Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds
Abstract
Electrical fields are known to interact with human cells. This principle has been explored to regulate cellular activities for bone tissue regeneration. In this work, Saos-2 cells were cultured on conductive scaffolds made of biodegradable poly(L-lactide) and the heparin-containing, electrically conducting polypyrrole (PPy/HE) to study their reaction to electrical stimulation (ES) mediated through such scaffolds. Both the duration and intensity of ES enhanced cell proliferation, generating a unique electrical intensity and temporal “window” within which osteoblast proliferation was upmodulated in contrast to the downmodulation or ineffectiveness in other ES regions. The favourable ES intensity (200 mV/mm) was further investigated in terms of the gene activation and protein production of two important osteoblast markers characterised by extracellular matrix maturation and mineralisation, that is alkaline phosphatase (ALP) and osteocalcin (OC). Both genes were found activated and the relevant protein production increased significantly following ES. In contrast, ES in the down-modulation region (400 mV/mm) suppressed the production of both ALP and OC. This work demonstrated that important osteoblast markers can be modulated with specific ES parameters mediated through conductive polymer substrates, providing a unique strategy for bone tissue engineering. Bioelectromagnetics 34:189–199, 2013. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Electrophysiological phenomena are detectable in the form of electrical signals such as those in electrogenesis and electrodiagnosis. In terms of cell biology, electrical signals also exist across contiguous cytoplasmic regions both within and between cells [McCaig et al., 2005]. The electrophysiological characteristics of cells are already used to modify cellular activity. For example, cell growth, migration, wound healing and even tissue regeneration were reportedly affected by exogenous electrical fields or adscititious electrical stimulations (ES) mimicking the physiological direct current (DC) electric field [Song et al., 2002; McCaig et al., 2005].
The early attempt at using ES to treat bone fractures can be dated back to the mid-1800s [Mollon et al., 2008]. Fukada and Yasuda [1957] demonstrated a relationship between electricity and callus formation. More recently, researchers have shown that bone can be regenerated in the presence of appropriate ES conditions and scaffolds. For example, Aaron et al. [2004] reported that electrical and electromagnetic fields are able to stimulate growth factors systematically. Wang et al. [2006] demonstrated that capacitively coupled electric fields could upregulate bone morphogenetic proteins (BMPs). Martino et al. [2008] reported a significant increase in mineral nodule formation following pulsed electromagnetic field stimulation. Biophysical stimuli simultaneously enhanced the secretion and expression of bone formation markers such as alkaline phosphatase (ALP) and osteocalcin (OC) [Torricelli et al., 2003; Wiesmann et al., 2004]. However, these studies were not conductive scaffold-based and used either capacitive coupling or inductive coupling, which affected not only the lesion but also the surrounding healthy tissue. In literature, the clinical efficacy of electrical bone stimulators remains inconclusive. However, a recent survey based on 268 valid responses among 753 Canadian orthopaedic surgeons demonstrated that 45% of Canadian orthopaedic surgeons actually use bone stimulators to manage tibia fractures, of which about 50% are electrical bone stimulators [Busse et al., 2008]. Furthermore, 80% of the respondents thought that bone stimulators would reduce the healing time of bone. The electrical stimulators used in clinics are mostly non-invasive and based on either low frequency pulse electromagnetic fields or capacitive electrodes [Meng et al., 2011]. Implantable electrodes are also used but to a lesser extent.
One of the key challenges in bone tissue engineering is the development of new strategies that combine scaffold and stimulatory cues to provide localised and guided regeneration. In addition to biological stimuli, physical cues such as mechanical force and electrical field have also been investigated. To apply electrical fields to cells seeded on a scaffold, it is rational to render the scaffold electrically conductive. For this reason, electrically conductive scaffolds or substrates made of conductive and biodegradable polymers are thought to be appropriate. Polypyrrole (PPy) is one of the most studied conducting polymers because of its relatively high environmental stability [Tian et al., 2006], versatile electrical properties [Yakuphanoglu and Aydin, 2007] and easy preparation [Vernitskaya and Efimov, 1997; Meng et al., 2010]. It has become an interesting material in biomedical engineering due to its good biocompatibility both in vitro [Wong et al., 1994; Zhang et al., 2001] and in vivo [Jiang et al., 2002; Wang et al., 2004; Ramanaviciene et al., 2007] as well as its unique intrinsic electric conductivity. PPy has been modified with bioactive molecules and coated onto electrodes to record or stimulate cellular functions [Wadhwa et al., 2006; Lee et al., 2009; Richardson et al., 2009]. In our previous studies, we designed and synthesised biodegradable conductive PPy/poly(L-lactide) (PPy/PLLA) composites for use as a substrate to mediate ES to fibroblasts and neurons [Meng et al., 2008; Shi et al., 2008a]. Instead of using electrodes, which may generate a cytotoxic redox reaction at the electrode surface and ionic movement in the culture medium between electrodes, the conductive substrate is integrated into a close circuit, with cells cultured on it. Through this approach, we have recently reported a significant increase in pro-inflammatory cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8) [Shi et al., 2008b] as well as increased cell viability following an ES of 50 mV/mm for 24 h to human skin fibroblasts [Shi et al., 2008a].
Inflammation is a normal step in wound healing during which angiogenesis takes place and enzymes are released to attack cell debris and foreign materials. Being able to upregulate inflammation in a controlled fashion is therefore highly desirable to study the angiogenesis inside scaffold implants and the degradation of the scaffold. The electrical current or voltage used in our experiment has been in the range of microamps and millivolts, which cannot and should not be compared with the parameters used in non-invasive bone stimulators. In our method, cells are in direct contact with a semiconductive substrate, in contrast to non-invasive stimulators where a very high voltage is required to generate an electrical field across dielectric substances (such as air) and penetrate tissues before reaching target cells. The electric potential gradients used in our conductive polymer-mediated experiments were originally identified by our group and are also within the wide range of reported values [Meng et al., 2011].
We have also demonstrated the enhanced electrical stability of PPy by using heparin as the dopant in PPy synthesis to improve the durability of this conductive composite [Meng et al., 2008], thus enabling long-term ES in applications such as bone regeneration. The heparin molecules not only increased the electrical stability of PPy but also improved cell adhesion to the conductive composites.
Pure PPy has been reported to be an excellent substrate for bovine bone marrow stromal cell attachment, proliferation and differentiation [Shastri and Pishko, 1998], in which the PPy film serves as an anode by applying a constant electrical field of 20 V/m for 1 h. For osteoblasts, however, there have been no reports on conductive polymer-mediated ES experiments. The objective of this study was to demonstrate that ES mediated through a conductive polymer substrate is a valid approach to modulate osteoblast growth for bone regeneration.
MATERIALS AND METHODS
Preparation of the Conductive Biodegradable PPy/HE/PLLA Membranes
The conducting PPy/HE particles were chemically synthesised, carefully washed and blended with the PLLA solution at a weight ratio of 5:95 (PPy:PLLA) to cast biodegradable conductive membranes [Meng et al., 2008]. Briefly, the conducting PPy/HE particles were synthesised using H2O2 and FeCl3 (Laboratoire Mat, Quebec City, QC, Canada) as the oxidant (Fenton's reagent) in a water-in-oil (chloroform; 3:7) emulsion system containing heparin (EMD; Biosciences, La Jolla, CA). The ratio of Fe3+:pyrrole:H2O2 was 2:1:2. Dodecylbenzenesulfonic acid sodium salt (DBS; Sigma–Aldrich, St. Louis, MO) was used as the emulsifier at a 1% concentration. The PPy/HE/PLLA membranes (5% PPy in weight) were prepared by blending PPy/HE particles with PLLA (Hycail, Noordhorn, The Netherlands) in a chloroform solution and casting onto a polytetrafluoroethylene (PTFE) plate, followed by washing and thorough drying.
Electrical Cell Culture Device
Figure 1 illustrates the cross section of one cell culture well of a homemade eight-well cell culture plate. The well was constructed by pressing a four-walled poly(methyl methacrylate) (PMMA) hollow cubic block (2.0 × 2.0 × 3.0 cm3) against a large PMMA substrate, forming a cubic well. A conductive PPy/HE/PLLA membrane was assembled to the bottom of the cubic well, with two ends exposed outside the well for electrical connection. Water tightness between the cubic well and the substrate plate was achieved with the help of a medical-grade polyurethane elastic sheet (Tecoflex 80A, Lubrizol, Wickliffe, OH) between the PPy/HE/PLLA membrane and the PMMA cubic well. The two ends of the conductive membrane exposed outside the well were in contact with two copper electrodes that were wired to a power source and monitoring device. The top of the well was covered while allowing air exchange. For each eight-well plate, four replications were used for the ES (experimental) group and four for the non-ES (control) group, and were considered as one experiment.
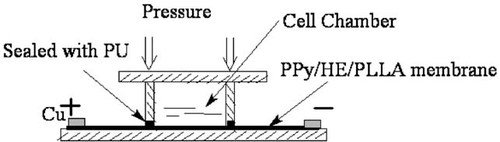
Illustration of the homemade electrical cell culture device.
Compared to previous experiments [Shi et al., 2008a, b], significant changes were made to our homemade electrical cell culture device. The electrodes are now outside the cell culture well, which completely avoids the exposure of electrodes to the culture medium. Thus, neither electrode redox products nor ionic currents would be generated in the culture medium, allowing us to perform long-term continuous ES without worrying about cytotoxic electrolytic products or ionic currents disturbing the culture medium composition.
Cell Culture and Electrical Stimulation
Prior to cell seeding, the PPy/HE/PLLA membranes and the specially designed electrical cell culture plates were sterilised with ethylene oxide (EO) gas for 24 h at 37 °C according to standard industrial procedures. Then, the PPy/HE/PLLA membranes were assembled and pre-incubated in the electrical cell culture devices with Dulbecco's Modified Eagle's Medium (DMEM) for 2 days to wash out any possible leachables from the membranes and to ensure that there was no leakage of the culture medium. The DMEM was refreshed after 24 h. Osteoblast-like Saos-2 cells (ATCC, Manassas, VA) were seeded in 3 ml of medium per chamber on the PPy/HE/PLLA membranes at a density of 50 × 103 cells per chamber (12,500 cells/cm2) and were incubated under 5% CO2 at 37 °C. The cell culture medium was prepared with a 3:1 mixture of Dulbecco–Vogt's Modified Eagle's (DME) medium and Ham's F12 (Invitrogen Life Technologies, Burlington, ON, Canada) supplemented with 24.3 µg/ml of adenine, 10 µg/ml of human epidermal growth factor (Chiron, Emeryville, CA), 0.5 µg/ml of amphotericin B (Sigma–Aldrich Canada, Oakville, ON, Canada), 0.4 µg/ml of hydrocortisone (Calbiochem, La Jolla, CA), 5 µg/ml of bovine insulin, 2 × 10−9 M of 3,3′5′-triiodo-L-thyronine (Schwarz/Mann, Cleveland, OH), 100 U/ml of penicillin, 25 µg/ml of streptomycin (Schering, Pointe-Claire, QC, Canada) and 10% fetal calf serum (NCS; Fetal Clone II, Hyclone, Logan, UT). Prior to applying ES, we allowed the cells to adhere and grow for 48 h without altering the cell culture medium. After the renewal of the culture medium at 48 h, the experimental membranes were connected to a DC constant potential source using electrodes. Four potential intensities were applied to the conductive membranes, that is 100, 200, 300 and 400 mV/mm. The cells were stimulated for 2, 4, 6 and 8 h at each ES intensity. Following exposure to each ES condition, cells were left to recover for another 48 h prior to staining or harvesting. Each control group has the same total cell culture time as its corresponding ES group including the ES period. At least three experiments were performed for each ES condition.
Saos-2 Adhesion on the PPy/HE/PLLA Membranes With ES
Owing to the opaqueness of the conductive membranes, Hoechst staining was used to visualise cell attachment. After cell seeding onto the conductive membrane and culture, the membranes were washed three times with phosphate-buffered saline (PBS) and transferred into six-well plates. Three millilitre Hoechst reagent (Molecular Probes, Eugene, OR) in 1 µg/ml PBS was added to each well. The plates were kept at 20 °C for 15 min before being washed again. The stained cells were observed and photographed using an epifluorescence microscope (Axiophot; Zeiss, Oberkochen, Germany).
Ultrastructural Analysis Using a Scanning Electron Microscope (SEM)
The osteoblasts on the membranes were washed with PBS and then fixed with 4% paraformaldehyde in PBS at 20 °C for 15 min. The cells were washed again in distilled water and then dehydrated in graded ethanol solutions (from 35% to 100%). The specimens were then dried at the ambient temperature and sputter-coated with gold. The surface morphology of the cells was investigated using a JSM-6360LV SEM (JEOL, Tokyo, Japan).
Cell Proliferation Measured With Sulforhodamine B (SRB)
Cell proliferation was analysed by measuring total cell membrane proteins with SRB [Papazisis et al., 1997; Vichai and Kirtikara, 2006; Ricard et al., 2007]. The PPy/HE/PLLA membranes were transferred to Petri dishes and 9 ml of warm culture medium (37 °C) was added to each membrane. Three millilitre of 10% (w/v) cold trichloroacetic acid (TCA) was then used to fix the cells on each PPy/HE/PLLA membrane at 4 °C for 2 h, followed by five washes with deionised water. After air drying, each membrane was incubated in 3 ml of 0.02% (w/v) SRB in 1% acetic acid at room temperature (22°C) for 30 min. The SRB solution was then removed and each membrane was carefully washed five times with 3 ml of 1% acetic acid and air dried. To solubilise the SRB bound to the cell membrane proteins, 2 ml of unbuffered Tris-base (10 mM) solution was added to the test membrane, which was then shaken in a plate shaker for at least 15 min. Thereafter, 6 × 200 µl of solution from each test membrane were transferred to a 96-well plate. The optical density (OD) at 550 nm was determined using a microplate reader (Model 680; Bio-Rad Laboratories, Mississauga, ON, Canada).
Effect of ES on ALP and OC Gene Expression
Following exposure to ES and the recovery period, the cells were trypsinised and collected for ribonucleic acid (RNA) extraction, and for ALP and OC expression analyses. Total cellular RNA content was extracted using the Illustra RNAspin Mini Kit (GE Health Care UK, Buckingham, UK), while RNA concentration, purity and quality were determined by means of the Experion system and RNA StdSens analysis kit according to the manufacturer's instructions (Bio-Rad, Hercules, CA). RNA (1 µg from each condition) was reverse transcripted into cDNA using Maloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen Life Technologies) and random hexamers (Amersham Pharmacia Biotech, Baie d'Urfé, QC, Canada). Reverse transcriptase (RT) conditions were 10 min at 65 °C, 1 h at 37 °C and 10 min at 65 °C. Quantitative polymerase chain reaction (qPCR) was carried out as described [Bahri et al., 2009]. The mRNA transcript quantity was measured using the Bio-Rad CFX96 real-time PCR detection system. Reactions were performed using a PCR supermix from Bio-Rad (iQ SYBR Green supermix). Primers (Table 1) were added to the reaction mix at a final concentration of 250 nM. Five microlitres of each cDNA sample were added to a 20 µl PCR mixture containing 12.5 µl of iQ SYBR Green supermix (Bio-Rad), 0.5 µl of specific primers (ALP and OC; Medicorp, Montréal, QC, Canada) and 7 µl of RNase and DNase free water (MP Biomedicals, Solon, OH). Every reaction was performed in a BioRad MyCycler Thermal Cycler.
| Gene name | GeneBank no. | Primer sequence | Product size (bp) |
|---|---|---|---|
| OC | NM_199173 | Sense: 5′-TAGTGAAGAGACCCAGGCGC-3′ | 107 |
| Antisense: 5′-CACAGTCCGGATTGAGCTCA-3′ | |||
| ALP | NM_000478 | Sense: 5′-GGGAACGAGGTCACCTCCAT-3′ | 72 |
| Antisense: 5′-TCGTGGTGGTCACAATGCC-3′ | |||
| GAPDH | NM_002046 | Sense: 5′-GGTATCGTCGAAGGACTCATGAC-3′ | 188 |
| Antisense: 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′ |
For the qPCR, the cycle threshold (CT) was automatically determined using the accompanying Bio-Rad CFX manager. Thermocycling conditions for the ALP were 5 min at 95 °C, followed by 45 cycles at 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s, with each reaction performed in triplicate. For the OCs, the thermocycling conditions were 95 °C for 3 min, followed by 45 cycles at 95 °C for 10 s, 63 °C for 10 s and 72 °C for 30 s, with each reaction also performed in triplicate. The specificity of each primer pair was determined by the presence of a single melting temperature peak. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) produced uniform expression levels varying by <0.5 CTs between sample conditions and was therefore used as a reference gene for this study. Data were analysed using the CT mean to obtain the normalised fold induction.
Measurement of ALP Activity and OC Production
Because ES modulates osteoblast growth, we deemed it necessary to examine the effect of ES on osteoblast-specific markers such as ALP and OC. In this context, we investigated ALP mRNA expression following exposure to 200 and 400 mV/mm of ES. ALP enzymatic activity of the cells cultured in the presence and absence of ES for 2, 4, 6 and 8 h was measured by reading the p-nitrophenyl phosphate (PNPP) hydrolysis kinetics in the presence of ALP, according to the manufacturer's instructions (Thermo Fisher Scientific, Rockford, IL). To do so, the culture medium was removed and the membranes were rinsed twice with 1 ml PBS, followed by lysing the cells with 0.5 ml of 0.1% Triton X-100. The lyasate (25 µl) reacted with 100 µl of PNPP solution at room temperature (22 °C) for 30 min before the addition of 50 µl of 2 M NaOH to stop the reaction. The absorbance at 415 nm was measured using the Bio-Rad microplate reader.
To quantify the OC protein levels, supernatants collected from cell cultures were analysed by sandwich enzyme-linked immunosorbent assay (ELISA). Once the collection of supernatants was cleared by centrifugation, we measured the OC in triplicate using an ELISA kit (Invitrogen, Camarillo, CA) according to the manufacturer's instructions. To do so, we performed quantitative analyses using 50 µl of diluted or undiluted supernatants. Following incubation, the plates were read at 450 nm using the Bio-Rad microplate reader. The minimum detectable concentration was under 0.4 ng/ml, according to the manufacturer. The experiments were repeated four times and the mean ± standard deviation (SD) are presented.
Statistical Analysis
All experiments were performed at least three times. Experimental values are given as mean ± SD. Data-significant comparisons were performed using the one-way ANOVA test. Significance was defined as a P-value < 0.05.
RESULTS
Cell Adhesion on the Conductive Membrane
Under an electrical potential gradient of 200 mV/mm, Hoechst staining (Fig. 2) showed apparently similar cell density and distribution on the conductive substrates for all ES exposure periods, that is 2, 4, 6 and 8 h, which were also similar to that on the conductive substrates cultured in the same homemade plate but with no ES applied (Fig. 2). For the cells exposed to a high electrical potential gradient at 400 mV/mm, there were more cell aggregations compared to the cells at 200 mV/mm.
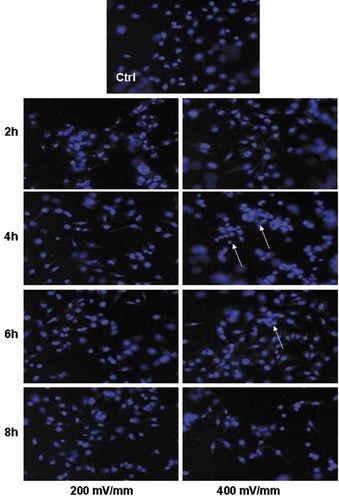
Saos-2 cell adhesion on the PPy/HE/PLLA membranes at 2–8 h with ES of 200 and 400 mV/mm, showing the comparable cell distribution and density on the membranes at 200 mV/mm ES and the non-ES controls (Ctrl), and the aggregation of cells at 400 mV/mm (arrows; original magnification: 300×).
In the ultrastructural analysis, as shown in Figure 3, cells attached and spread on both ES and non-ES groups. Compared with the non-ES group, the cells at 200 mV/mm elongated and spread normally, with the frequent formation of long pseudopods. This shows that cell attachment and spreading were not affected throughout the 8 h ES at 200 mV/mm. In contrast, cells under high ES intensity of 400 mV/mm frequently appeared round and revealed fewer and shorter pseudopods, showing a clear impact of the high ES on cell morphology and ultrastructure.
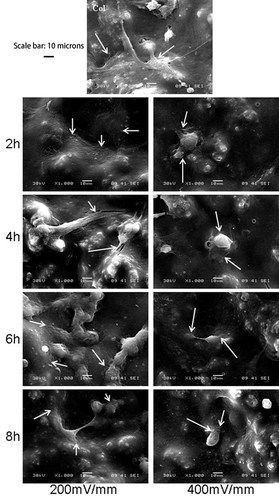
SEM photomicrographs showing normal Saos-2 cell attachment and spreading on the PPy/HE/PLLA surface at 200 mV/mm (arrows), and round cells and less pseudopodia at 400 mV/mm (arrows).
ES Modulated Osteoblast Proliferation
The OD of the ES groups was normalised against their respective control groups, which was considered as 1 (Fig. 4). At the electrical field strength of 100 mV/mm, cell proliferation was only mildly affected, with no significant difference (P > 0.05) compared to that observed in the non-ES control (Fig. 4A). A significant upregulation of Saos-2 growth was obtained at 6 h with 200 mV/mm ES and the doubling time of Saos-2 is about 43 h. Indeed, after only 48 h of ES, the Saos-2 proliferation became 120% higher than the non-ES control group [P ≤ 0.05, degree of freedom (D.F.) = 12; Fig. 4B]. However, after exposure to a high ES intensity of 300 mV/mm, Saos-2 growth was significantly downregulated as early as 2 h, to around 85–80% of control between 2 and 6 h and to around 60% of control at 8 h (P ≤ 0.05 at 2, 4 and 6 h, P ≤ 0.01 at 8 h, D.F. = 12). At 400 mV/mm, the downregulation of cell growth became even more significant after only 2 h of ES; the OD was around 80% of the control (Fig. 4D). This further decreased to close to 60% of the control after an exposure of 6 h (Fig. 4D). Our data therefore suggest that only optimal intensity ES promoted Saos-2 growth, whereas a high intensity ES reduced cell growth.
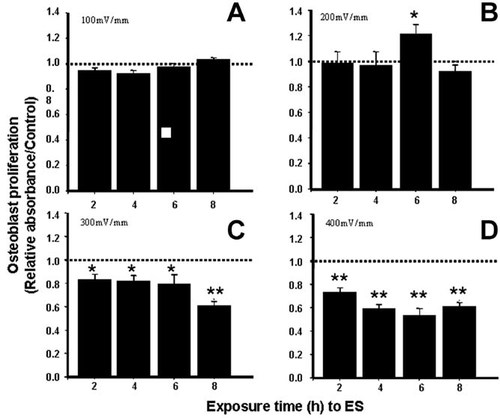
Osteoblast proliferation measured with sulforhodamine B after various ES conditions showing (A) unchanged cell proliferation at 100 mV/mm for all the stimulation periods; (B) significantly upregulated cell proliferation at 200 mV/mm for 6 h and (C,D) significantly downregulated proliferation at ≥300 mV/mm (n = 12). Total culture period: 4 days plus ES. ES tests are normalised against corresponding non-ES controls. Dashed lines represent the respective controls (*P ≤ 0.05, **P ≤ 0.01).
ES Modulated Osteoblast Expression and Production of ALP
As shown in Figure 5, exposure to ES downregulated ALP mRNA expression. At 200 mV/mm, the most significant effect (P ≤ 0.05) was observed after exposure for 2 and 4 h, while a longer exposure had no significant effect. At 400 mV/mm of ES, no significant effect on ALP gene activation/inhibition was observed (P > 0.05, D.F. = 12).
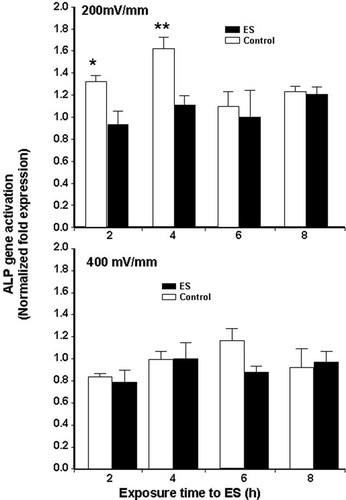
ALP gene expression measurement by real time RT-PCR, showing that ALP gene activation was not detected 2 days after ES (*P ≤ 0.05, **P ≤ 0.01).
We sought to determine whether ES modulated ALP production because moderate ES intensity (200 mV/mm) reduced ALP mRNA expression after 2 and 4 h. Measurements were performed by means of a pNPP assay. As shown in Figure 6, the ALP concentration (OD values) significantly increased (P ≤ 0.05) after 6 and 8 h of exposure to 200 mV/mm. However, when osteoblasts were exposed to 400 mV/mm, ALP activity was significantly reduced (P ≤ 0.05) after only 4 h. The data suggest that a low intensity ES promoted ALP production by osteoblasts, whereas a high intensity ES had no effect or slightly downregulated ALP production.
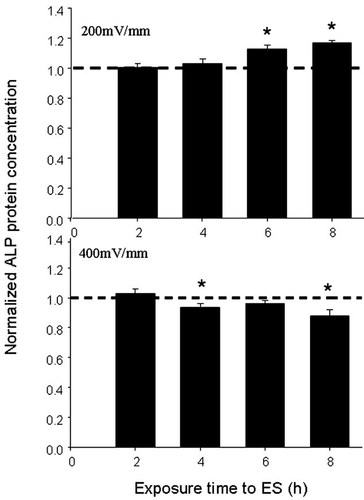
ALP protein secretion measurement by ELISA, showing significantly increased ALP activity at 200 mV/mm (∼120%) and inhibited activity at 400 mV/mm. ES tests are normalised against corresponding non-ES controls (*P ≤ 0.05).
ES Promoted Osteoblast Expression and Production of OC
OC is an osteoblast-secreted, non-collagenous protein present in bone and dentin. Because of its active role in bone mineralisation [Hauschka and Wians, 1989], OC is often used as a marker in the bone formation process. Because ES may play an active role in the bone formation process, we investigated the effect of ES on OC mRNA expression. Unstimulated osteoblasts expressed a basal OC level (Fig. 7). Upon exposure to ES at 200 mV/mm, OC mRNA expression was indeed modulated. At the early exposure times (2 and 4 h), its mRNA expression remained unchanged compared to that of the controls, whereas at 6 and 8 h of exposure the OC gene was significantly (P ≤ 0.05) activated. An exposure of 400 mV/mm had no significant effect on OC mRNA expression.
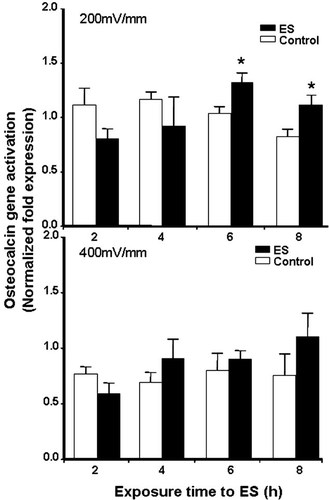
OC gene expression measurement by real time RT-PCR, revealing that OC gene expression was significantly upregulated at 200 mV/mm but not at 400 mV/mm (*P ≤ 0.05).
To determine whether the effect of ES on OC mRNA expression was reflected in the secretion of proteins, we measured OC levels in the culture supernatants and found that Saos-2 constitutively secreted approximately 25 pg/ml of OC (Fig. 8). Upon exposure to ES at 200 mV/mm, the OC level was upregulated. Indeed, OC concentration increased to 40 pg/ml (160%, P ≤ 0.01) following exposure to ES for 2 h. This upmodulation was also found at 4 and 8 h of stimulation, where the concentration of OC increased from 35 pg/ml in the control to almost 45 pg/ml in the ES-exposed cells at 8 h (129%, P ≤ 0.01). At a high ES (400 mV/mm), the OC was apparently reduced with no significant difference. Overall, these results show that ES at 200 mV/mm upregulated OC gene activation and promoted OC secretion by osteoblasts.
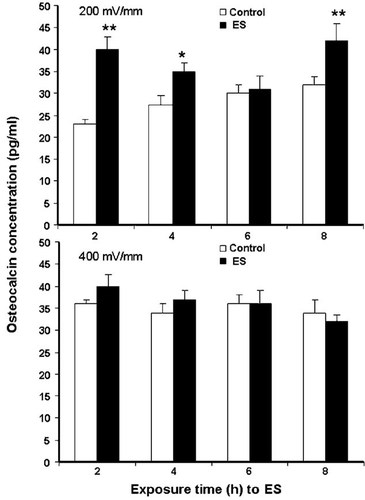
OC protein secretion analysis by ELISA, showing that OC secretion was strongly upregulated at 200 mV/mm (∼160% at 2 h and 129% at 8 h) and was not significantly affected at 400 mV/mm (*P ≤ 0.05, **P ≤ 0.01).
DISCUSSION
One of the major issues in electrically stimulated cell cultures is determining the optimal ES parameters, which may include intensity, duration, frequency and form. With the understanding that these parameters may be cell type- and indicator-specific, we sought to identify an effective ES “window” for bone cells using Saos-2 as the model and proliferation as the primary indicator. The results in Figure 2 clearly indicate a significant increase in cell proliferation after 6 h of ES at 200 mV/mm intensity for the Saos-2 cells cultured on the conductive PPy/HE/PLLA membranes. Furthermore, when the potential gradient was as high as 300 and 400 mV/mm, cell proliferation became suppressed (Fig. 4) compared to that observed in the control groups, regardless of the duration of the ES. On the other hand, a weak ES, such as 100 mV/mm, showed no significant impact on cells. However, this does not exclude the possibility that a longer stimulation time or a multiple ES at 100 mV/mm could be effective. As confirmed in Figure 3, our study shows that an ES “window” does in fact exist for osteoblasts, probably in the range between >100 and <300 mV/mm, where positive modulation of cell proliferation is indeed possible. Furthermore, cell proliferation can be negatively modulated using a potential gradient higher than 300 mV/mm. Therefore, the optimal exposure time of the cells to ES may be approximately 6 h; nevertheless, precaution should be exercised in different experimental settings because cell and tissue response to ES depends not only on intensity and duration but also on other parameters such as frequency and the nature of the ES (electrical, ionic or electromagnetic) [Aaron et al., 2006].
ALP is one of the most important osteoblast markers and is specific to the maturation period of the extracellular matrix. This marker catalyses the hydrolysis of phosphomonoester bonds and plays a crucial physiological role in the metabolism of phosphoethanolamine, inorganic pyrophosphate and pyridoxal 5′-phosphate [Whyte et al., 2002]. ALP is also regarded as a key player in bone mineralisation [Aubin, 1998]. In this context, we used different ES intensities to show that ES significantly upmodulated ALP production following 6 and 8 h of exposure at 200 mV/mm and downmodulated it at 400 mV/mm (Fig. 6). Previous studies have also reported an upregulation of ALP following exposure of ROS 17/2.8 cells to an electrical field [Vander Molen et al., 2000], although this was not carried out on a conductive scaffold. The undetected ALP gene activation was probably because of the measuring time, 2 days following ES, which might not be optimal to detect ALP gene activation.
As one of the osteoblast-specific genes and a late-stage marker of bone cell differentiation, OC is the one of the most abundant proteins present in bone, second only to collagen type I [Hauschka and Wians, 1989]. With its ability to bind calcium ions (Ca2+) and hydroxylapatite, OC is thought to play an important role in the mineralisation phase of the bone formation process. Moreover, studies have reported that it was significantly upregulated in both extracellular matrix synthesis and mineralisation periods [Hauschka and Wians, 1989; Stein et al., 1996; Ryoo et al., 1997]. The gene expression and production of this key bone protein were upregulated following exposure to 200 mV/mm, while no significant effect was detected at 400 mV/mm.
In the literature, both the positive and negative effects of electrical cues on bone protein production have been reported. For example, Nakamura et al. [2009] found increased OC expression in the gaps between electrically prepolarised hydroxyapatite (HA) bone plates and cut cortical bone surfaces, particularly in the vicinity of the latter following exposure to the polarised surface charges, and concluded that the static electric charge on the HA surface induced OC expression. On the other hand, Denaro et al. [2008] showed that a continuous static electromagnetic field for 3–14 days downregulated the expression of Rous-2 and collagen type I, with no effect on OC expression. Although these studies all involve electrical cues, their results are not necessarily comparable because of the differences in the type of electrical cues used and how they were applied and sensed by cells or the extracellular matrix.
According to Curtze et al. [2004], ES could disrupt the transmembrane potential and the subsequent opening of calcium channels. As a result, the change in intracellular Ca2+ would lead to dramatic structural cytoskeleton remodelling and changes in contractile forces showing as cellular traction. However, cells may not respond to ES in the first minutes after the application of an electric field. Cellular traction occurs, on average, 10 min after ES and this period varies widely depending on the ES intensity. The 100–400 mV/mm ES intensity used in this study is close to the value used by Brighton's group [Brighton et al., 2001; Wang et al., 2006]. In their studies, they harvested cells 48 h following ES.
We must thus take a closer look at the molecular mechanisms in terms of how cells and the extracellular matrix detect and react to electrical cues applied through various approaches such as a constant DC electric field, pulsed electromagnetic field and ionic electric field. Only molecular-level quantitative research may possibly unify the effect of the apparently very different ES processes. In fact, studies in recent years focusing on cell membrane sensory proteins have probably elucidated part of these mechanisms. It is well known that cellular activities are tightly related to the transmembrane potential, which normally lies at about 70 mV [Lodish et al., 2000]. The cell transmembrane potential is regulated by various membrane sensory proteins, such as “voltage-sensing proteins” reportedly responsive to an exogenous electrical field [Bezanilla, 2008]. The reported effect of ES on calcium channels is probably mediated by those surface voltage sensing proteins. It is well known that a flux of Ca2+ through the cell membrane has a significant impact on various cellular behaviours. The conformation of membrane receptors may change upon exposure to the electric field, similar to responding to signalling molecules, causing the downstream biological reactions. Because receptor–ligand interaction is, at the molecular level, electrical by nature (interaction of electrons), we may hypothesise that the specificity of receptor–ligand interactions could be replaced by a group of specific EF parameters such as frequency and intensity. Future studies should ideally involve molecular biologists and electrical engineers/physicists to calculate or measure the key electrical parameters (electric field, magnetic field, electric current and ionic current) of the cell membrane, and how membrane proteins are affected by those parameters. Finally, primary osteoblasts are preferred in future studies because they are more relevant to normal bone healing.
The scaffold is a critical component in tissue engineering-based bone regeneration. This work demonstrated that an electrically conductive scaffold may provide a unique approach to introduce the electrical field as a cue to modulate osteoblasts and hopefully enhance bone repair and bone regeneration in the future.
CONCLUSION
This study showed that ES through a conductive scaffold modulates osteoblast activity. An optimal DC potential gradient of 200 mV/mm, with an exposure time between 2 and 8 h, upregulates osteoblast proliferation and the expression and production of its markers specific to the maturation (ALP) and mineralisation (OC) stages of bone formation. These results highlight the possibility of combining ES and conductive scaffolds as a new strategy in bone tissue engineering. Further investigations will better define the cellular and molecular mechanisms involved in how cells and the extracellular matrix sense electrical cues.
Acknowledgements
The authors would like to thank Séverine Curt for her technical assistance in the biological experiments.




