Hypomagnetic Field Exposure Affecting Gut Microbiota, Reactive Oxygen Species Levels, and Colonic Cell Proliferation in Mice
Grant sponsors: National Natural Science Foundation of China, grant numbers: 42074073, 41621004; Strategic Priority Research Program of the Chinese Academy of Sciences, grant number: XDA17010501.
Abstract
The gut microbiota has been considered one of the key factors in host health, which is influenced by many environmental factors. The geomagnetic field (GMF) represents one of the important environmental conditions for living organisms. Previous studies have shown that the elimination of GMF, the so-called hypomagnetic field (HMF), could affect the physiological functions and resistance to antibiotics of some microorganisms. However, whether long-term HMF exposure could alter the gut microbiota to some extent in mammals remains unclear. Here, we investigated the effects of long-term (8- and 12-week) HMF exposure on the gut microbiota in C57BL/6J mice. Our results clearly showed that 8-week HMF significantly affected the diversity and function of the mouse gut microbiota. Compared with the GMF group, the concentrations of short-chain fatty acids tended to decrease in the HMF group. Immunofluorescence analysis showed that HMF promoted colonic cell proliferation, concomitant with an increased level of reactive oxygen species (ROS). To our knowledge, this is the first in vivo finding that long-term HMF exposure could affect the mouse gut microbiota, ROS levels, and colonic cell proliferation in the colon. Moreover, the changes in gut microbiota can be restored by returning mice to the GMF environment, thus the possible harm to the microbiota caused by HMF exposure can be alleviated. © 2022 Bioelectromagnetics Society.
INTRODUCTION
The geomagnetic field (GMF) maintains a habitable environment for organisms on Earth by preventing radiation from solar winds and cosmic high-energy particles and preventing oxygen and water from escaping into space [Hong, 1995; Wei et al., 2014]. However, the decrease or elimination of GMF, the so-called hypomagnetic field (HMF), may affect the evolution of life on Earth [Wei et al., 2014; Channell and Vigliotti, 2019; Erdmann et al., 2021]. Organisms on Earth may be exposed to HMF environments, such as magnetic shielding chambers, which have many applications in hospitals and research fields. In addition, the HMF environment is also a risk factor for the health of astronauts during space exploration missions [Mo et al., 2014].
Previous studies have shown that HMF exposure could cause many negative biological effects on the animal, plants, and microbes [Binhi and Prato, 2017; Zhang et al., 2021b], even harmful to humans’ cognition and memories [Binhi and Sarimov, 2009; Binhi and Prato, 2017]. HMF exposure could affect the resistance to antibiotics of microorganisms, which decreased the resistance against antibiotics of Escherichia coli strains isolated from human subjects, and also negatively affected the growth, multiplication rate, sporulation, and potassium content of Bacillus subtilis [Creanga et al., 2004; Poiata et al., 2009; Obhodas et al., 2021]. Moreover, it has been revealed that HMF exposure affected the morphogenesis of microscopic fungi, namely, the appearance of spiral growth instead of fractal [Panina et al., 2019]. North-seeking and south-seeking magnetotactic bacteria could obviously coexist in HMF (<0.2 µT) and even, when the oxidation-reduction gradient configuration is suitable, in the GMF [Zhang et al., 2010].
In recent years, growing evidence suggests that the mammalian gut microbiota has been linked to host metabolism and immunity, as one of the key elements contributing to host health and diseases [Thursby and Juge, 2017; Dupont et al., 2020; Paik et al., 2022]. The disorders of gut microbiota are associated with many diseases and cancers either directly or indirectly [Hooper and Gordon, 2001; Human Microbiome Project, 2012]. Its diversity is influenced by many factors including environmental extremes (noise, cold/heat, microgravity, and high altitude) [Li et al., 2015; Cui et al., 2018; Jin et al., 2018; Rothschild et al., 2018; Bo et al., 2019; Chevalier et al., 2020; Sun et al., 2020]. For example, cold acclimation could cause a significant alteration in cecal microbiota in male Brandt's voles, and further gut microbiota-host neurotransmitters (norepinephrine) crosstalk via cAMP signaling regulates energetics and thermogenesis [Cryan et al., 2019], while warmth prevents bone loss through enhancing bacterial polyamine biosynthesis, resulting in higher total polyamine levels in vivo [Chevalier et al., 2020]. The possible mechanism of influence is related to reactive oxygen species (ROS), that is, external environmental factors could induce changes in ROS levels and leads to oxidative stress in intestinal tissue [Chi et al., 2021], which could further affect the gut microbiota [Oliveira et al., 2011; Yang et al., 2015]. Recent studies have revealed that a microgravity environment can also affect the community structure of gut microbiota, their functional pathways, and expression of certain pro-inflammatory factors, thereby leading to gut microbiota disturbance, impairment of the intestinal barrier function, and an increased risk of colitis [Li et al., 2015; Jin et al., 2018]. The effects of the elimination of GMF on gut microbiota are important to understand the roles of HMF in general, which, however, remain poorly understood.
In this study, we sought to determine whether long-term HMF exposure could affect the composition and function of the gut microbiota in mice. Fecal microbiota diversity and composition, gut oxidative stress, and colonic cell proliferation were determined. We found that approximately 95% of bacteria were composed of phyla Firmicutes and Bacteroidota both in GMF and HMF groups. Long-term exposure to HMF altered the structure of the gut microbiota based on the β-diversity analysis. Eight-week (8 w) HMF-exposed mice had a distinct bacterial community from GMF-exposed control mice at phylum/family/genus levels. The concentrations of short-chain fatty acids (SCFAs) in HMF-exposed mice showed a decreasing trend compared with the GMF group. Moreover, we found that ROS levels and colonic cell proliferation were significantly increased in the mid-colon of HMF-exposed mice based on the immunofluorescence analysis. This is the first in vivo finding that long-term HMF exposure could affect the mouse gut microbiota, ROS levels, and colonic cell proliferation. This study highlights that HMF is a stressor that modulates gut microbiota composition and function and that GMF plays an important role in maintaining host-microbiota balance in mammals.
MATERIALS AND METHODS
Animals
Adult 6-week-old male C57BL/6J mice (Beijing HFK Bio-Technology, Beijing, China) were purchased and housed in the experimental coils to acclimate to the exposure environments for two weeks before starting the magnetic field exposure experiments. All animals were housed at a constant temperature (23 ± 1 °C) with a 12: 12 h light: dark cycle. Water and food were given ad libitum. Mice were randomly allocated to three experimental groups: (i) GMF group (sham-exposure), (ii) HMF group, and (iii) RGMF group (returning to GMF [RGMF] for a 4-week GMF exposure after 8-week HMF exposure) (Fig. 1). The number of animals used for 16 S amplicon sequencing is nine individuals in each group. Four animals in each group were used for immunofluorescence analysis. The body mass of mice was checked when samples were taken. All animal experiments were performed double-blind. All procedures and husbandry were performed according to protocols approved by the Institutional Animal Care and Use Committee at the Institute of Geology and Geophysics, Chinese Academy of Science.
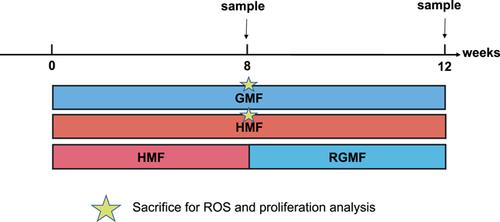
 sacrifice for ROS and colonic cell proliferation after 8-week GMF and HMF exposures.
sacrifice for ROS and colonic cell proliferation after 8-week GMF and HMF exposures.Magnetic Fields and Environmental Parameters
To ensure uniform, stable GMF background, the experimental HMF environment was set up in the laboratory of the Beijing National Observatory of Space Environment, which was generated by the double-wrapped coils system (Fig. S1A). It is composed of three nested sets of orthogonal square coils. Each coil contained two matched sets of windings to allow operation in active or sham mode. In active mode, currents in paired windings were parallel to generate the magnetic field that could cancel the GMF, thus obtaining a near-zero magnetic field (HMF). In sham mode, currents ran antiparallel, yielding no measurable external field, so the GMF was left. Importantly, in sham mode, ohmic heating and magneto-mechanical effects are similar to those in active mode [Kirschvink, 1992; Wang et al., 2019]. The magnetic strength and electromagnetic field inside the coils of the HMF and GMF environment were measured by Mag-13MS sensors combined with the Spectramag-6 (Bartington Instruments, Witney, UK) during the experiments. HMF had a high uniformity with a strength of 31.1 ± 2.0 nT (mean ± SEM). For GMF control, the magnetic field intensity was 55,547.8 ± 1.5 nT (Fig. S1B and C). During the exposure experiments, because both the HMF and GMF coils were placed in the same room, the other environmental parameters inside the two coils were similar, with no significant difference. The noise level was 39.9 ± 0.2 dB. The light intensity was 11.1 ± 0.6 lux. The temperature was 20.6 ± 0.7 °C, and the humidity was 43 ± 2%.
16S rRNA Gene Profiling
Fecal samples were collected by placing the mice into a sterile cage and collecting fecal pellets. All collected fecal samples were immediately stored at −80 °C. DNA was extracted from the fecal pellets using a QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. This study mainly focuses on intestinal bacteria, because the function and host interaction of intestinal bacteria are well-studied and deeply understood. Therefore, it is a good subject to study the effect of HMF on gut microbiota.
The V3–V4 regions of the 16S rRNA gene were PCR-amplified for Illumina NovaSeq. 6000 (PE250) sequencing. Library constructs and sequencing were performed by Novogene (Novogene, Beijing, China). Sequence processing was performed using Quantitative Insights Into Microbial Ecology version 2 (QIIME2, 2021.11 release) [Bolyen et al., 2019]. Briefly, the demultiplexed paired-end fastq files were imported into the QIIME2 software suite. Then, Cutadapt was used to remove primer sequences and discard the unmatched reads [Martin, 2011]. The reads were joined using VSEARCH [Rognes et al., 2016], quality-filtered, denoised, and chimera-removed using deblur plugin in Qiime2 [Amir et al., 2017]. Clustering of operational taxonomic units (OTUs) was conducted at a minimum sequence similarity of 99% using VSEARCH. Taxonomic annotation of OTUs was performed according to the Silva database [Quast et al., 2013]. The phylogenetic tree was constructed using Qiime2 phylogeny fasttree2 [Price et al., 2010]. The α-diversity and weighted UniFrac distance metrics were calculated through the QIIME2 diversity plugin. The α-diversity of species in the gut microbiome is measured by their richness (number) and evenness (relative proportions). Four α-diversity indices (Shannon, Pielou's evenness, observed OTUs, Faith's PD) were used to measure the richness and evenness of bacterial taxa in different groups. Wilcoxon rank-sum test was used to calculate significant differences in the α-diversity indices. β-diversity analysis was performed using principal coordinate analysis (PCoA) based on the weighted UniFrac distances between samples.
PCoA analyses were conducted using vegan and ggplot2 packages in R [Oksanen et al., 2013; Villanueva and Chen, 2019; R Core Team, 2021]. Differential abundance analysis is performed based on the relative abundance of more than 1% in samples. To predict functional profiles of microbial communities, PICRUSt2 software was accessed to generate the differential pathways based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and the result was visualized using the STAMP package [Kanehisa and Goto, 2000; Parks et al., 2014; Douglas et al., 2020]. The original 16S rRNA fastq data are available under NCBI BioProject accession number PRJNA828136.
Tissue Preparation and Immunofluorescence Analysis
Mice were deeply anesthetized via isoflurane inhalation and transcardially perfused with saline followed by 4% paraformaldehyde (PFA). Mid-colon tissues were obtained and post-fixed in 4% PFA overnight and then equilibrated in 30% sucrose buffer. Colon tissues were sectioned in the coronal plane on a freezing microtome at a thickness of 8 µm. The sections were transferred to a glass microscope slide and stored at −20 °C. The tissue sections were pre-blocked with blocking buffer TBS++ (TBS containing 3% donkey serum and 0.3% Triton X-100) for 1 h at 37 °C, followed by incubation with primary antibodies diluted in blocking buffer for 24 h at 4 °C. After washing three times, sections were incubated with the secondary antibody for 1 h at 37 °C. To detect bromodeoxyuridine (BrdU, B5002; Sigma-Aldrich, St Louis, MO) incorporation, the tissue sections were pretreated with 2 M HCl for 20 min at 37 °C, incubated with borate buffer (pH 8.5) for 30 min, and subjected to immunocytochemistry analyses. All sections were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, B2261; Sigma–Aldrich). The primary antibody used was rat anti-BrdU (1:1000, ab6326; Abcam, Cambridge, UK) [Hewitt et al., 2021; Xu et al., 2021]. The fluorescent secondary antibody used was donkey anti-rat Alexa Fluor 488 (1:500, A21208; Invitrogen, Carlsbad, CA) [Yang et al., 2016]. After staining, sections were mounted, coverslipped, and then maintained at 4 °C in the dark until imaging. Images were acquired on an Olympus FV1000 multiphoton confocal system (Olympus, Tokyo, Japan) with a multitrack configuration.
BrdU and Dihydroethidium (Hydroethidine) Administration
For the in vivo colonic cell proliferation assay, the mice received BrdU (200 mg/kg) injections and were sacrificed 2 h post-BrdU injection. The details of immunohistochemistry were as described above. For the quantification of BrdU+ cells, every 150th section was selected for staining. Eight sections were analyzed per mouse. The number of BrdU+ cells was counted in immunofluorescence images. The tissue area was determined by DAPI staining. The density of BrdU+ cells was calculated by dividing the number of BrdU+ cells by the tissue volume.
In order to measure the endogenous ROS levels, administration of the ROS-sensitive dye dihydroethidium (HEt, 37291, 25 mg/kg; Sigma–Aldrich) was performed by intraperitoneal injection 4 h prior to perfusion fixation. The intraperitoneal injection was used to administer agents to mice because it is simple and least stressful to the animal. The details of immunohistochemistry were as described above. Every 150th section was selected for staining. Fluorescence images of sections were acquired using equal identical laser power, exposure time, sensitivity, and resolution. The colon tissue sections were analyzed using ImageJ software (NIH, Bethesda, MD) Relative intensities of fluorescence were measured and exported by ImageJ software.
SCFAs Measurement
Fecal SCFAs were determined by gas chromatography-mass spectrometry (GC-MS). Briefly, 200 mg feces were homogenized with 1.2 ml of phosphate buffer (pH 7.3) and centrifuged at 4 °C at 16,000g for 15 min. The supernatants were filtered through a 0.22 µm nylon filter (EMD Millipore, Billerica, MA). An aliquot (200 µl) of the supernatants was acidified by adding 0.1 ml 50% (v) sulfuric acid. After vortexing and standing for 2 min, the organic acids were extracted by adding 0.4 ml of diethyl ether, and supernatants were measured by GC on an Agilent 6890 (Agilent Technologies, Santa Clara, CA) equipped with flame ionization, thermal conductivity detectors, capillary columns, and GC ChemStation software.
Statistical Analysis
The statistical analysis of SCFAs, fluorescence intensity, and BrdU+ cells was conducted by the Student's t-test using SPSS software (SPSS 26.0; SPSS Inc., Chicago, IL). Data were valued within a confidence interval of 95%. A P-value of less than 0.05 was considered statistically significantly different (P < 0.05). Differences in microbial relative abundance were assessed by one-way ANOVA followed by post-hoc Tukey's multiple comparisons test (SPSS). Alpha diversity differences were analyzed using Kruskal–Wallis post-hoc tests (SPSS). The statistical significance of β-diversity was determined by the PERMANOVA test in the QIIME2 pipeline and Benjamini and Hochberg method was used to correct the p-value (which is called q-value) for multiple comparisons. The LDA effect size (LEfSe) analysis was performed for the quantitative analysis of biomarkers among each group (https://huttenhower.sph.harvard.edu/galaxy/). The linear discriminant analysis (LDA) effect size (LEfSe) statistical analysis was performed on the online interface Galaxy (http://huttenhower.sph.harvard.edu/lefse/) with an α value less than 0.05 and an LDA score greater than 2. The statistical analysis of the KEGG functional categories was conducted by White's no-parametric t-test with Storey FDR correction using STAMP software.
RESULTS
Long-term HMF Exposure Affected the Diversity of Bacterial Taxa in the Fecal Samples
No significant difference in α-diversity indices was found between HMF and GMF groups after 8- and 12-week exposure (P > 0.05); however, after RGMF, there is a significant difference between GMF and RGMF group reflecting species richness (Number of observed OTUs) and phylogenetic diversity (Faith's PD) (Fig. S2). However, there are significant differences in fecal bacterial community structure between HMF-exposed and GMF-exposed mice (8- and 12-week exposures) based on β-diversity analyses (Fig. 2A), which suggested that long-term HMF exposure alters the β-diversity of fecal bacterial microbiota. Interestingly, RGMF for 4 weeks after 8-week HMF exposure, there is no difference in β-diversity of fecal bacterial microbiota between GMF- and RGMF-exposed mice, and a significant difference appeared between HMF- and RGMF-exposed mice (Fig. 2B). These results clearly revealed that the β-diversity changes induced by 8 w-HMF exposure could be restored by RGMF environment for 4 weeks. There were no significant differences in mouse body mass at different time points (Fig. S3).
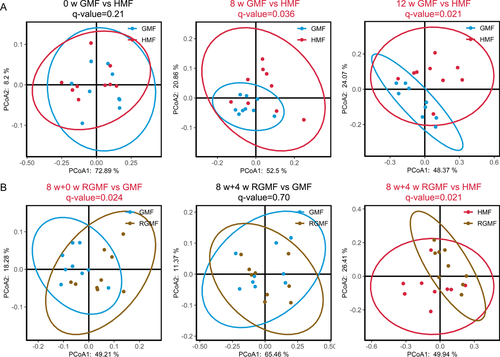
Long-term HMF Exposure Affected the Taxonomic Composition of Fecal Bacterial Microbiota
Differential abundance analyses of bacterial taxa of fecal microbiota were performed at different ranking levels from phylum to genus. Results showed that Firmicutes and Bacteroidota were the most abundant bacterial phyla in all samples (Fig. S4). At the phylum level, significant differences were observed in Bacteroidota, which decreased to 68.9% in abundance after 8-w HMF exposure (compared with 76.5% in GMF, P = 0.007), and the proportions of Firmicutes were increased without a significant level after 8-week HMF exposure (P > 0.05). After 12-week HMF exposure, the proportions of Bacteroidota also decreased (from 62.5% to 51.4%) without a significant level, while the proportion of Firmicutes significantly increased compared with GMF control (P = 0.003) (Fig. 3). After returning to GMF for 4 weeks, the above differences at phylum level disappeared between the GMF group and RGMF group (Figs. 3 and 4A).
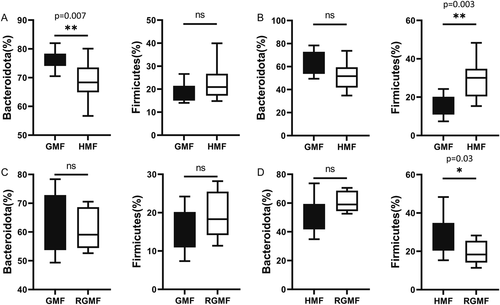
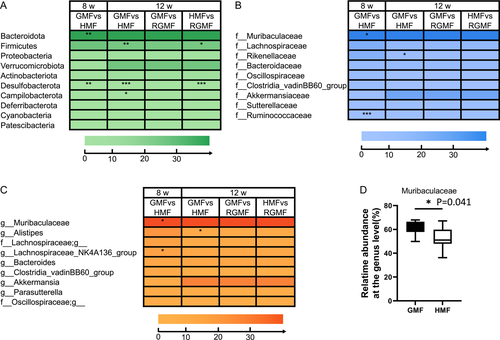
At the family level, Muribaculaceae and Ruminococcaceae were significantly decreased after 8-week HMF exposure. Rikenellaceae was significantly decreased after 12-week HMF exposure (Fig. 4B). At the genus level, the proportion of Muribaculaceae (s24-7) was significantly decreased, whereas NK4A136_group (Lachnospiraceae) was significantly increased in the 8 w-HMF groups compared with GMF group (Fig. 4C). Alistipes was significantly decreased after 12-week HMF exposure (Fig. 4C). Especially, the relative abundance of S24-7 (Muribaculaceae) was dominant in the gut of mice (Fig. 4D). In addition, based on linear discriminant analysis effect size (LEfSe, LDA score > 2, P < 0.05, relative abundance 0.1–1%), the genus Roseburia and Ruminococcus were decreased, while the genus Odoribacter and Desulfovibrio were increased in the HMF group compared with GMF group (Fig. S5). After returning to GMF from 8-week HMF, Muribaculaceae, which had the highest relative abundance of the total genera, showed no significant differences between GMF and 4-week RGMF groups (P = 0.99). After returning to GMF for 4 weeks, the differences at family and genus levels disappeared between the GMF group and RGMF group (Figs. 3 and 4B and C).
Long-term HMF Exposure Affected the Functional Potential of Microbiota and SCFAs Levels
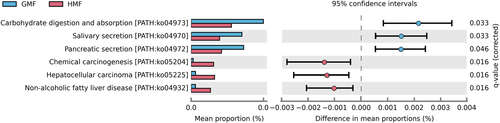
We identified potential functional differences between the GMF group and the HMF group at 8-week exposure by looking at the differentially abundant predicted genes from PICRUSt2 and KEGG database. At KEGG pathway level 3, functional pathways related to Chemical carcinogenesis, Hepatocellular carcinoma, and Non-alcoholic fatty liver disease pathway were enriched in the HMF group, compared with GMF controls. Conversely, carbohydrate digestion and absorption, Salivary secretion, and Pancreatic secretion pathway were enriched in the GMF group (Fig. 5).
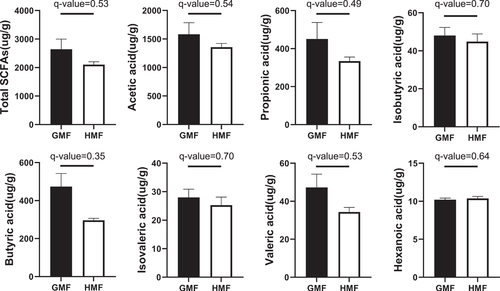
To test whether HMF affects the metabolic capacity of the gut microbiota, we used GC-MS to examine the contents of seven kinds of SCFAs, including acetate, propionate, and butyrate, and so forth, within the colonic content samples. Results of GC-MS showed that, although the differences in SCFAs did not reach statistical significance, the concentrations of SCFAs in HMF-exposed mice showed a decreasing trend compared with the GMF group (Fig. 6), which is closely related to the difference in carbohydrate degradation and metabolism pathway (Fig. 5).
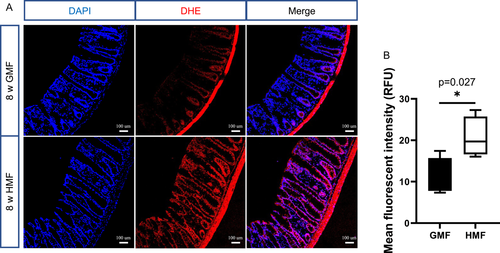
The HMF Exposure Increased ROS Levels in Epithelial Layers of Colon
To directly measure the cellular ROS content in the epithelial layers of the colon in vivo, we performed in situ ROS labeling by injecting ROS-sensitive dye hydroethidine into adult mice. As shown in Fig. 7, compared with GMF-exposed mice, the endogenous ROS levels were significantly increased in the epithelial layers of the colon in the HMF-exposed mice (P = 0.027).
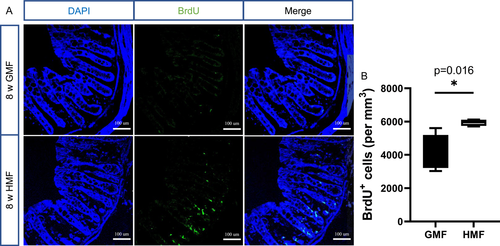
The HMF Exposure Increased Colonic Cell Proliferation
We tested the effect of exposure to HMF on colonic cell proliferation in the colon of adult mice. The mice were injected with BrdU and sacrificed 2 h later to examine proliferation during exposure to GMF or HMF. Compared to GMF control, there was an increase in the numbers of BrdU+ labeled proliferating cells in the crypt of HMF-exposed mice at 8 weeks of exposure (Fig. 8).
DISCUSSION
Accumulating evidence has revealed that gut microbiota is affected by both genetic and environmental factors [Chevalier et al., 2020; Li et al., 2020; Wang et al., 2020]. As one of the important environmental factors, the diminishing or disappearing GMF could affect living organisms at different levels of biological organization [Dubrov, 1978; Binhi and Prato, 2017; Erdmann et al., 2021]. However, it is unclear whether and how long-term HMF exposure affects gut microbiota in mammals. Here, our results show that (i) long-term HMF exposure affects the diversity and composition of mouse gut bacterial microbiota, and returning to the GMF environment could recover the changes in gut microbiota caused by HMF (Figs. 2-4); (ii) long-term HMF exposure may affect the metabolic potential of gut bacterial microbiota and reduce the content of SCFAs in the colon (Figs. 5 and 6); and (iii) long-term HMF exposure increases the ROS levels in epithelial layers and the number of cell proliferation in the colon (Figs. 7 and 8). The possible associations among the change of gut microbiota, high ROS levels in the colon, and increased number of colonic cell proliferation can provide some valuable hints to better understanding the effects of HMF on the gut microbiota in mice.
The relative abundances of S24-7 and Ruminococcus decreased after 8-, or 12-week HMF exposure compared with those in the GMF control at the family level (Fig. 4B). The bacterial family S24-7 are generally considered to be associated with complex carbohydrate degradation due to the presence of a substantial and versatile set of carbohydrate-active enzymes in this family, and Ruminococcus is also functionally associated with carbohydrate metabolism [Ormerod et al., 2016; La Reau and Suen, 2018; Park et al., 2022]. Therefore, the changes in the relative abundances of S24-7 and Ruminococcus coincide with the decrease in carbohydrate digestion function (Fig. 5). Desulfovibrio is generally sulfate-reducing bacteria in the intestinal microbiota, belonging to the proinflammatory bacteria [Liu et al., 2021], which was increased after 8 w-HMF exposure in this study. The changes in the genus of Desulfovibrio may lead to the inflammation in the gut [Chen et al., 2019]. While we also note that long-term HMF exposure could increase the relative abundances of some anti-inflammatory bacteria (such as Lachnospiraceae_NK4A136_group), which is likely in response to high ROS stress.
Our results showed a decreasing tendency in the concentrations of the total SCFAs, and the lowest q-value was calculated based on the concentration of butyrate between the two groups (Fig. 6). The main butyrate producers in the gut belonging to the Firmicutes phylum (Roseburia) decreased in this study, although Odoribacter (Bacteroidota) showed an increase after 8-week HMF exposure, which often contributed to the overall butyrogenic pool [Duncan et al., 2002]. These changes in the butyrate-producing bacteria may cause a decrease in butyrate content, as shown in the GC-MS result (Fig. 6). Undigested carbohydrates are fermented by the gut microbiota into the SCFA acetate, propionate, and butyrate, which are beneficial to reducing inflammation, enhancing immunity and stimulating epithelial cell growth for the host [Macfarlane and Macfarlane, 2012]. Especially, SCFA butyrate has anti-inflammatory and anti-oxidative effects and improves gut barrier function, thus playing a role in oxidative stress in the healthy colonic mucosa [Hamer et al., 2009; Hamer et al., 2010; Zhou et al., 2021]. In this study, the trend of decreased butyrate in HMF-exposed mice may attenuate its role in anti-inflammatory and antioxidant effects in the colon.
To our knowledge, this is the first in vivo finding that long-term HMF exposure could affect the mouse gut microbiota, the ROS levels, and the colonic cell proliferation, although we currently could not determine their relationships. Recent data have demonstrated that gut epithelia contacted by enteric commensal bacteria rapidly generate ROS in response to microbial signals [Marciano and Vajro, 2017]. While HMF exposure also could directly affect cellular ROS levels as potential regulatory molecules and thereby alter physiological and biological processes in organisms [Martino and Castello, 2013; Barnes and Greenebaum, 2015; Van Huizen et al., 2019; Zhang and Tian, 2020].
Based on previous results, we speculate two possible relationships among the observed phenotypes caused by HMF exposure (Fig. 9). One possible relationship is the changes in gut microbes induced by HMF exposure could stimulate the rapid ROS generation in epithelial cells. The NoxO1-controlled activity of Nox1 was previously shown to contribute to proper epithelial homeostasis and renewal in the gut of mice [Moll et al., 2018]. It has been previously shown that microbial-elicited ROS could mediate increased cellular proliferation [Reedy et al., 2019]. Therefore, high ROS may further modulate colonic cell proliferation in the colon (Figs. 7 and 8), serving as critical second messengers [Mo et al., 2014; Channell and Vigliotti, 2019; Erdmann et al., 2021]. The other possibility is the production of ROS directly induced by HMF exposure further mediates colonic cell proliferation, which is the response of gut cells to HMF, independent of gut microbiota.
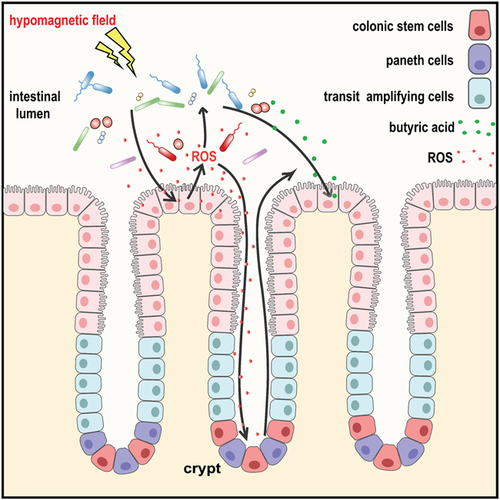
In this study, HMF exposure significantly increased the ROS level in mouse colonic epithelial cells, while in our previous study HMF exposure induced a significant reduction of ROS level in the hippocampal neural stem cells in mice. The different effects of HMF on ROS levels have also been reported in cell studies that HMF exposure increased or decreased ROS levels dependent on different types of cells [Martino and Castello, 2011; Fu et al., 2016; Wang and Zhang, 2017]. So we speculate that the different cell types may lead to the different responses of endogenous ROS levels to the HMF in our studies. Increased ROS levels in epithelial cells may be a response to HMF-induced changes in gut microbiota, which would be absent in animal brains. However, the exact underlying mechanism remains unclear.
Alternatively, gut microbes are also capable of synthesizing neuroactive compounds such as serotonin, dopamine, histamine, and gamma-aminobutyric acid from amino acid precursors [Karl et al., 2018]. These compounds are thought to impact cognition and behavior via the gut-brain axis [Dinan and Cryan, 2017]. Thus, the changes in gut microbiota induced by HMF exposure may contribute to cognition dysfunction of the central neural system in mice [Zhang et al., 2021a]. Since the relationship between host and bacteria is really complicated, additional microbiota transfer and antibiotic treatment experiments in the future are needed to prove whether ROS and colonic cell proliferation in the host intestine depend on the gut microbiota and the contribution of gut microbiota to central neural system function in mammals.
ACKNOWLEDGMENTS
The authors would like to thank the Beijing National Observatory of Space Environment (Institute of Geology and Geophysics, Chinese Academy of Sciences) for help in the exposure experiment, Xueying Zhang and Tongwei Zhang for valuable suggestions on the experiment, and Ying Zhang for help with measuring environmental parameters.




