Maternal residential exposure to agricultural pesticides and birth defects in a 2003 to 2005 North Carolina birth cohort
Supported in part by an appointment to the Internship/Research Participation Program at Office of Research and Development (National Center for Environmental Assessment), U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA. This research was supported in part by grants from the National Institute of Environmental Health Sciences (T32ES007018, P30ES010126, R01ES020619), National Birth Defect Prevention Study CDC funds, and the NSF Graduate Research Fellowship Program grant 0646083.
Abstract
Background
Birth defects are responsible for a large proportion of disability and infant mortality. Exposure to a variety of pesticides have been linked to increased risk of birth defects.
Methods
We conducted a case–control study to estimate the associations between a residence-based metric of agricultural pesticide exposure and birth defects. We linked singleton live birth records for 2003 to 2005 from the North Carolina (NC) State Center for Health Statistics to data from the NC Birth Defects Monitoring Program. Included women had residence at delivery inside NC and infants with gestational ages from 20 to 44 weeks (n = 304,906). Pesticide exposure was assigned using a previously constructed metric, estimating total chemical exposure (pounds of active ingredient) based on crops within 500 meters of maternal residence, specific dates of pregnancy, and chemical application dates based on the planting/harvesting dates of each crop. Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals for four categories of exposure (<10th, 10–50th, 50–90th, and >90th percentiles) compared with unexposed. Models were adjusted for maternal race, age at delivery, education, marital status, and smoking status.
Results
We observed elevated ORs for congenital heart defects and certain structural defects affecting the gastrointestinal, genitourinary and musculoskeletal systems (e.g., OR [95% confidence interval] [highest exposure vs. unexposed] for tracheal esophageal fistula/esophageal atresia = 1.98 [0.69, 5.66], and OR for atrial septal defects: 1.70 [1.34, 2.14]).
Conclusion
Our results provide some evidence of associations between residential exposure to agricultural pesticides and several birth defects phenotypes. Birth Defects Research (Part A) 106:240–249, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
Birth defects are a leading cause of infant mortality and childhood and adult disability in the United States (US) (Decoufle et al., 2001; Matthews and MacDorman, 2013). Over 1000 specific birth defects have been identified, encompassing all organ systems. Approximately 1 in 33, or 3%, of births are diagnosed with any anomaly (Mai et al., 2013), although the prevalence of specific birth defects varies considerably. Risk factors for birth defects are largely unknown and may vary by specific defect. Some known notable risk factors are genetics, maternal age, infections, certain medications such as thalidomide, nutritional factors such as lack of folate, and environmental factors such as pesticides (CDC, 2014).
Pesticides are known reproductive and neurotoxic agents, and have been shown to be teratogenic in animals (Shepard and Lemire, 2004). Several epidemiologic studies have found elevated associations between pesticide exposures before or during pregnancy and various birth defects (Kristensen et al., 1997; Shaw et al., 1999, 2014; Garry et al., 2002; Heeren et al., 2003; Rull et al., 2006; Meyer et al., 2006; Ochoa-Acuna and Carbajo, 2009; Agopian et al., 2013a, 2013b; Carmichael et al., 2013, 2014b; Kielb et al., 2014). These studies, along with others that observe null or inverse associations, vary in factors such as birth defects examined, specific pesticides studied, and how pesticide exposure is captured.
The ways in which pesticide exposures have been estimated include: questionnaires, detailed biomarker or dust sampling, and residential proximity. Questionnaire or survey data will often use paternal or maternal occupation, which is easy to acquire but encompasses more potential exposures than just pesticides (Kristensen et al., 1997; Shaw et al., 1999; Garry et al., 2002). Some studies use self-reported use of pesticides at home or work, which has the benefits of being an individual exposure that is relatively simple to acquire, but some people may not know what types or amounts of pesticides they are using (Shaw et al., 1999; Kielb et al., 2014). Persistent pesticide analytes (e.g., glyphosate and maneb) in serum and amniotic samples of pregnant women, and in dust samples from home environments have also been examined (Bradman et al., 1997; Bradman et al., 2003; Barr et al., 2010); these methods are perhaps the most accurate for individual-level exposures. However, these samples are difficult and expensive to get and analyze and have yet to be used to examine associations with birth defects.
Others studies use residential proximity to agricultural cropland or county-level pesticide use estimates, which reflect environmental exposures but may not include detailed pesticide information, adequate spatial resolution, or timing of pesticide use (Meyer et al., 2006; Ochoa-Acuna and Carbajo, 2009; Agopian et al., 2013a, 2013b). In California (CA), pesticide application data have been matched to areas of 1 square km and pesticide exposures around maternal residences were estimated using crop data; these exposures are more accurate than other options but are limited to study populations in CA as such detailed pesticide application data is not available elsewhere (Rull and Ritz, 2003; Rull et al., 2006; Carmichael et al., 2013, 2014b; Shaw et al., 2014; Yang et al., 2014). This method of exposure quantification offers better detail about potential pesticide exposures than survey or occupational data, while being less intensive in terms of cost and effort to acquire.
In this hypothesis-generating study, we examine associations between individual birth defects in a population from North Carolina (NC) and pesticide exposures during preconception and early pregnancy using a metric of pesticide exposure created to balance efficiency with improved quantification of residential proximity to total pesticide applications.
Materials and Methods
Study Population
The study population for our analysis is the same as that used in Warren et al. (2014), and is based on geocoded birth records of 335,729 singleton live births delivered in NC from January 1, 2003, to December 31, 2005. The following were excluded: women with a residence at delivery outside of NC (n = 23,829; 7.10%); births with a gestational age of <20 or > 45 weeks (n = 84; 0.03%); and women missing data on demographic covariates of interest (n = 6910; 2.06%) (Warren et al., 2014). The final population used in the analysis was 304,906 singleton live births.
Outcome
Birth records were linked to the NC Birth Defects Monitoring Program (NCBDMP), an active population based surveillance system that collects information from medical records about all medically diagnosed birth defects through the first year of life in NC (Warren et al., 2014; NCSCHS, 2015). The NCBDMP includes information of specific types of birth defects including neural tube, cardiovascular, gastrointestinal, genitourinary, and musculoskeletal defects; in our analysis 42 birth defect phenotypes were examined as outcomes.
Exposure
Development of the pesticide metric we used as the exposure variable was described previously (Warren et al., 2014). Briefly, NC crop maps were available for years 2002 and 2008 to 2010; crop maps for years 2003 to 2005 were extrapolated from these maps (Warren et al., 2014). A 500-m buffer was created around each residence at birth and the amount of crop acreage within the buffer was calculated (Warren et al., 2014). Pesticide application data from the National Agricultural Statistics Service was combined with earliest planting and last harvesting dates of each crop and active chemical half-life data to create a probable window of application and to determine if that window overlaps with 1 month before conception through the 3rd month of pregnancy (Warren et al., 2014). Finally, quantities and types of pesticide active ingredients applied during the relevant window in each woman's buffer zone were estimated; quantities were summed across types to give a final amount of pounds of pesticides (Warren et al., 2014). As all active ingredients are summed for this analysis, exposure is in pounds of total pesticides. Unexposed women either had no crops within their buffer, or the relevant window of pregnancy occurred during times when no pesticide applications were expected to occur. This method was considered a “hybrid” metric, falling between a simple crop-only exposure metric and a more difficult to compute metric that uses data on chemical/crop specific application dates.
Analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression models. In lieu of testing null hypotheses (OR = 1), we examined patterns of OR estimates and focused on their precision. Cases were those infants with birth defect diagnoses from the NCBDMP, and were categorized based on the Centers for Disease Control and Prevention/British Pediatric Association coding system. Infants with more than one defect could have been categorized into multiple phenotype groups. Cases were analyzed in two ways: any birth having the specified defect under analysis (all cases), wherein other birth defects may also be present, or as a birth having only the birth defect examined (isolated cases). Controls were any infant free of diagnosed birth defects. For hypospadias, analyses were performed only in male infants. Exposures were investigated as a priori specified categories of exposed (<10th percentile, 10–<50th percentile, 50–<90th percentile, and ≥90th percentile) versus unexposed. These categories were chosen due to an interest in the extremes of the exposure distribution, and we also believe they capture the range of exposure with sufficient flexibility around that interest. We identified potential confounders a priori based on previous literature and knowledge of factors influencing birth defects and potential pesticide exposure through maternal residence. From the birth certificate, we included maternal race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, Other), maternal education (less than high school [<HS], high school graduate [HS], and greater than high school [HS]), marital status (married, unmarried), maternal age (<20, 20–<35, ≥ 35), and smoking status (ever, never).
We also performed several sensitivity analyses. We examined a more difficult to compute pesticide metric that estimates earliest and latest possible pesticide application dates based on crop and pest phenology or specified NC information, information that is often generally unavailable (Warren et al., 2014). This was done to examine how the “hybrid” and more accessible metric, used in the main analyses, performs compared with the more informational and computationally intensive metric. Because we lacked information on pesticides applied to tobacco crops, we also examined only women with no tobacco crops in their buffer. Finally, we examined women without diabetes recorded during pregnancy, as some defects may be affected by diabetic status. All analyses were performed in SAS 9.4 (Cary, NC).
Results
Descriptive statistics for the study population are presented in Table 1. There were 298,548 controls and 6358 cases in our analysis; of the cases, 4634 had a single (isolated) congenital anomaly. While race/ethnicity groupings and smoking status were evenly distributed among cases and controls, case mothers had higher proportions of diabetes, unmarried status, and lower educational attainment. The proportion of male infants was higher among cases compared with controls. Case numbers varied from 19 (anencephaly) to 1364 (patent ductus arterious) (Table 2). The median value for the pesticide metric among all the exposed (cases and controls) was 1.60 lbs (10th percentile: 0.06 lbs; 90th percentile: 39.25 lbs). A total of 49,069 controls and 982 cases were considered unexposed (i.e., no pesticides applied to crops, or no crops, within 500 m of maternal residence).
| Controls | Cases | |
|---|---|---|
| Variable | n = 298,548 | n = 6,358 |
| Race/ethnicity | ||
| Non-Hispanic White | 179,856 (60.24) | 3,826 (60.18) |
| Non-Hispanic Black | 65,284 (21.87) | 1,461 (22.98) |
| Hispanic | 41,527 (13.91) | 850 (13.37) |
| Other | 11,881 (3.98) | 221 (3.48) |
| Maternal smoking status | ||
| Non-smoker | 261,483 (87.58) | 5,494 (86.41) |
| Smoker | 37,065 (12.42) | 864 (13.59) |
| Maternal diabetic status | ||
| Non-diabetic | 290,340 (97.25) | 6,075 (95.55) |
| Diabetic | 8,208 (2.75) | 283 (4.45) |
| Infant sex | ||
| Female | 146,146 (48.95) | 2,314 (36.4) |
| Male | 152,402 (51.05) | 4,044 (63.6) |
| Parity | ||
| Primiparous | 123,055 (41.22) | 2,642 (41.55) |
| Multiparous | 175,493 (58.78) | 3,716 (58.45) |
| Maternal educational attainment | ||
| Less than high school | 66,235 (22.19) | 1,532 (24.1) |
| High school | 85,791 (28.74) | 1,910 (30.04) |
| More than high school | 146,522 (49.08) | 2,916 (45.86) |
| Maternal marital status | ||
| Married | 191,015 (63.98) | 3,919 (61.64) |
| Unmarried | 107,533 (36.02) | 2,439 (38.36) |
| Maternal age at delivery | ||
| <20 years | 33,851 (11.34) | 779 (12.25) |
| 20 - < 35 years | 228,261 (76.46) | 4,679 (73.59) |
| 35 or more years | 36,436 (12.2) | 900 (14.16) |
| Birth defect | All cases | Isolated cases | Birth defect | All cases | Isolated cases |
|---|---|---|---|---|---|
| Anencephaly | 19 | 14 | Choanal atresia | 50 | 30 |
| Spina bifida | 101 | 74 | Biliary atresia | 26 | 23 |
| Encephalocele | 22 | 17 | Renal agenesis | 146 | 88 |
| Hydrocephalus | 237 | 151 | Tracheal esophageal fistula/esophageal atresia | 84 | 29 |
| Microcephalus | 158 | 106 | Anorectal stenosis | 144 | 54 |
| Anophthalmia/ microphthalmia | 46 | 13 | Hypertrophic pyloric stenosis | 592 | 551 |
| Congenital cataract | 60 | 41 | Hirschsprung's disease | 77 | 62 |
| Truncus arteriosus | 28 | 6 | Obstructive genitourinary defect | 680 | 482 |
| Transposition of the great vessels | 82 | 7 | Upper limb deficiency | 84 | 41 |
| Tetralogy of Fallot | 123 | 34 | Lower limb deficiency | 39 | 20 |
| Pulmonary valve atresia | 215 | 60 | Gastroschisis | 85 | 71 |
| Tricuspid valve atresia | 46 | 1 | Omphalocele | 37 | 11 |
| Ventricular septal defect | 1110 | 458 | Congenital diaphragmatic hernia | 70 | 24 |
| Atrial septal defecta | 1020 | 323 | Trisomy 13 | 26 | 3 |
| Atrioventricular septal defect/endocardial cushion defectb | 63 | 11 | Trisomy 21 | 327 | 130 |
| Aortic valve stenosis | 67 | 14 | Trisomy 18 | 38 | 10 |
| Coarctation of the aorta | 156 | 30 | Hypospadias | 936 | 856 |
| Hypoplastic left heart syndrome | 75 | 15 | Epispadias | 39 | 30 |
| Ebstein's anomaly | 25 | 5 | Anotia/microtia | 50 | 31 |
| Patent ductus arteriosusc | 1364 | 382 | Amniotic band sequence | 30 | 13 |
| Cleft palate | 166 | 117 | |||
| Cleft Lip with/without Cleft palate | 243 | 194 |
- a Secundum atrial septal defects, separate from patent foramen ovale.
- b Infants diagnosed with Down's syndrome/trisomy 21 were excluded from this case group.
- c In infants with a birth weight >2500 g.
Cardiovascular Defects
ORs and 95% CIs for cardiovascular defects are shown in Figure 1. Patent ductus arteriosis (PDA), in which the fetal blood vessel connecting the pulmonary artery to the aorta fails to close, and atrial septal defect (ASD), a defect in the wall dividing the left and right atria of the heart, had elevated ORs, although only at the highest level of exposure (all cases analysis: ORs [95% Cis] for 90th percentile versus unexposed PDA: 1.50 [1.22, 1.85]; ASD: 1.70 [1.34, 2.14]). Hypoplastic left heart syndrome (HLHS), in which only one side of the heart is capable of pumping, had elevated ORs that shift away from the null in the isolated case analysis compared with the all case analysis, but CIs are very large and there is no particular pattern with increasing exposure levels. HLHS ORs (95% CIs) in ascending order were: 1.23 (0.40, 3.76); 1.35 (0.60, 3.03); 2.05 (0.94, 4.44); and 1.43 (0.49, 4.13). Other cardiovascular defects had ORs consistent with the null or insufficient numbers of isolated cases to determine if patterns were consistent across both analyses (e.g., tricuspid valve atresia).
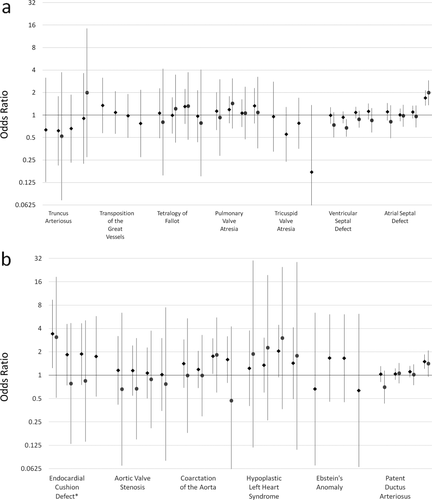
Odds ratios and 95% confidence intervals for cardiovascular birth defects and pesticide exposure. Pesticide exposure levels from left to right are: <10th percentile, 10th to < 50th percentile, 50th to <90th percentile, and 90th percentile and above, all compared with unexposed. Estimates for all case analyses are shown in green diamonds and isolated case analyses are shown in purple circles. In some instances there were insufficient numbers (due to small overall case numbers or no cases falling within an exposure category) to estimate ORs and no effects are shown. Numerical ORs and 95% CIs are reported in Supplementary Table S1.
Gastrointestinal Defects
Results for gastrointestinal defects are shown in Figure 2. Tracheal esophageal fistula/esophageal atresia (TEF), in which there is an abnormal connection between the trachea and the esophagus, had elevated ORs across exposure categories. ORs for TEF did not change with increasing pesticide levels: all case OR<10th percentile 1.95 (0.68, 5.56); OR > 90th percentile 1.98 (0.69, 5.66). Hypertrophic pyloric stenosis (HPS), a narrowing of the opening between the stomach and the intestine, also had elevated ORs, although only for pesticide levels at the 50 to 90th percentile and above the 90th percentile (respectively: 1.41 [1.09, 1.82]; 1.71 [1.25, 2.35]). ORs for Hirschsprung's disease, in which the large intestine has no nerves and cannot function, increased with increasing levels of pesticides (in order: 1.04, [0.35, 3.03]; 1.17 [0.55, 2.49]; 1.55 [0.75, 3.19]; 2.22 [0.94, 5.24]). Other gastrointestinal defects showed associations consistent with the null.
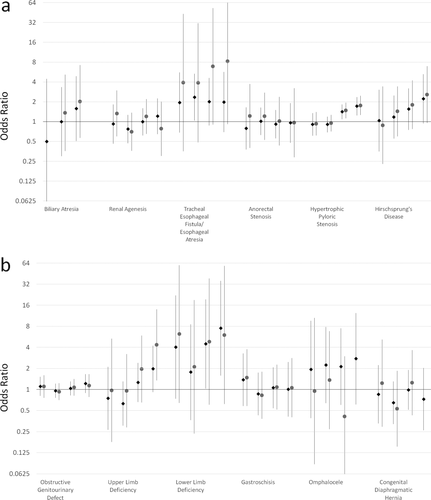
Odds ratios and 95% confidence intervals for gastrointestinal (a) and musculoskeletal (b) defects and pesticide exposure. Pesticide exposure levels from left to right are: <10th percentile, 10th to <50th percentile, 50th to < 90th percentile, and 90th percentile and above all compared with unexposed. Estimates for all case analyses are shown in green diamonds and isolated case analyses are shown in purple circles. In some instances there were insufficient numbers to estimate ORs and no effects are shown. Numerical ORs and 95% CIs are reported in Supplementary Table S1.
Musculoskeletal Defects
Both upper and lower limb deficiencies had elevated ORs, although both have large CIs (Fig. 2). Associations for upper limb deficiencies were elevated for exposure levels above the 50th percentile. Lower limb deficiencies ORs were elevated at all exposure levels, although without any clear pattern. ORs for other musculoskeletal defects were consistent with the null.
Orofacial Clefts
Results for orofacial clefts are shown in Figure 3. Cleft palate and cleft lip with or without cleft palate had elevated ORs for some levels of exposure but no discernable patterns with increasing levels of pesticides.
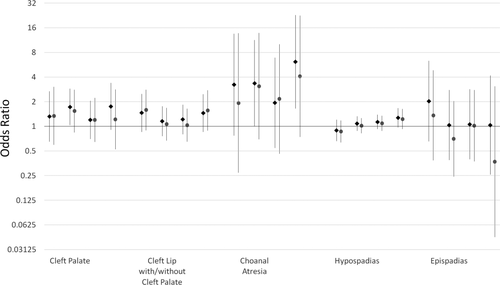
Odds ratios and 95% confidence intervals for orofacial, respiratory, and genitourinary defects and pesticide exposure. Pesticide exposure levels from left to right are: <10th percentile, 10th to <50th percentile, 50th to <90th percentile, and 90th percentile and above all compared with unexposed. Estimates for all case analyses are shown in green diamonds and isolated case analyses are shown in purple circles. Numerical ORs and 95% CIs are reported in Supplemental Table S.1.
Respiratory Defects
Choanal atresia, a blockage of the back of the nasal passage, had elevated ORs at all levels of exposure, as shown in Figure 3.
Genitourinary Defects
Hypospadias, a ventrally abnormally placed urethra in males, showed small increases in ORs with increasing levels of pesticide exposure. Epispadias, a dorsally abnormally placed urethra, had null associations. Results are shown in Figure 3.
Other Birth Defect Categories
Of the remaining birth defect categories, central nervous system defects, chromosomal defects, and amniotic band sequence had no associations with pesticide levels (Fig. 4). For eye defects, both anophthalmia/microphthalmia and congenital cataracts had inconsistent patterns of effect between all and isolated case analyses (Fig. 4). ORs for anotia/microtia were inverse (i.e., as estimated exposures increased, odds of birth defects decreased), consistently across pesticide levels in the isolated case analysis (Fig. 4).
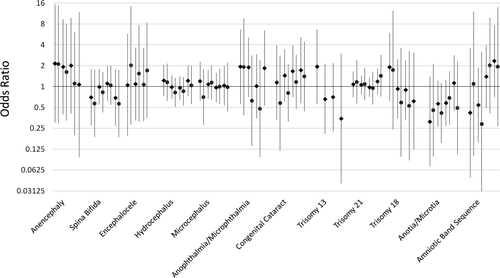
Odds ratios and 95% confidence intervals for central nervous system, eye, chromosomal, and other defects and pesticide exposure. Pesticide exposure levels from left to right are: <10th percentile, 10th to <50th percentile, 50th to < 90th percentile, and 90th percentile and above all compared with unexposed. Estimates for all case analyses are shown in green diamonds and isolated case analyses are shown in purple circles. In some instances there were insufficient numbers to estimate ORs and no effects are shown. Numerical ORs and 95% CIs are reported in Supplemental Table S.1.
Sensitivity Analyses
Analyses using the more difficult to compute pesticide metric, which uses more in-depth pesticide timing data to create the exposure variable, had results that were very similar to the results shown above using the “hybrid” pesticide metric, which uses crop planting and harvesting dates to estimate exposure (Supplementary Table S2, which is available online). Analyses examining populations with women without tobacco in their crop buffer and women without diabetes recorded during pregnancy were similar to the presented results (Supplementary Table S2).
Discussion
We examined associations between individual birth defects in a population from NC and pesticide exposures during preconception and early pregnancy using a novel metric of residential agricultural pesticide exposure. Our analyses suggest that several birth defects may be associated with exposure to pesticides in early pregnancy. Of particular interest are several congenital heart defects (i.e., ASD, PDA, HLHS), as well as structural defects affecting the gastrointestinal (i.e., TEF, HPS, Hirschsprung's disease), genitourinary (i.e., hypospadias), and musculoskeletal systems (i.e., upper limb defects, lower limb defects,), and other malformations (i.e., choanal atresia, anotia/microtia [inverse association]).
Several other studies have examined agricultural crops or pesticides around maternal residence, although the particular defects studied vary among them. Many of these studies also take place in California, likely due to the improved pesticide data available there, and examine several different pesticides in association with various birth defects. Such heterogeneity in study design can make it difficult to draw direct comparisons of the results from different studies as well as to evaluate the consistency of results across studies. Several studies reported results consistent with those reported here: Carmichael et al. (2014a) found elevated associations with HLHS and ASD; Shaw et al. (2014) found no associations with gastroschisis; and Carmichael et al. (2013) found mixed results with hypospadias. These California based studies all used the method of exposure assessment described in Rull and Ritz (2003), wherein pesticide use reports were linked to land survey data; a 500 m or 1000 m buffer was created around each residence, and amount of pesticide active ingredients were estimated within each buffer.
Additionally, in studies that used crops near maternal residence as a surrogate of exposure to agricultural pesticides, Ochoa-Acuna et al. (2009) observed elevated associations between limb defects and residential proximity to cornfields in Indiana, and Winston et al. (2014) reported that maternal residence in block groups with greater than 5% crop cover was associated with increased odds of hypospadias. Other studies using alternative ways to discern pesticide exposure have also found similar results; for example Winchester et al. (2009) found elevated associations between several birth defects and months of conception where pesticide concentrations in water supplies were highest. Overall, the limited consistency in these observations provides some evidence for associations between exposure to agricultural pesticides and the risk of specific cardiovascular, genitourinary, and musculoskeletal defects. Additional research using more refined exposure assessment techniques is warranted to further investigate these associations.
There are also several studies with results inconsistent with the associations we observed. Some studies observed elevated associations where we observed no associations. In a California study, again using the Rull and Ritz (2003) exposure assessment method, Yang et al. (2014) found elevated ORs for anencephaly, spina bifida, and cleft lip with or without cleft palate with different pesticides, while we did not observe consistent, elevated associations for these defects. Carmichael et al. (2014b) also found elevated associations for some congenital heart defects (e.g., Tetralogy of Fallot, pulmonary valve stenosis, PDA) although, with the exception of PDA, these were not the same congenital heart defects for which we observed elevated associations (i.e., ASD, HLHS). Some of these differences may be due to Carmichael et al.'s (2014a) inclusion of fetal deaths in their study population. Rull et al. (2006) found elevated ORs for neural tube defects, spina bifida, and anencephaly with different pesticides, again using their previously developed method (Rull and Ritz, 2003); although due to low exposure prevalence they were restricted to dichotomous exposure comparisons (Rull et al., 2006).
There is also a study of hypospadias in Arkansas that found strongly inverse associations with increasing total pesticides within 500 m of maternal residence at birth while we found slightly elevated associations (Meyer et al., 2006). There are several possible explanations for these differences. One is that many estimates are unstable due to low case numbers. The California studies examined many specific chemicals whereas we examined total pesticide exposure, thus our metric may be smoothing over some potential elevated or inverse associations due to homogenization of exposure. For those studies that examine total pesticides as the exposure of interest, the differing ways of metric construction/exposure estimation may contribute to observed differences in ORs. It is also possible that pesticide profiles differ in the different areas under study, and this may lead to varied associations.
Because we examined total pesticide exposure, potential mechanisms on birth defects are many and varied. Some pesticides (e.g., atrazine, alachlor, and chlorpyrifos) are endocrine disruptors, whereas others are known developmental toxicants (e.g., bifenthrin, diuron). Endocrine disruptors may act through estrogenic or antiandrogenic pathways to disrupt normal hormone-dependent development. Developmental toxicants disrupt growth and organ formation. Prenatal exposure to sulfoxaflor, a chemical with insecticide properties, has been implicated in limb defects (limb contracture: forelimb flexure, hindlimb rotation, and bent/misshapen clavicle bones) in rats by binding with aChR receptor during fetal development (Ellis-Hutchings et al., 2014). Other potential mechanisms of pesticide action in chronic diseases may also play a role, such as: genetic or germ cell damage or epigenetic changes from preconception exposures; inhibition of electron chain transport activity or mitochondrial dysfunction; oxidative stress; or protein regulation (Mostafalou and Abdollahi, 2013). These pesticide modes of action are linked to chronic diseases, such as diabetes, neurodegenerative disorders, and cancer, but may also result in birth defects through cell damage, disrupted signaling, or disrupted energy production during growth and organogenesis. It is also possible that high levels of pesticide exposure may lead to increased severity or lethality of some birth defects.
Strengths of our study include the population-based design that captures all singleton live births in NC, and complete case ascertainment through the 1st year of life. Birth defects reported on birth certificates have been found to have low sensitivity (Watkins et al., 1996; Boulet et al., 2011) and will only capture the most severe and easily apparent birth defects; using the complete case ascertainment through the 1st year of life, therefore, reduces bias due to misclassification. We used a novel metric of pesticide exposure (Warren et al., 2014) that balances computational efficiency and data accessibility with improved information on pesticides for areas where data may be sparse or incomplete. The pesticide exposure metric relied upon crop phenology, pesticide application records, and maternal residential location, and included timing of pesticide exposure, which allowed us increase the specificity of the exposure estimate and decrease potential bias of the effect estimates. Our study was conducted in NC, a state in which the associations between pesticides and birth defects have been understudied. We also examined individual birth defects rather than grouping by organ system, as etiologies may differ even within systems.
Related to examination of individual defects, a limitation of our study is that many birth defects have small case numbers, leading to potential instability in effect estimates. Our use of live birth data may introduce potential bias, particularly for those defects that typically result in termination or fetal loss (e.g., anencephaly, trisomy 13, trisomy 18). The true population at risk of birth defects is all conceptions reaching implantations. Using live births as the cohort base may result in attenuation of effect estimates. However, data on pregnancies reaching conception are not available, and live births provide a reasonable stand-in. Some birth defects may be the result of inadequate development time (i.e., preterm birth), particularly certain heart defects such as ASD or PDA.
Due to the use of registry data, there is the issue of potential unmeasured confounding; some variables, such as parental occupation, are known risk factors for birth defects and may also be associated with area of residence. We were unable to adjust for such variables in this analysis; however, studies that do capture this and other detailed information, for example the National Birth Defects Prevention Study, would be able to. Births with ungeocodable maternal residences were excluded from our analysis; many of these are likely to be rural routes or PO boxes, which may fall more often in rural areas where pesticide exposure is likely to be more common. We use maternal residence at birth with the assumption that this is the residence throughout pregnancy; however, many women do move during pregnancy. Reported rates of mobility between the time of conception and delivery vary widely, anywhere from approximately 9 to 32% (Shaw and Malcoe, 1992; Bell and Belanger, 2012). However, the median distance of residential moves tends to be relatively short, and thus it is likely that those women who do move during pregnancy move to similar areas, as has been shown in air pollution research (Lupo et al., 2010; Bell and Belanger, 2012). However, it is difficult to say whether this holds true for moving to areas with similar crop profiles, and how this movement would change effect estimates, other than the introduction of exposure misclassification. We also use estimates of total pesticide exposure, which may mask effects that are specific to a particular pesticide.
Conclusions
Our study is a hypothesis-generating analysis that uses a novel metric combining accessible data on agricultural crops and pesticide use. We found associations between estimated total pesticide exposure and several congenital heart defects (i.e., ASD, PDA, HLHS), as well as structural defects affecting the gastrointestinal (i.e., TEF, HPS, Hirschsprung's disease), genitourinary (i.e., hypospadias), and other systems (i.e., choanal atresia, upper limb defects, lower limb defects, anotia/micotia). Future directions include a closer examination of these birth defects with specific pesticide active ingredients. There is a clear need to understand the contributions of these factors, especially preventable exposures, in birth defect etiology to develop and optimize prevention strategies.
Acknowledgments
The authors thank K. Meyer for her work on pesticide exposure and hypospadias, and D. Lobdell and M. Patel for their helpful reviews and comments.




