Exposure system to study hypotheses of ELF and RF electromagnetic field interactions of mobile phones with the central nervous system
Abstract
A novel exposure system for double-blind human electromagnetic provocation studies has been developed that satisfies the precision, control of fields and potential artifacts, and provides the flexibility to investigate the response of hypotheses-driven electromagnetic field exposure schemes on brain function, ranging from extremely low frequency (ELF) to radio frequency (RF) fields. The system can provide the same exposure of the lateral cerebral cortex at two different RF frequencies (900 and 2140 MHz) but with different exposure levels at subcortical structures, and also allows uniform ELF magnetic field exposure of the brain. The RF modulation and ELF signal are obtained by a freely programmable arbitrary signal generator allowing a wide range of worst-case exposure scenarios to be simulated, including those caused by wireless devices. The maximum achievable RF exposure is larger than 60 W/kg peak spatial specific absorption rate averaged over 10 g of tissue. The maximum ELF magnetic field exposure of the brain is 800 A/m at 50 Hz with a deviation from uniformity of 8% (SD). Bioelectromagnetics 33:527–533, 2012. © 2012 Wiley Periodicals, Inc.
Previous research has shown that 30 min exposure to pulse-modulated radiofrequency (RF) electromagnetic fields (EMF) approximating the exposure from mobile phones affects the sleep electroencephalogram (EEG) when applied prior to sleep [Regel et al., 2007; Schmid et al., 2012]. Significant effects have been observed for pulse-modulated exposures below peak spatial specific absorption rates (psSAR) of 2 W/kg (whole head), where the brain and skin temperature increase is estimated to be about 0.14 and 0.4 °C and remains below 0.25 and 0.6 °C, respectively [Hirata and Shiozawa, 2003]. No effects were observed when continuous wave (CW) fields with identical psSAR values were applied [Huber et al., 2002].
The objective of this study was to develop an advanced exposure system to empower researchers to investigate a number of hypotheses derived from the above-mentioned body of experimental evidence. Specifically, this includes the hypotheses that biological responses to RF exposure depend on the modulation frequency or time-domain characteristics of the modulation, peak-to-average ratios (PAR), intermittency, exposed functional subregions of the central nervous system (CNS; e.g., cortex vs. subcortical brain regions), and combined RF and extremely low frequency (ELF) magnetic field exposures. The developed system has to comply with the minimal requirements of reproducibility [Kuster et al., 2004]. A comparison of the exposure pattern between an actual mobile phone and a patch antenna can be found elsewhere [Boutry et al., 2008].
The exposure system is based on planar patch antennas (SPA860/65/9/0/V and SPA2000/80/8/0/V, Huber + Suhner, Herisau, Switzerland), which are used for the carrier frequencies of 900 and 2140 MHz, and are fixed on low reflecting, low-loss stands (wood) at a height of 130 cm from the floor. In order to blind the person and supervisor, the 900 and 2140 MHz antennas were mounted inside closed identical boxes of low dielectric material (εr = 2.5, σ < 0.15 mS/m). These boxes were then fixed together with Helmholtz-like coils for ELF exposure on the wooden stand (Fig. 1). The subject's head is supported by styrofoam mounts so that the ear canal is situated horizontally at the center and vertically 42 mm below the center of the antenna patch. The distance between the antenna and the head is 110 mm (900 MHz) and 180 mm (2140 MHz). The ELF exposure is realized using two Helmholtz-like coils on each side of the head. Although their rectangular shape (330 mm × 390 mm) and higher separation distance (257 mm ± 20 mm, depending on the subject's anatomy) is different from the original Helmholtz design, sufficient homogeneity of the magnetic field is achieved (Fig. 2). The ear canal is 82 mm below the center line between the coils. The electric current through the dual windings (2 × 23 windings per coil) is either parallel or anti-parallel for ELF exposure and sham exposure, respectively. The coils are powered by a voltage-controlled current source (VCCS, SPEAG, Zurich, Switzerland).
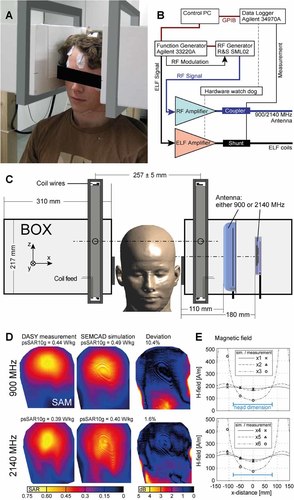
A: Subject within the exposure system with attached Helmholtz-like coils. B: Schematic of the exposure system hardware. C: Boxed antenna enclosures with antenna positions (either the 900 or 2140 MHz antenna), coil housings, and “Ella” [Christ et al., 2010]. D: SAM head surface SAR exposure comparison of measurements and simulations. E: Simulated and measured values of the ELF magnetic field (measurement positions ×1–×6 indicated in Fig. 2).
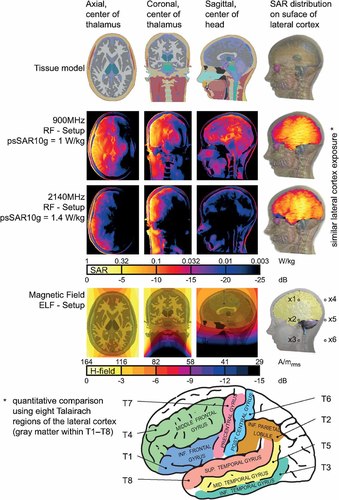
Discretized tissue model, SAR and magnetic field distribution in three orthogonal cuts, and brain schematic with selected Talairach regions (T1–T8). Maximum SAR is not present in depicted slices. Illustration shows RF exposure of the left hemisphere.
The signal for the RF modulation envelope and the ELF magnetic field is generated by an arbitrary function generator (Agilent 33220A, Agilent Technologies, Santa Clara, CA), allowing any 14-bit, 50 MSa/s, 64 k-point waveform. An RF generator is used for modulations up to 50 kHz (SML02, Rohde & Schwarz, Munich, Germany). The ELF current source allows frequency components up to 1.3 kHz, which is sufficient to simulate the main ELF components generated by the currents in mobile phones [Ebert, 2009].
The whole system is computer controlled and monitored using a custom-made software package written in C++. The applied RF power is controlled via bi-directional couplers and calibrated Schottky diodes (ACSP-2663NZC15, Advanced Control Components, Eatontown, NJ). A set of randomized exposure conditions can be defined to allow a fully double-blind protocol. Forward and reflected power is continuously monitored, controlled, and logged (10 s sampling). The system aborts immediately if the expected forward power is exceeded by more than 10%. A watchdog safety hardware circuit prevents exposure in case of a software failure, and RF amplifiers operate 1 dB below their maximum output power.
Numerical dosimetry and optimization were conducted within the simulation platform SEMCAD X V13.4 (SPEAG), applying the finite-difference time-domain (FDTD) solver in combination with the SEMCAD extension for the analysis of exposure levels at functional subregions of the brain (1105 regions in the Talairach space) [Crespo-Valero et al., 2011]. The reference and uncertainty analyses were performed with the 26-year-old female “Ella” from the Virtual Family [Christ et al., 2010; www.itis.ethz.ch/vip]. The dielectric tissue properties were assigned according to literature values that have recently been updated [Hasgall et al., 2011]. The computational space consisted of approximately 60 million voxels (10 million voxels for the head), with a minimal voxel size of 0.2 mm (x, y, z direction) in the antenna and a maximum size of 0.9 mm in the head. For numerical variability evaluations, two additional anatomical head models were used: the 34-year-old male adult “Duke” [Christ et al., 2010] and the 40-year-old European female “HR-EF1”, with a coarser than state-of-the-art resolution of 1 mm in the ear region and 3 mm for the rest of the head. The latter was included for comparison with the precedent exposure system described by [Huber et al., 2003]. The magneto quasi-static low frequency solver of SEMCAD X was applied to analyze the ELF magnetic field coil system, using a 2.5 mm grid resolution and low frequency current sources for the two coils.
Experimental validation was performed by comparing the RF exposure of the specific anthropomorphic mannequin (SAM) in simulations with measurements obtained from the near-field scanner DASY5 NEO (SPEAG) equipped with a calibrated dosimetric SAR probe (ET3DV6, SPEAG) and tissue-simulating liquid (HSL-U10, SPEAG). The ELF magnetic field was validated using a gauss meter equipped with a temperature-regulated magnetic field probe (FH49 with HS-ZOA71-3208-05-T, Magnet-Physik, Cologne, Germany). Comparison results are shown in Figure 1.
The dosimetric results of the RF system are summarized in Table 1. The configuration was optimized to achieve a similar exposure at the level of the lateral cerebral cortex with a maximal exposure difference in the thalamus. This was obtained by selecting the two carrier frequencies of 900 and 2140 MHz, and optimizing for minimal differences between the average SAR induced in eight selected Talairach regions (grey matter within T1-T8; Fig. 2) by varying the distance of the patch antennas from the head. The best results were achieved with distances of 110 mm (900 MHz) and 180 mm (2140 MHz), with a psSAR10g ratio of 1.4 (e.g., values of 1 W/kg at 900 MHz and 1.4 W/kg at 2140 MHz). The optimization resulted in an average SAR in the thalamus that was eight times lower at 2140 MHz than at 900 MHz, while maintaining a similar cortex exposure. Figure 2 illustrates the SAR distribution for both carrier frequencies in three orthogonal cuts. The experimental validation resulted in differences well within the combined numerical and experimental uncertainty (see also Fig. 1).
| Both hemispheres | Exposed hemisphere | Non-exposed hemisphere | Variatione | Uncertaintye | Talairach regionsf | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ga | Av.b | SDc | Lossd | 1 g | Av. | SD | Loss | 1 g | Av. | SD | Loss | 1 g | Av. | 1 g | Av. | No | Av. | Var | Unc | |
| W/kg | W/kg | W/kg | mW | W/kg | W/kg | W/kg | mW | W/kg | W/kg | W/kg | mW | % | % | % | % | W/kg | % | % | ||
|
System [Huber et al., 2003 ] |
Ella, psSAR10g = 1 W/kg, 900 MHz | T1 | 0.33 | 15 | 3 | |||||||||||||||
| Grey matter | 1.06 | 0.16 | 0.2 | 116 | 1.06 | 0.29 | 0.22 | 102 | 0.18 | 0.04 | 0.03 | 14 | 12 | 20 | 5 | 3 | T2 | 0.44 | 22 | 4 |
| White matter | 0.72 | 0.12 | 0.13 | 51 | 0.72 | 0.2 | 0.13 | 44 | 0.12 | 0.03 | 0.03 | 7 | 17 | 27 | 3 | 5 | T3 | 0.71 | 18 | 2 |
| Grey + White matter | 1.02 | 0.15 | 0.18 | 167 | 1.02 | 0.26 | 0.2 | 146 | 0.18 | 0.04 | 0.03 | 21 | 12 | 21 | 7 | 3 | T4 | 0.17 | 17 | 5 |
| Thalamus | 0.31 | 0.18 | 0.07 | 2 | 0.31 | 0.23 | 0.06 | 2 | 0.15 | 0.13 | 0.02 | 0.9 | 35 | 35 | 15 | 16 | T5 | 0.64 | 16 | 3 |
| Brain (without CSF)g | 1.32 | 0.15 | 0.18 | 201 | 1.32 | 0.26 | 0.2 | 174 | 0.18 | 0.04 | 0.04 | 27 | 21 | 22 | 4 | 4 | T6 | 0.36 | 18 | 5 |
| Brain (with CSF) | 1.33 | 0.18 | 0.24 | 284 | 1.33 | 0.3 | 0.28 | 241 | 0.29 | 0.05 | 0.05 | 43 | 16 | 22 | 4 | 3 | T7 | 0.28 | 17 | 3 |
| Total head | 1.72 | 0.13 | 0.24 | 605 | 1.72 | 0.23 | 0.31 | 525 | 0.29 | 0.04 | 0.05 | 80 | 16 | 16 | 8 | 1 | T8 | 0.57 | 16 | 2 |
| 900 MHz system | Ella, psSAR10g = 1 W/kg | T1 | 0.37 | 15 | 3 | |||||||||||||||
| Grey matter | 1.06 | 0.19 | 0.22 | 135 | 1.06 | 0.33 | 0.24 | 117 | 0.22 | 0.05 | 0.04 | 18 | 12 | 20 | 5 | 3 | T2 | 0.69 | 22 | 4 |
| White matter | 0.72 | 0.14 | 0.14 | 61 | 0.72 | 0.24 | 0.14 | 51 | 0.16 | 0.04 | 0.04 | 10 | 17 | 27 | 3 | 5 | T3 | 0.64 | 18 | 2 |
| Grey + White matter | 1.03 | 0.17 | 0.2 | 196 | 1.03 | 0.3 | 0.21 | 168 | 0.21 | 0.05 | 0.04 | 28 | 12 | 21 | 7 | 3 | T4 | 0.25 | 17 | 5 |
| Thalamus | 0.37 | 0.21 | 0.07 | 3 | 0.37 | 0.25 | 0.08 | 2 | 0.18 | 0.17 | 0.02 | 1.2 | 35 | 35 | 15 | 16 | T5 | 0.66 | 16 | 3 |
| Brain (without CSF) | 1.19 | 0.17 | 0.19 | 228 | 1.19 | 0.29 | 0.21 | 193 | 0.21 | 0.05 | 0.04 | 35 | 21 | 22 | 4 | 4 | T6 | 0.59 | 18 | 5 |
| Brain (with CSF) | 1.42 | 0.2 | 0.27 | 327 | 1.42 | 0.34 | 0.32 | 272 | 0.42 | 0.07 | 0.07 | 55 | 16 | 22 | 4 | 3 | T7 | 0.42 | 17 | 3 |
| Total head | 1.55 | 0.14 | 0.26 | 648 | 1.55 | 0.24 | 0.33 | 549 | 0.42 | 0.04 | 0.06 | 99 | 16 | 16 | 8 | 1 | T8 | 0.63 | 16 | 2 |
| 2140 MHz system | Ella, psSAR10g = 1.4 W/kg (value optimized for similar outer cortex exposure) | T1 | 0.25 | 36 | 15 | |||||||||||||||
| Grey matter | 1.13 | 0.13 | 0.20 | 90 | 1.13 | 0.24 | 0.22 | 83 | 0.08 | 0.01 | 0.01 | 7 | 16 | 38 | 6 | 7 | T2 | 0.56 | 37 | 3 |
| White matter | 0.56 | 0.07 | 0.10 | 31 | 0.56 | 0.13 | 0.11 | 28 | 0.06 | 0.01 | 0.01 | 3 | 15 | 39 | 6 | 12 | T3 | 0.43 | 14 | 3 |
| Grey + White matter | 1.02 | 0.11 | 0.17 | 120 | 1.02 | 0.20 | 0.20 | 111 | 0.08 | 0.01 | 0.01 | 10 | 14 | 38 | 6 | 9 | T4 | 0.25 | 35 | 9 |
| Thalamus | 0.07 | 0.03 | 0.02 | 0 | 0.07 | 0.04 | 0.02 | 0 | 0.02 | 0.01 | 0.01 | 0 | 45 | 60 | 10 | 11 | T5 | 0.52 | 27 | 7 |
| Brain (without CSF) | 1.02 | 0.10 | 0.17 | 132 | 1.02 | 0.18 | 0.20 | 122 | 0.08 | 0.01 | 0.01 | 10 | 14 | 37 | 6 | 7 | T6 | 0.50 | 37 | 6 |
| Brain (with CSF) | 1.29 | 0.13 | 0.21 | 195 | 1.29 | 0.22 | 0.27 | 175 | 0.24 | 0.03 | 0.04 | 20 | 21 | 37 | 13 | 8 | T7 | 0.36 | 37 | 9 |
| Total head | 3.72 | 0.17 | 0.42 | 778 | 3.72 | 0.31 | 0.56 | 717 | 0.34 | 0.03 | 0.06 | 62 | 10 | 26 | 4 | 4 | T8 | 0.48 | 35 | 11 |
- a Peak spatial SAR averaged over a 1 g cube of tissue (psSAR1g).
- b Tissue averaged SAR.
- c Standard deviation of the averaged SAR.
- d Total power losses.
- e Variation and uncertainty for k = 1 or coverage factor of 66%.
- f Talairach regions according to Figure 2, only grey matter included.
- g Cerebrospinal fluid.
The ELF magnetic field over the volume of the brain has a standard deviation of 8%, except for the marginally exposed medulla oblongata (Fig. 2), and the achievable peak exposures are 800 A/m at 50 Hz and 140 A/m at 1 kHz. Sham exposure (anti-parallel currents) is >43 dB below the ELF exposure. The average deviation between the ELF simulations and measurements remains below 7% (Fig. 1).
All exposures are compliant with the safety guidelines defined by the International Commission on Non-Ionizing Radiation Protection [ICNIRP, 1998], that is, maintaining the basic restriction of 2 W/kg psSAR10g for RF exposures and complying with the summation of the multifrequency ELF field with the corresponding reference levels.
The uncertainty and variability analyses for the dosimetric study were performed following the concept of the National Institute of Standards and Technology (NIST) [Taylor and Kuyatt, 1994]. The uncertainty analysis included: (a) ±10% variation of the tissues' relative permittivity and conductivity; (b) discretization of the head, estimated with half and double voxel sizes; and (c) segmentation of the head anatomy, comparing the left and right hemisphere. The variation analysis included: (a) changes in antenna position relative to the head (±5 mm in x direction, ±10 mm in y, z direction); (b) different head size, assessed by scaling the reference head model (“Ella”) by ±10%; and (c) use of two additional head models, relevant for the representation of the inter-subject and age-related structural differences of the population. The European female model “HR-EF1” made the most significant contribution to the variability (Table 1).
In conclusion, a novel system for double-blind human electromagnetic provocation studies has been developed and fully characterized. It satisfies the precision, control of fields and potential artifacts, and provides the exposure flexibility necessary to investigate the response of hypotheses-driven EMF exposure schemes on brain function. It allows the exposure of the CNS to ELF magnetic fields and RF fields of different carrier frequencies (900 and 2140 MHz). Very similar induced field distributions in the lateral cortex are provided at both radio frequencies and uniform exposure at ELF. Furthermore, modulation and ELF signals are obtained by a freely programmable arbitrary signal generator, allowing a wide range of worst-case exposure scenarios to be simulated with peak RF exposures of up to 60 W/kg and ELF exposures up to 800 A/m at 50 Hz. The system is therefore suitable for testing a wide range of hypotheses of EMF effects on brain function.




