Early prenatal exposure to air pollution and its associations with birth defects in a state-wide birth cohort from North Carolina
The authors have no conflicts of interest to declare. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA.
Abstract
BACKGROUND
Few studies have examined the potential relationship between air pollution and birth defects. The objective of this study was to investigate whether maternal exposure to particulate matter (PM2.5) and ozone (O3) during pregnancy is associated with birth defects among women living throughout North Carolina.
METHODS
Information on maternal and infant characteristics was obtained from North Carolina birth certificates and health service data (2003–2005) and linked with information on birth defects from the North Carolina Birth Defects Monitoring Program. The 24-hr PM2.5 and O3 concentrations were estimated using a hierarchical Bayesian model of air pollution generated by combining modeled air pollution predictions from the U.S. Environmental Protection Agency's Community Multi-Scale Air Quality model with air monitor data from the Environmental Protection Agency's Air Quality System. Maternal residence was geocoded and assigned pollutant concentrations averaged over weeks 3 to 8 of gestation. Binomial regression was performed and adjusted for potential confounders.
RESULTS
No association was observed between either PM2.5 or O3 concentrations and most birth defects. Positive effect estimates were observed between air pollution and microtia/anotia and lower limb deficiency defects, but the 95% confidence intervals were wide and included the null.
CONCLUSION
Overall, this study suggested a possible relationship between air pollution concentration during early pregnancy and certain birth defects (e.g., microtia/anotia, lower limb deficiency defects), although this study did not have the power to detect such an association. The risk for most birth defects does not appear to be affected by ambient air pollution. Birth Defects Research (Part A) 97:696–701, 2013. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
A growing body of epidemiologic studies have examined whether air pollution exposure is associated with various birth outcomes. Some studies have examined if air pollutants during the relevant time in pregnancy are related to birth defects (Gilboa et al., 2005; Hansen et al., 2009; Strickland et al., 2009; Marshall et al., 2010; Dadvand et al., 2011; Padula et al., 2013). Among those air pollutants that have been examined, particulate matter (PM) and ozone (O3) are two that have been observed to be associated with birth outcomes.
Studies of thoracic PM (PM10; with aerodynamic diameter ≤ 10 μm) and O3 concentrations and cardiac defects have reported mixed findings (Ritz et al., 2002; Gilboa et al., 2005; Hansen et al., 2009; Strickland et al., 2009; Dadvand et al., 2011). Studies that examined the association between PM concentrations and orofacial clefts or oral clefts reported no associations with PM10 (Ritz et al., 2002; Hwang and Jaakkola, 2008; Hansen et al., 2009; Marshall et al., 2010; Padula et al., 2013) or fine PM (PM2.5; aerodynamic diameter ≤ 2.5 μm) (Marshall et al., 2010; Padula et al., 2013). Previous studies have reported no associations between O3 concentration and orofacial clefts (Ritz et al., 2002; Gilboa et al., 2005; Hansen et al., 2009; Dolk et al., 2010; Marshall et al., 2010; Padula et al., 2013).
Many of these studies are limited in that they are only able to examine ambient air pollution exposure for women living within a certain distance of air monitoring stations. In addition, few studies have examined whether there is evidence of potential confounding of air pollution-birth outcome associations by other pollutants. In this study, we examine the association between various birth defects and predicted concentrations of PM2.5 and O3 in both single- and copollutant models in a large, geographically defined population of live births among women living throughout North Carolina.
MATERIALS AND METHODS
Study Population
The study population included all North Carolina resident singleton live births delivered during the period 2003 through 2005. We constructed a data file consisting of all North Carolina birth certificates matched to data from the North Carolina Birth Defects Monitoring Program, State Center for Health Statistics. The North Carolina Birth Defects Monitoring Program is a statewide, population-based surveillance program that uses active case-ascertainment to identify congenital malformations diagnosed among infants within the first year of life. Cases were those infants with birth defect diagnoses from the North Carolina Birth Defects Monitoring Program, and were categorized based on the Centers for Disease Control and Prevention/British Pediatric Association coding system. Infants with more than one defect could have been categorized into multiple phenotype groups. Comparison groups were births that did not have the individual phenotype being examined but could have either another defect or no defects. This method of selection for a comparison group was chosen because infants with one defect are still at risk for other defects. For the birth outcome, hypospadias, the comparison group was limited to male births. Information extracted from the birth certificates included maternal residence, age, education, parity, race/ethnicity, smoking during pregnancy, marital status, month prenatal care began, and infant sex. We analyzed selected birth defect phenotypes in relation to estimated maternal exposure to air pollutants. For all of the birth defects examined, analyses were restricted to those birth defects that had at least 30 cases.
Air Pollution Data
A hierarchical Bayesian model that combined modeled air pollution estimates from the U.S. Environmental Protection Agency's (EPA's) Community Multi-Scale Air Quality (CMAQ) model (which bases estimates on data from the EPA's National Emissions Inventory and meteorological and geographical factors) with data from the EPA's Air Quality System's monitoring network (McMillan et al., 2010) estimated concentrations for two of the criteria air pollutants: O3 and PM2.5. We obtained these data for the years of our study.
These air pollution estimates were predicted to 12- by 12-km grid cells for the entire spatial geography of North Carolina. We used maternal residence at birth, which was obtained from birth records, as a surrogate for residence during early pregnancy. Addresses were geocoded and matched to the corresponding grid cell using ArcGIS (version 9.3). Over 90% of addresses were successfully matched to a grid cell.
The predicted air pollution concentrations from the CMAQ model are reported as hourly estimates. These estimates were averaged across weeks 3 through 8 (inclusive) of pregnancy using the date of a woman's last menstrual period (or clinical estimate of gestation if information on last menstrual period was not available). This developmental period was chosen because this is when the majority of major structural birth defects are thought to occur (Moore and Persaud, 1998).
Exclusion Criteria
For this study, 361,105 records for live-born infants were obtained. The exclusion criteria included multiple factors and a birth may have been excluded for more than one reason. We excluded nonsingleton births (n = 12,083), infants whose gestational age was either unknown, less than 20 weeks, or greater than 45 weeks (n = 237), infants whose gestational age was implausible for their birth weight (Alexander et al., ) (n = 1439), and births associated with an implausible date for mother's last menstrual period (n = 12). Births were also excluded if maternal age was less than 15 years, greater than 50 years, or unknown (n = 821). Additionally, births were excluded if the maternal residence at birth was outside of North Carolina or missing (n = 524). We also excluded infants with chromosomal anomalies, as these may be associated with the birth defects of interest in this study and are unlikely to be related to air pollution exposure (n = 745). Among the remaining individuals in the dataset, 22,485 (6.5%) were excluded because they were not able to be geocoded to the 12 × 12 km grid covering North Carolina. The final study population was 322,969 (89% of all birth records obtained for the study).
Statistical Analysis
Binomial regression was used to examine the association between a one-unit change in the interquartile range for PM2.5 and O3 concentrations (4 μg/m3 and 19 ppb, respectively) and each birth defect category in single and copollutant models. Control for confounding was performed by including the following variables, which were chosen a priori based on previous research and their possible associations with the exposures and outcomes: maternal age (5-year age categories), maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, American Indian, other), and rural–urban continuum codes (RUCC; metropolitan urbanized counties [RUCC: 1, 2, 3], nonmetropolitan urbanized [RUCC: 4, 5], less urbanized [RUCC: 6, 7], thinly populated [RUCC: 8, 9]) (USDA, 2008). Maternal age and RUCC were included as ordinal variables and race/ethnicity was coded using dummy variables. Single-pollutant models were run for PM2.5 and O3 concentrations and then both pollutants were included in a single model to examine copollutant effects.
Additional confounders (maternal education, parity, maternal smoking during pregnancy, marital status, prenatal care began in first trimester, and season of birth) were considered in adjusted models but decreased the precision and did not substantially affect the results. Therefore, these results are not presented.
The defects for which the potential for an association seemed strongest were further examined in additional models (i.e., magnitude of effect estimate was >1.3 or 95% confidence interval [CI] did not include 1). To assess if the highest exposure, in general, demonstrated an increased risk, the exposure concentrations were divided into tertiles or quartiles based on the exposure distribution in the comparison population for that defect. Tertile cutpoints were chosen if the sample size for cases was small (n < 50). Copollutant models were run adjusting for maternal race, maternal age, and RUCC category.
RESULTS
Approximately 2% of infants included in the study had at least one birth defect. The majority of women in the study identified as non-Hispanic white (Table 1). Most women were between 20 and 29 years old. As expected, most residences were in metropolitan urbanized counties. Mean concentrations of O3 and PM2.5 were 40.71 ppb and 14.03 μg/m3, respectively. The correlation between O3 and PM2.5 concentrations, weighted by the exposure distribution among the study population, was 0.44.
| O3 (ppb), weeks 3–8 | ||
|---|---|---|
| Mean (SD) | 40.74 (11.14) | |
| Median | 42.15 | |
| IQR | 30.19, 49.32 | |
| Range | 16.54, 74.99 | |
| PM2.5 (µg/m3), weeks 3–8 | ||
| Mean (SD) | 14.03 (3.05) | |
| Median | 14.17 | |
| IQR | 12.15, 16.08 | |
| Range | 3.49, 27.25 | |
| N | % | |
| Maternal age | ||
| 15–19 years | 36,101 | 11.18 |
| 20–24 years | 86,461 | 26.77 |
| 25–29 years | 87,461 | 27.08 |
| 30–34 years | 74,007 | 22.91 |
| 35–39 years | 32,579 | 10.09 |
| 40–50 years | 6,360 | 1.97 |
| Maternal race/ethnicity | ||
| Non-Hispanic White | 190,528 | 59.05 |
| Non-Hispanic Black | 71,646 | 22.20 |
| Hispanic | 47,523 | 14.73 |
| American Indian | 8,863 | 2.75 |
| Other | 4,104 | 1.27 |
| RUCC category | ||
| 1) Metropolitan urbanized counties | 233,895 | 72.74 |
| 2) Nonmetropolitan urbanized counties | 54,503 | 16.95 |
| 3) Less urbanized counties | 25,044 | 7.79 |
| 4) Thinly populated counties | 8,112 | 2.52 |
- PM2.5, particulate matter; O3, ozone; IQR, interquartile range; RUCC, rural–urban continuum codes.
Table 2 presents the results of binomial regressions for single- and copollutant models. No associations were observed between PM2.5 or O3 concentrations and most birth defect groups. Positive effect estimates were found for air pollution concentration and microtia/anotia, but due to the small number of cases (n = 44), the 95% CIs were wide and included the null (relative risk for PM2.5, 1.38 (95% CI, 0.91–2.09); relative risk for O3, 1.37 (95% CI, 0.83–2.26)). Similarly, the effect estimates for PM2.5 and lower limb deficiency defects (n = 39) were elevated, but did not reach statistical significance (relative risk, 1.38; 95% CI, 0.89–2.13). O3 concentration was not associated with atrial septal defects and pyloric stenosis in single pollutant models, but positive associations were observed in copollutant models with PM2.5. Thus, there appears to be an interplay between O3 and PM2.5 in the copollutant model. PM2.5 concentration was inversely associated with these defects in both single and copollutant models. O3 was inversely associated with the risk of hydrocephalus in single and copollutant models.
| PM2.5 per 4 µg/m3 (weeks 3–8) | O3 per 19 ppb (weeks 3–8) | ||||||
|---|---|---|---|---|---|---|---|
| N | Single pollutant - crude | Single pollutant - adjusted | Copollutant - adjusted | Single pollutant - crude | Single pollutant - adjusted | Copollutant - adjusted | |
| Spina bifida | 105 | 0.95 (0.74–1.21) | 1.02 (0.79–1.32) | 1.07 (0.80–1.44) | 0.90 (0.65–1.25) | 0.89 (0.64–1.24) | 0.86 (0.59–1.24) |
| Hydrocephalus | 224 | 1.03 (0.87–1.23) | 1.00 (0.84–1.20) | 1.11 (0.90–1.36) | 0.80 (0.64–1.00) | 0.80 (0.64–1.01) | 0.76 (0.59–0.98) |
| Anophthalmia/ microphthalmia | 35 | 0.97 (0.63–1.50) | 0.89 (0.57–1.41) | 0.97 (0.58–1.62) | 0.79 (0.45–1.40) | 0.79 (0.45–1.39) | 0.80 (0.43–1.52) |
| Congenital cataract | 60 | 1.00 (0.72–1.39) | 1.09 (0.77–1.54) | 1.07 (0.73–1.58) | 1.08 (0.70–1.66) | 1.09 (0.71–1.69) | 1.05 (0.65–1.71) |
| Microtia/anotia | 44 | 1.46 (0.98–2.19) | 1.38 (0.91–2.09) | 1.29 (0.80–2.07) | 1.38 (0.84–2.29) | 1.37 (0.83–2.26) | 1.18 (0.67–2.09) |
| Transposition of great vessels | 84 | 1.08 (0.81–1.43) | 1.05 (0.78–1.40) | 0.99 (0.72–1.37) | 1.16 (0.81–1.67) | 1.15 (0.80–1.66) | 1.16 (0.77–1.74) |
| Tetralogy of Fallot | 104 | 1.04 (0.81–1.34) | 0.99 (0.76–1.29) | 1.00 (0.74–1.35) | 0.96 (0.69–1.34) | 0.96 (0.70–1.33) | 0.96 (0.67–1.39) |
| Ventricular septal defect | 1034 | 0.98 (0.91–1.07) | 0.95 (0.87–1.03) | 0.94 (0.85–1.03) | 0.99 (0.89–1.10) | 0.99 (0.89–1.10) | 1.03 (0.92–1.16) |
| Atrial septal defect | 934 | 0.80 (0.74–0.87) | 0.77 (0.71–0.84) | 0.73 (0.66–0.80) | 0.98 (0.88–1.09) | 0.98 (0.88–1.09) | 1.17 (1.03–1.32) |
| Endocardial cushion defect/ atrioventricular septal defect | 65 | 1.05 (0.76–1.45) | 1.02 (0.73–1.43) | 0.94 (0.65–1.38) | 1.18 (0.78–1.79) | 1.18 (0.78–1.79) | 1.22 (0.77–1.95) |
| Pulmonary valve atresia/stenosis | 216 | 0.99 (0.83–1.18) | 1.01 (0.84–1.22) | 0.95 (0.77–1.17) | 1.12 (0.89–1.41) | 1.15 (0.92–1.45) | 1.19 (0.92–1.54) |
| Tricuspid valve atresia/stenosis | 39 | 0.96 (0.64–1.45) | 0.93 (0.60–1.43) | 0.98 (0.60–1.59) | 0.83 (0.48–1.42) | 0.85 (0.50–1.47) | 0.87 (0.47–1.59) |
| Aortic valve stenosis | 62 | 0.88 (0.64–1.21) | 0.96 (0.69–1.33) | 0.95 (0.66–1.38) | 0.99 (0.65–1.51) | 0.98 (0.64–1.51) | 1.01 (0.63–1.63) |
| Hyperplastic left heart syndrome | 67 | 0.91 (0.67–1.25) | 1.06 (0.76–1.47) | 1.01 (0.70–1.46) | 1.14 (0.76–1.72) | 1.16 (0.76–1.76) | 1.15 (0.72–1.84) |
| Coarctation of aorta | 141 | 1.04 (0.84–1.29) | 1.06 (0.85–1.32) | 0.97 (0.76–1.24) | 1.25 (0.95–1.66) | 1.25 (0.94–1.66) | 1.27 (0.93–1.75) |
| Cleft palate | 154 | 0.97 (0.79–1.19) | 1.01 (0.82–1.25) | 1.08 (0.85–1.37) | 0.87 (0.67–1.15) | 0.87(0.66–1.14) | 0.83 (0.62–1.13) |
| Cleft lip with or without cleft palate | 241 | 0.88 (0.74–1.03) | 0.92 (0.77–1.08) | 0.93 (0.77–1.12) | 0.92 (0.74–1.14) | 0.91 (0.73–1.13) | 0.95 (0.75–1.21) |
| Esophageal atresia/ tracheoesophageal fistula | 77 | 0.90 (0.68–1.21) | 0.90 (0.67–1.22) | 0.96 (0.68–1.34) | 0.82 (0.56–1.20) | 0.81 (0.55–1.19) | 0.83 (0.54–1.28) |
| Anorectal atresia/stenosis | 140 | 0.96 (0.77–1.19) | 0.93 (0.75–1.17) | 0.91 (0.71–1.18) | 1.01 (0.76–1.34) | 1.01 (0.76–1.34) | 1.06 (0.77–1.45) |
| Pyloric stenosis | 625 | 0.84 (0.76–0.93) | 0.89 (0.81–0.99) | 0.85 (0.76–0.95) | 1.06 (0.93–1.21) | 1.06 (0.93–1.21) | 1.16 (1.00–1.35) |
| Renal agenesis | 153 | 1.08 (0.88–1.34) | 1.08 (0.86–1.34) | 1.07 (0.84–1.37) | 1.05 (0.80–1.38) | 1.05 (0.80–1.37) | 1.01 (0.74–1.36) |
| Obstructive genitourinary defect | 696 | 1.08 (0.98–1.19) | 1.08 (0.98–1.20) | 1.06 (0.95–1.19) | 1.09 (0.96–1.24) | 1.09 (0.96–1.24) | 1.06 (0.92–1.22) |
| Hypospadias | 978 | 0.95 (0.87–1.03) | 0.98 (0.90–1.07) | 1.00 (0.91–1.10) | 0.96 (0.86–1.07) | 0.95 (0.86–1.06) | 0.95 (0.85–1.08) |
| Deficiency defect – upper limbs | 86 | 0.96 (0.73–1.27) | 0.98 (0.74–1.31) | 0.92 (0.67–1.27) | 1.14 (0.79–1.63) | 1.14 (0.80–1.64) | 1.20 (0.80–1.80) |
| Deficiency defect – lower limbs | 39 | 1.18 (0.77–1.79) | 1.38 (0.89–2.13) | 1.59 (0.98–2.58) | 0.85 (0.50–1.46) | 0.86 (0.50–1.49) | 0.67 (0.37–1.22) |
| Gastroschisis | 90 | 0.80 (0.62–1.04) | 0.94 (0.71–1.23) | 0.93 (0.69–1.26) | 0.97 (0.68–1.38) | 0.97 (0.68–1.40) | 1.01 (0.68–1.51) |
| Omphalocele | 31 | 1.05 (0.66–1.67) | 1.08 (0.67–1.75) | 1.02 (0.59–1.75) | 1.19 (0.65–2.17) | 1.19 (0.65–2.17) | 1.18 (0.60–2.32) |
| Diaphragmatic hernia | 71 | 0.88 (0.65–1.18) | 0.85 (0.62–1.16) | 0.78 (0.55–1.10) | 1.11 (0.75–1.65) | 1.11 (0.75–1.66) | 1.28 (0.82–2.01) |
- Adjusted for maternal race (indicator), maternal age, and rural–urban continuum codes category.
- PM2.5, particulate matter; O3, ozone.
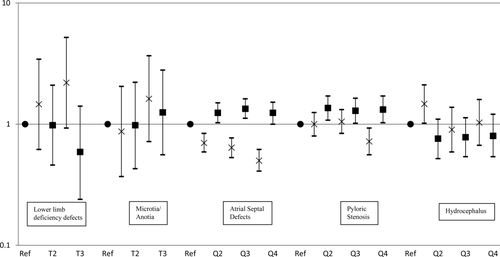
Results for the examination of categorized exposures are displayed in Figure 1. The results are similar to those observed when modeling exposure as a continuous variable. Increased effect estimates were detected among the highest tertile of O3 and PM2.5 concentrations for microtia/anotia and PM2.5 concentration and lower limb deficiency defects, but again, precision was poor. Also, for atrial septal defects and pyloric stenosis, the associations with O3 and PM2.5 concentrations are in opposite directions. However, the inverse association observed between O3 concentration and hydrocephalus in the continuous model is reduced when O3 concentration is examined in quartiles.
DISCUSSION
Overall, the majority of associations between air pollutants and birth defects were null. For some defects, positive associations were observed with increasing concentrations of one pollutant, whereas negative associations were observed with increasing concentrations of the other. The results are similar to other studies that have explored the associations between PM and O3 concentration and birth defects. Studies of PM10 and O3 concentrations have reported findings that are positive, null, or inverse for varying cardiac defects (Ritz et al., 2002; Gilboa et al., 2005; Hansen et al., 2009; Strickland et al., 2009; Dadvand et al., 2011). Additionally, studies conducted in several locations, including southern California (Ritz et al., 2002), England (Dolk et al., 2010; Dadvand et al., 2011), and Australia (Hansen et al., 2009), reported no association between PM10 concentration and cardiac defects. However, a study from Texas reported a positive association between atrial septal defects and PM10 concentration (Gilboa et al., 2005), and a study from Atlanta, Georgia, reported a positive association between PM10 concentration and patent ductus arteriosus (Strickland et al., 2009). Other cardiac defects examined in these two studies did not demonstrate an association with PM10 concentration. In a meta-analysis of cardiac defects, including the above-mentioned studies, a positive association between PM10 and atrial septal defects was observed, but no association was apparent for the other cardiac defects examined (Vrijheid et al., 2011). A study conducted in Southern California observed positive associations with some cardiac defects, specifically aortic artery and valve defects and O3, in the second month of pregnancy but associations were not observed for other cardiac defects (Ritz et al., 2002). Two studies in the United States detected no positive associations between O3 concentration and cardiac defects and instead reported a few inverse associations for some of the specific defects under study (ventricular septal defects [Gilboa et al., 2005]; right ventricular outflow tract defects in a sensitivity analysis restricted to infants with only one cardiac defect [Strickland et al., 2009]). A study in Northeast England reported no associations between O3 concentration and congenital heart disease unless the analysis was restricted to women living near an air monitor, which then resulted in positive findings (Dadvand et al., 2011). Similarly, a study in Australia reported positive associations for O3 concentration with pulmonary artery and valve defects but only when restricted to those women living near a monitor (Hansen et al., 2009). No associations were observed in this study for other congenital cardiac defects. When examining atrial septal defects, ventricular septal defects, and conotruncal defects in a meta-analysis, no association was observed with O3 concentration (Vrijheid et al., 2011).
We observed no association between PM2.5 or O3 and cleft palate and/or lip. Other studies that have examined the association between oral clefts and PM2.5 concentration also observed no association (Marshall et al., 2010; Padula et al., 2013). PM10 exposure has also been evaluated for its effect on the risk of cleft lip and/or palate. Among four studies conducted in the United States (Ritz et al., 2002; Gilboa et al., 2005; Marshall et al., 2010; Padula et al., 2013), only one reported a positive association but this association was only statistically significant in the third quartile of exposure (Gilboa et al., 2005). Studies in Taiwan and England also observed no association for PM10 concentration and oral clefts (Hwang and Jaakkola, 2008; Dolk et al., 2010). An inverse association with cleft palate was reported in a study from Australia but the association was null for cleft lip or for cleft lip with or without cleft palate (Hansen et al., 2009). Similar to our work, three studies in the United States reported no associations between O3 concentration and orofacial clefts (Ritz et al., 2002; Gilboa et al., 2005; Padula et al., 2013). A fourth study in the United States also observed no association with oral clefts except for a positive association with cleft palate only when the analysis was restricted to women living in close proximity to the air monitors (Marshall et al., 2010). International studies also reported mixed findings, with a study from Australia demonstrating no association between O3 concentration and cleft lip or palate (Hansen et al., 2009) and a study from Taiwan reporting a positive association with oral clefts and concentrations during first and second months of pregnancy but not in the third month of pregnancy (these results were robust to inclusion of other pollutants) (Hwang and Jaakkola, 2008). Many of these studies were included in a meta-analysis conducted in 2011 that demonstrated a slight association between O3 concentration and orofacial defects (odds ratio, 1.10 [95% CI, 0.99–1.21] per 10 ppb) (Vrijheid et al., 2011). Although the 95% CIs of our estimates include the odds ratios from the meta-analysis, our point estimates are below those of the overall estimate.
Similar to our results, no associations were demonstrated between either PM2.5 or O3 concentrations and gastroschisis in a study conducted in the San Joaquin Valley of California (Padula et al., 2013). This study did report an inverse association between O3 concentration and neural tube defects that we did not observe. Neither study detected an association between PM2.5 concentration and neural tube defects.
We found a positive effect estimate for microtia/anotia and both air pollutants and a positive effect estimate for lower limb deficiency defects and PM2.5 concentration. However, the precision was poor and no definitive statement can be made regarding an association. In addition, it seems unlikely that there would be an effect of air pollution on limb deficiency for only the lower and not the upper limbs. To our knowledge, no research has examined the association between microtia/anotia or limb deficiency defects and air pollution. Further research will be useful for determining whether there truly is an effect of air pollution on defects related to ear and/or limb development.
This study has some limitations. One limitation is that the ambient air pollution information is based on residential address, which may not adequately characterize personal exposure. A woman's actual exposure to ambient air pollution would vary by time spent outdoors, time spent in locations other than the home, commuting time, etc. In addition, information was not available on maternal residence during early pregnancy. If a woman resided in a different location for the first part of her pregnancy, then her residence at birth may not accurately describe her air pollution exposure during weeks 3 through 8 of pregnancy. Reported rates of mobility between the time of conception and delivery vary widely, anywhere from approximately 9 to 32% (Bell and Belanger, 2012). However, the median distance moved tends to be relatively short and, thus, would be less likely to result in misclassification of one's exposure status where ambient exposures such as air pollution are concerned (Lupo et al., 2010; Bell and Belanger, 2012). Finally, our study contained multiple comparisons, and it is possible that the positive and inverse associations reported could be due to chance. Further research will be needed to confirm these associations.
This study also has multiple strengths. The use of the CMAQ data allowed for the inclusion of all births in North Carolina, whereas most studies include individuals living near an air monitor and therefore exclude births in nonurban areas. The CMAQ data used in this analysis also combined model estimates with air monitor measurements, creating information that is more complete than using either of these individually. Finally, the data on birth defects were obtained from a statewide population-based surveillance program using active case-finding, which provided relatively complete and reliable information on birth defects in the study area.
In summary, we did not observe associations between PM2.5 or O3 concentrations and most birth defects examined. We observed some positive and some inverse associations that may be due to associations with other pollutants, confounding, or multiple testing. Further research is needed to explore the associations between air pollution and certain birth defects.
ACKNOWLEDGMENTS
We thank Valerie Garcia and EPA's Atmospheric Modeling and Analysis Division for providing CMAQ data; Fred Dimmick, Dave Holland, and EPA's Ecosystems Research Division for providing the fused CMAQ data; and Mark S. Murphy for his assistance in analyzing CMAQ data in ArcGIS 9.3. We also thank Dianne Enright for geocoding the North Carolina birth certificate data.




