which have not yet been found to be affected by TH. | Amphibian skin metamorphosis | |
| sseKer2 ↓ | |||
| Rana larval type I ↓ | |||
| K6 ↑ | Hypothyroid states result in poorer wound healing, suggesting a therapeutic role for TH in wound healing, mediated in part by affecting the keratins taking part in epidermal cell proliferation. | Wound healing promotion | |
| K16 ↑ | |||
| K17 ↑ | |||
| K15 ↑ | Since mutations in the KRT14 gene may lead to EBS, T3-based treatment strategies might become interesting agents in the management of patients with EBS, by utilizing K15 as a substitute keratin for K14. | EBS | |
| K6 ↑ | Human HFs are direct targets for TH, and this is accompanied by modulation of the recognized TH-responsive keratins in the ORS. | Hair abnormalities | |
| K14 ↓ |
Vitamin D
The main role of vitamin D was considered until recent years to regulate calcium and bone metabolism through its active metabolite, 1,25(OH)2D3 (D3), affecting the intestines, kidneys, and bone.48 However, recent years have revealed that D3 exerts many other important physiological effects, including immunoregulation and protection against oxidative stress, UV radiation, infectious agents, and cancer.48 The epidermis is the major site for D3 synthesis, mediated by sunlight.48 The epidermal keratinocytes, in addition to many other cells residing in the skin, have the required enzymes to metabolize vitamin D, and keratinocytes also contain the receptor for D3.49
The effects of D3 on keratinocytes show a shift from the hyperproliferative cell compartment toward the differentiating cell compartment.50-52 These effects led to its utilization in psoriasis treatment: Calcipotriol, a vitamin D derivative, inhibits keratinocyte proliferation and induces keratinization and cornified envelope formation in psoriasis.53 This is achieved by switching the basal progenitor keratinocytes toward a differentiating pathway, instead of the typical hyperproliferative pathway seen in psoriasis.50 These effects are accompanied by up-regulation of the differentiation-associated keratins.54, 55 In part, these effects might be secondary, i.e., not related directly to keratin expression modulation by vitamin D, but rather reflecting an effect of vitamin D on the cell cycle and general differentiation programs.
The effects of D3 have been mainly related to calcium homeostasis, and D3 was found to induce several essential components in the calcium signaling pathway.56 These include for example calcium receptor and phospholipase C, which are required for calcium responsiveness. The increasing calcium levels then inhibit keratinocyte proliferation and induce terminal differentiation.54, 55 In addition, vitamin D may up-regulate a number of key proteins involved directly in differentiation via its specific nuclear receptor, a transcription factor that belongs to the steroid hormone receptor superfamily and that exerts its effects by binding to specific DNA sequences–the vitamin D response elements (VDRE).57
Recently, VDREs have been found in the promoters of K15 and a large number of hair keratins and KAPs, thus constituting transcriptional targets for vitamin D in synergism with β-catenin, a key component of the Wnt signaling pathway.58 Due to this direct regulation, vitamin D promotes β-catenin-induced HF differentiation, without affecting proliferation. The importance of vitamin D in hair growth is evident in patients with hereditary vitamin D receptor (VDR) deficiency (vitamin D-dependent rickets type II), who may suffer from alopecia.59 This role was also demonstrated in nude mice, as application of vitamin D led to a dramatic stimulation of hair growth, associated with increased expression of several hair keratins.60
Through its differentiating properties and effects on keratins, vitamin D may also have anti-cancer properties. The absence of VDR led to induction of undifferentiated basal cell carcinomas,58 and a keratin gene was found to be up-regulated by D3 in a model for human colon cancer cells.61 The recognized D3 effects on keratin regulation are summarized in Table 4.
| Keratin regulation | Comment | Clinical effect | References | |
|---|---|---|---|---|
| K1 ↑ | Vitamin D and its metabolites are potent stimulators of epidermal keratinocyte differentiation, up-regulating differentiation associated keratins and down-regulating the hyperproliferative associated ones. Psoriasis, a disease characterized by hyperproliferative keratinocytes overexpressing the proliferation-associated keratins, is an exceptional therapeutic target for D3. | Psoriasis treatment | ||
| K10 ↑ | ||||
| K15 ↑ | ||||
| K6 ↓ | ||||
| K14 ↓ | ||||
| K16 ↓ | ||||
| K27 ↑ | K38 ↑ | Vitamin D leads to β-catenin induction of HF differentiation, without affecting proliferation. This is achieved through direct induction of numerous hair keratin genes. In nude mice, treatment with vitamin D led to hair growth that was associated with hair shaft keratins up-regulation. | Hair growth | |
| K31 ↑ | K71 ↑ | |||
| K33b ↑ | K81 ↑ | |||
| K34 ↑ | K83 ↑ | |||
| K35 ↑ | K85 ↑ | |||
| K36 ↑ | K86 ↑ | |||
| K37 ↑ | ||||
| K13 ↑ | Using a microarray analysis, D3 was found to up-regulate K13 in a model for human colon cancer cells. | Anti-cancer activity | ||
Androgens
Endocrine hair research has centered on the well-documented, major effects of androgens on hair growth, and indeed, dihydrotestosterone synthesis and the androgen receptors have become important therapeutic targets.62 The pilosebaceous unit responds to androgens in a highly localized, HF subpopulation- and gender-dependent manner.5 The question arises, therefore, to what extent do any of the recognized effects of androgens in skin biology and pathology63 reflect a modulation of keratin expression.
Only very scant information is available on this issue. Androgens regulate K37, a protein that is generally not seen in the large HFs in the terminal anagen phase of scalp hair, but primarily in the central cortex cells of vellus HFs, and that is constitutively expressed in the medullary cells of male and female sexual HFs.64 During puberty, circulating androgens induce medullated beard, axillary, and pubic hairs from small, unmedullated vellus hairs. In addition, the nuclei of medullary cells of beard hairs express androgen receptors. This suggests an androgen-controlled expression of the KRT37 gene.65 Indeed, the promoter of KRT37 was found to contain an androgen transactivatable-response element.65
Additional pointers come from studies on prostate epithelium, where androgens have been speculated to regulate keratin expression.66, 67 The molecular mechanisms that stand behind this regulation are generally unknown. One candidate is the transcriptional regulator Hoxc13. The human Hox proteins constitute a large family of transcriptional regulators.68 This family has been linked to hair growth and development, and demonstrates differential gene expression in developing and cycling hair. Members of this family are probably involved in the control of HF patterning during morphogenesis, as well as in hair-cycle-specific events. Both Hoxc13-deficient and -overexpressing mice exhibit severe hair growth and patterning defects. In humans, HOXC13 is known to be involved in controlling the expression of several hair keratin genes.69 In addition, studies on prostate epithelium revealed that androgens increase the expression of the Hox13 genes in the developing rat ventral prostate.70 However, direct, definitive evidence for a regulation of keratin expression and/or synthesis by androgens is still missing for the vast majority of keratins.
Estrogens
The situation is rather similar with estrogens. Multiple constituents and functional aspects of human skin physiology are influenced by estrogens. For example, estrogens regulate skin aging, pigmentation, sebum production, and skin cancer.71 The expression of some keratin genes in extracutaneous epithelial tissues appears to underlie estrogen regulation. These tissues include, for example, the colon epithelium and the mammary gland.72, 73 Human scalp skin HFs are also largely affected by estrogens, which mainly act as hair growth inhibitors.72 Studying RNA extracted from organ-cultured fronto-temporal human scalp skin HFs, Conrad et al.72 screened 1,300 genes selected for their relevance in cutaneous biology and major skin diseases for a modulation of their expression by estrogen. The authors noted that transcript levels for several keratin genes, KRT2, KRT14, KRT15, KRT17, KRT19, KRT37, and KRT75, were stimulated by 17-β-estradiol.
The human vaginal epithelium is an interesting representative for the effect of estrogen on keratin expression, as the epithelial characteristics are constantly changing with menstrual cycle and during pregnancy. Indeed, K1, a differentiation marker, is not detectable at the end of pregnancy in the suprabasal cells of the epithelium, reflecting a completely suppressed epidermal differentiation.73 As the levels of the female sex steroids such as estrogens are significantly increased in pregnant women, a role for estrogens in down-regulating epidermal differentiation has been suggested.73 However, direct evidence for a regulation of K1 by estrogens is not yet available. The effects of sex steroids on keratin regulation are summarized in Table 5.
| Sex steroids | Tissue | Keratins up-regulated | Keratins down-regulated | Comments | References |
|---|---|---|---|---|---|
| Androgens | Vellus hair follicles | K37 | Correlates with the development of sexual HFs during puberty. | ||
| Prostate | K18 | K8 | In an enriched basal progenitor population prostate epithelial cells, the androgen analogue R1881 promoted differentiation, presumably by the induction of K18 expression. | ||
| Mammary gland | K14 | Androgens are considered protectors of the mammary gland. Gene expression analysis found K14 to be up-regulated. | |||
| Estrogens | Colon | K20 | In estrogen receptor β–/– mice, expression of the differentiation marker K20 in colon epithelium was significantly decreased. | ||
| Mammary gland | K18 | In a culture model for normal human mammary gland, the addition of estrogen reversed the decline in K18 expression. | |||
| MCF-7 breast cancer cells | K19 | Up-regulation of KRT19 gene expression may contribute to the cytoskeletal and nuclear matrix reorganization in these cells, thus potentially increasing their metastatic potential. | |||
| Vaginal epithelium | K1 | K1 is not detectable at the end of pregnancy, a state of significantly increased estrogen levels, thus reflecting a completely suppressed epidermal differentiation. | |||
| Human scalp HFs | K2, K14, K15, K17, K19, K37, K75 | Micro-array results from organ-cultured fronto-temporal human scalp skin HFs. |
Conclusions and perspectives
Keratins are a family of type I and II intermediate filaments that serve crucial roles in skin physiology. A large number of hormones are recruited to control keratin expression. These encompass primarily steroid hormones such as GCs, RA and vitamin D directly via a response element in the promoter of these genes.28, 41, 74 Other hormones that are well-documented direct keratin-expression modulators, such as THs, still need to be fully explored in the context of dermatological therapy. Given that human skin and its constituent cell populations have recently been identified as major, non-classical sites of synthesis for numerous neuroendocrine mediators, such as PRL, corticotropin-releasing hormone, the POMC derivatives ACTH, β-endorphin, α-MSH, thyroid stimulating hormone, and thyrotropin releasing hormone,1, 44, 75, 76 it is reasonable to ask whether these also impact keratin expression. In fact, the existing, although few, pointers that selected neuroendocrine signals regulate keratin expression, directly or indirectly (Table 6), and the growing awareness of potential neuroendocrine controls of HF stem cells1 suggest that this may soon develop into a particularly exciting frontier of endocrine keratin expression research.
| Keratin regulation | Description | Clinical effect | References |
|---|---|---|---|
| Parathyroid hormone-related protein (PTHrP) | |||
| K2 ↑ | PTHrP is important in nipple induction and morphogenesis. This is accompanied by changes in keratin expression, with K17 immunoreactivity even observed in myoepithelial cells beneath the epidermis and around the lactiferous duct. | Mammary gland development | |
| K6 ↑ | |||
| K17 ↑ | |||
| TSH | |||
| K5 ↑ | Normal human scalp skin and HFs express TSH receptor mRNA, and immunoreactivity was observed in mesenchymal skin compartments in situ. Stimulation of the TSH receptor led to up-regulation of K5 immunoreactivity in hair matrix keratinocytes. | Hair follicle growth | |
| β-endorphin | |||
| K16 ↑ | Although K16 is a marker for hyperproliferation, no increase in keratinocyte proliferation was observed in β-endorphin treated skin. Therefore, it was hypothesized that the µ-opiate receptor system is actively involved in skin differentiation, but not in proliferation. | Psoriasis | |
| K16 ↑ | In acute wounds there is a balanced expression of ligand and µ-opiate receptor, which leads to increased expression of K16. In chronic wounds there is disturbance of this balance, leading to changes in differentiation and impaired wound healing. | Wound healing | |
| Prolactin | |||
| K8 ↑ | Knockout mice lacking the PRL receptor showed decreased expression of keratins K8, K17, K18, and K19 during early pregnancy, accompanied by failure to develop normal mammary glands. | Mammary gland development | |
| K17 ↑ | |||
| K18 ↑ | |||
| K19 ↑ | |||
As we have seen, there are numerous indirect endocrine controls of keratin expression/synthesis through the modulation of keratinocyte proliferation and differentiation, which then activate distinct programs for the coordinated expression of proliferation/differentiation-related keratins (summarized in Fig. 3). This, and the fact that many steroid hormones in skin are now recognized to exert non-classical and/or non-genomic effects not based on the interaction of steroid-receptor complexes with a specific hormone-response element in the promoter region of a given keratin gene63, 77 make it exceedingly difficult to definitively distinguish between direct and indirect endocrine controls of keratin expression. This remains one of the most critical tasks for future keratin research, which also needs to identify those that really matter most under physiological conditions.
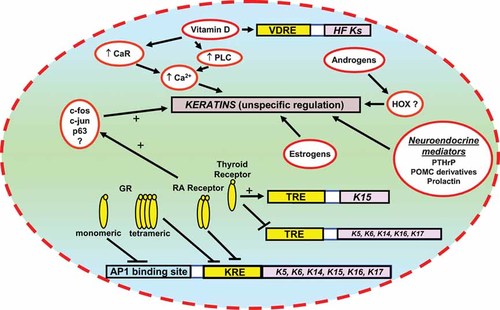
Summarizing scheme of the different regulatory pathways of hormones on keratin expression.
Along the same lines, given the paramount importance of GCs, vitamin D and retinoids in the current clinical management of skin diseases, another important, yet largely unmet, research challenge is to clarify the basis for the multiple keratin expression changes reported in the epidermis of patients treated with these steroid hormones.42, 50, 51, 78 It is necessary to determine to what extent they reflect direct, hormone-induced changes in keratin expression and/or synthesis, or whether they result from endocrine modulation of epidermal keratinocyte proliferation, apoptosis, differentiation, and secretory activities or of inflammatory cell activities, which then alter keratin expression secondarily. Recently, the promiscuous signaling character of steroid hormone receptors,79 many of which probably bind and become activated by several, and sometimes numerous, compounds, has become increasingly appreciated. Therefore, it will also be important to dissect how the, often clinically used, co-administration of recognized keratin-modulatory hormones (such as GCs, calcitriols and retinoids) affects epidermal keratinocyte keratin expression patterns in vitro and in vivo. Available human skin and HF organ culture assays,72, 80 in conjunction with the study of isolated human keratinocytes, offer an attractive research tool for addressing these questions.
More systematic dissection of the—as yet far too incompletely characterized—endocrine controls of keratin expression may also render a wide range of skin and hair diseases that are recognized to show pathogenetically important, inherited abnormalities of keratin expression6, 19 more accessible to endocrine-treatment strategies. Until effective gene correction therapies for clinically often devastating keratin gene mutations are available, the second-best option may well be to use the already widely employed and readily available hormonal treatment modalities (topically or systemically) to up-regulate the synthesis of such keratin(s) that can partially compensate for the defective mutant keratin.
Acknowledgements
The authors are grateful to Dr. Yusur Al-Nuaimi for reviewing the manuscript. This review was supported in part by a Minerva Fellowship (Y.R.) and by the Authority for Research and Development, Hebrew University of Jerusalem (A.Z.).




