Altered localization of gene expression in both ectoderm and mesoderm is associated with a murine strain difference in retinoic acid–induced forelimb ectrodactyly†
Presented at the 46th Annual Meeting of the Teratology Society, Tucson, AZ, June 26th, 2006.
Abstract
BACKGROUND:
Defects in digit number or fusion as a teratogenic response are well documented in humans and intensively studied in various mouse models. Maternal exposure to excess levels of all-trans-retinoic acid (RA) at gestational day 9.5 induces postaxial ectrodactyly (digit loss) in the murine C57BL/6N strain but not in the SWV/Fnn strain.
METHODS:
Whole-mount in situ hybridization was used to examine the differential expression of limb patterning genes at the transcriptional level between the two mouse strains following the maternal exposure to a teratogenic level of RA. The detection of a gene with altered expression was followed by either the evaluation of other genes that were synexpressed or with an assessment of downstream genes.
RESULTS:
In the C57BL/6N limb bud following maternal RA administration, gene-specific perturbations were observed within hours of the RA injection in the posterior pre-AER (apical ectodermal ridge) (Fgf8, Dlx3, Bmp4, Sp8, but not Dlx2 or p63), whereas these genes were normally expressed in the SWV/Fnn limb bud. Furthermore, although RA caused comparable reductions of Shh expression between the strains in the 12 h after administration, some Shh downstream genes were differentially expressed (e.g., Gli1, Ptc, and Hoxd13), whereas others were not (e.g., Fgf4, Bmp4, and Gremlin).
CONCLUSIONS:
It is proposed that altered gene expression in both pre-AER and mesoderm is involved in the pathogenesis of postaxial digit loss, and that because the alterations in the pre-AER occur relatively early in the temporal sequence of events, those changes are candidates for an initiating factor in the malformation. Birth Defects Research (Part A) 2007. © 2007 Wiley-Liss, Inc.
INTRODUCTION
Congenital malformations of the distal extremities in humans exist in various phenotypes (Manouvrier-Hanu et al., 1999). Although limb development has several critical parameters such as size, position, and orientation, the incidence of the defects altering the fusion and the number of digits is particularly high (Talamillo et al., 2005). These malformations, including adactyly (total loss of digits), syndactyly (fusion of digits), polydactyly (addition of digits), and ectrodactyly (loss of specific digits), are thought to be a consequence of perturbation in the molecular and cellular aspects of limb development.
The tetrapod limb has long served as an exquisite model for the study of pattern formation due not only to convenience in observation and experimental manipulations but also to its lucid three-dimensional polarity. Indeed, vertebrate limb patterning and outgrowth are regulated by the complex interplay of a number of signaling molecules that originate in signaling centers (for review, see Capdevila and Izpisúa Belmonte, 2001; Tickle, 2002). The zone of polarizing activity (ZPA), the mesoderm at the posterior margin of the limb bud, directs anteroposterior (thumb to little finger) specification via sonic hedgehog (Shh) signaling (Chang et al., 1994; Riddle et al., 1993), perhaps in concert with other molecules. This is evidenced by the fact that an anterior ectopic implantation of the ZPA in the chick results in a mirror-image duplication of the digits (Tickle et al., 1975), and both Shh and retinoic acid (RA) can mimic this effect (Ogura et al., 1996; Summerbell, 1983). The apical ectodermal ridge (AER), a thickened epithelium located at the dorsoventral (knuckles to palm) interface at the distal tip of the limb bud, is another signaling center regulating proximodistal (shoulder to finger tip) limb outgrowth via predominantly, if not exclusively, Fgf signaling. This is delineated by the fact that, if excised, limb outgrowth is inhibited but exogenously supplied Fgf can rescue the function of the AER and the limb phenotype (Niswander et al., 1993). As a result of the activities of the molecules emanating from the signaling centers, a limb field obtains positional cues that instruct the appropriate limb patterning such that, for instance, the autopod develops at the distal end of the limb and the thumb is formed at the anterior end of the autopod. For these processes, other subsets of genes such as Hox genes are responsible. They direct the formation of position-dependent limb structures in response to the positional information. As indicated above, the molecular basis of limb patterning could be simply schematized with a small number of genes; however, actual limb patterning contains far more complicated signaling systems and interactions, partially due to the fact that some gene families have a number of members with overlapping functions, single genes may be involved in multiple pathways, and conversely, a single pathway can be mediated by multiple genes (Oberg et al., 2004). Furthermore, gene products function in networks as opposed to linear pathways. These features of the developing limb bud have hampered the elucidation of the genetic pathways/networks implicated in the development of limb malformations.
To obtain more insight into the mechanisms of limb dysmorphogenesis, limb malformations induced by teratogens have been studied using several experimental models, all of which have advantages. In particular, the mouse limb has been a valuable model of the human limb due to a variety of factors such as the highly analogous development and phenotype among mammalian species, availability and similarity of the whole genome sequences, relatively short gestational times, availability of mouse mutants and inbred strains, and the capability of genetic manipulations such as transgenic and knockout mice. Additionally, the sensitivity to teratogen-induced limb malformations is known to substantially depend on the strain of mouse (Biddle, 1988; Layton and Layton, 1979). For instance, C57BL/6 mice are highly susceptible to forelimb ectrodactyly triggered by acetazolamide, cadmium, carbon dioxide, ethanol, hyperthermia, and RA in contrast to SWV mice, which demonstrate relatively lower susceptibility (reviewed in Lee et al., 2004). In this regard, understanding the mechanisms of strain differences in teratogen-induced limb defects will partially define the role of genotype in teratogenesis as well as yield clues as to the genetic pathways involved in the induction of the malformation.
All-trans-RA, an active metabolite of vitamin A, has been implicated in limb morphogenesis of vertebrates. Whereas endogenous production of RA is indispensable for proper limb development (Mic et al., 2004; Niederreither et al., 2002), toxicological levels of RA are known to cause a wide range of malformations in a dose- and stage-dependent manner (reviewed in Lee et al., 2004). One of the major pathways by which retinoids produce biological effects is by binding to nuclear receptors, including the RA receptors and retinoid X receptors (for review, see Mark et al., 2006). It has been shown by examining multiple retinoid ligands that teratogenic activity is associated with RA receptor agonists as opposed to retinoid X receptor agonists or retinoids that activate neither of these receptor types (Kochhar et al., 1996). Thus, alterations in limb development by exogenous RA may be due to changes in gene expression. Previously, it was reported that midgestational exposure to embryotoxic levels of RA induced a high incidence of postaxial ectrodactyly in the C57BL/6N (C57) strain but none in the SWV/Fnn (SWV) strain (Collins et al., 2006), demonstrating strain-specific susceptibility to this teratologic outcome. However, the pathway(s) altered by exogenous RA remain largely unknown in either strain. Of interest, it has been demonstrated that, among a number of murine strains, the C57BL/6J strain is the most sensitive to acetazolamide-induced forelimb ectrodactyly, whereas the SWV strain is the most resistant (Biddle, 1988; Biddle, 1975). Likewise, it is presumed that the C57 and SWV strains may be antipodal extremes in their forelimb ectrodactyly response to RA. From this perspective, neither of these two strains has a normal phenotypic response to teratogenic levels of RA.
The present study was designed to examine the expression of limb development genes following RA exposure in these two strains of mice. Using a whole-mount in situ hybridization (WISH) assay, both spatial and temporal expression patterns of genes involved in limb development were examined to document differential gene expression between the two strains following RA treatment. The investigation of a number of genes revealed the potential importance of altered gene expression in the RA-treated C57 limb bud to explain the strain difference in sensitivity to ectrodactyly following RA administration on day 9.5: posterior-specific reduced expression of a subset of genes localized in the AER precursor cells and the attenuation of Shh activity. Furthermore, two models are proposed to explain the induction of postaxial ectrodactyly: the direct perturbation of the AER precursor cells by RA, and perturbation of early Shh signaling from the ZPA to the apical ectoderm, both of which lead to AER disruption in the posterior domain.
MATERIALS AND METHODS
Mice and RA Administration
The animal protocols were approved by the UCLA Chancellor's Animal Research Committee in accordance with NIH guidelines. This study was conducted in the inbred C57BL/6NCrlBR (C57) and SWV/Fnn mouse strains. C57 mice for breeding were purchased from Charles River Laboratories (Hollister, CA). SWV mice were originally provided by Dr. Richard Finnell (Texas A&M University, College Station, TX) and subsequently maintained in a breeding colony at UCLA. Mice were housed in transparent polycarbonate cages maintained in climate-controlled rooms (23 ± 2°C, 30–70% humidity) under a 12 h alternating light/dark cycle (dark from 2100 to 0900) with tap water and Purina 5008 diet (St. Louis, MO) available ad libitum. To generate embryos, individual male mice were placed into cages with multiple female mice for the last 2 h of the dark cycle, 0700 to 0900. The detection of a vaginal plug was used as an indication of a successful copulation and 0900 was designated as the initiation of gestational day (GD) 0.
Prior to retinoid administration, female mice that had vaginal plugs after cohabitation were slightly anesthetized by exposure to ethyl ether in order to validate pregnancy by abdominal palpation. All-trans-RA (Sigma, St. Louis, MO) was dissolved in a small volume (8% of the final volume) of ethanol to the extent possible and then suspended in a larger volume (92% of the final volume) of soybean oil to create a dosing solution of 5 mg/mL that was administered at a dosing volume of 10 mL/kg, to yield a dose of 50 mg/kg. All the procedures, including dosing solution preparation and injection, were performed in a dark room under dim yellow light to prevent photoisomerization of RA.
For C57 mice, the retinoid was administered via an intraperitoneal (IP) injection to pregnant females on GD 9.5 (2100 on day 9). This scheme of RA administration (i.e., dose and timing) was based on a previous study conducted by Collins et al. (2006), in order to achieve a reasonably low level of lethality and a high level of teratogenicity in the embryos. Similarly, control animals were administered the vehicle (92% soybean oil and 8% ethanol) in a single dose with the same dosing volume of 10 mL/kg of body weight. Pregnant C57 mice were sacrificed at 2, 6, 12, 24, 36, 48, or 72 h after injection, equivalent to GDs 9.58, 9.75, 10.0, 10.5, 11.0, 11.5, and 12.5, respectively, by ethyl ether anesthesia followed by cervical dislocation.
For SWV embryos, an alternate temporal scheme was used to produce embryos and limb buds of an equivalent developmental stage to the C57 embryos, and limb buds were collected at the aforementioned gestational times. SWV mice were ∼2.5 somites behind in developmental timing when compared to C57 mice at GD 9.25 (Lee et al., 2005), which corresponds to approximately a 4 h duration in time because the embryonic mouse produces a somite approximately every 1.7 h during this period of development (Goedbloed and Smits-van Prooijie, 1986). Moreover, it has been shown that the resistant strain in this study could not be made sensitive by administering the retinoid at 12 h before or after GD 9.5 (Collins et al., 2006). Thus, to collect stage-matched limb buds, SWV pregnant females were treated with either RA or vehicle at 0100 on GD 9 (or 4 h after GD 9.5 or GD 9.67), and then embryos were harvested at equivalent time points to C57 mice. (SWV collection times were 4 h after the C57 embryo collection times or GD 9.75, 9.92, 10.17, 10.67, 11.17, 11.67, and 12.67).
Embryos were manually harvested into RNase-free cold PBS treated with diethylpyrocarbonate and fixed overnight in a solution of 4% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) in PBS at 4°C. Embryos were subsequently dehydrated through a graded series of methanol washes (25%, 50%, 75% and twice with 100%) in PBST (PBS containing 0.1% Tween-20) at 4°C for 5 min in each wash and stored at −80°C until needed for WISH assays.
WISH
WISH was performed as described by Hogan et al. (1994) with some modifications. Briefly, embryos were permeabilized with 10 μg/mL Proteinase K (Sigma) in PBST for varying durations, depending on the developmental stage of the specimen. Basically, 5 μg/mL of Proteinase K was used with a 5-min incubation for the genes expressed in the ectoderm or both ectoderm and mesoderm, and 10 μg/mL with stage-dependent incubation times for the genes expressed only in the mesenchyme. The basic stage-dependent incubation times for the mesenchymal genes are as follows: 8 min for 2-h embryos, 10 min for 6-h embryos, 13 min for 12-h embryos, 15 min for 24-h embryos, 20 min for 36-h embryos, 30 min for 48-h embryos, and 50 min for 72-h embryos. Additionally, the exact conditions for this process were determined empirically for each gene on the basis of the performance of the probe. After quenching digestion with 2 mg/mL glycine (Sigma) in PBST, the specimens were postfixed with 4% paraformaldehyde (Fisher Scientific) and 0.2% glutaraldehyde (Polysciences, Warrington, PA) in PBST for 20 min at room temperature. Then, embryos were incubated for 2 h at 63°C in hybridization buffer followed by an overnight incubation at 70°C with a digoxygenin-labeled cRNA probe. At the end of the hybridization, the embryos were rinsed once and washed twice for 30 min with Wash1 Solution (300 mM NaCl, 1× PE [10× PE: 0.5 M PIPES and 0.5 M EDTA], 1% SDS) at 50°C, then washed twice for 30 min with Wash2 Solution (50 mM NaCl, 1× PE, 0.1% SDS) at 50°C; treated with RNase A (100 μg/mL; Sigma) in RNase Buffer (500 mM NaCl, pH 7.2, 10 mM PIPES, 0.1% Tween-20); washed once for 30 min at 50°C with Wash3 Solution (300 mM NaCl, 1× PE, 1% SDS, 50% Formamide); washed once for 30 min at 50°C with Wash4 Solution (150 mM NaCl, 1× PE, 50% Formamide, 0.1% Tween-20); and rinsed once followed by a wash with Wash5 Solution (500 mM NaCl, 1× PE, 0.1% Tween-20) at 70°C for 20 min. RNA hybrids were detected by an immunohistochemical method using antidigoxigenin Fab/alkaline phosphatase conjugate (Roche, Indianapolis, IN). Color reactions were performed in BM Purple AP solution (Roche) at 4°C with gentle rocking for 12–72 h in the dark until satisfactory staining had developed.
cRNA probes were synthesized according to the standard procedure in the presence of digoxigenin–conjugated UTP (Roche). Subsequently, unincorporated free nucleotides were removed by spin filtration. Probes and their providers are listed in the Acknowledgments.
Fetal Skeletal Staining
Fetal skeletal staining was performed using a protocol previously described (Inouye, 1976; Kimmel and Trammel, 1981; Kuczuk and Scott, 1984). Mouse neonates isolated at GD 18.0 were killed via an overdose of ethyl ether and then scalded under hot (70°C) tap water to remove the skin, followed by evisceration. After fixation in 95% ethanol for 35 days, specimens were stained for cartilage with 0.015% Alcian Blue (Fisher Scientific) in a 1:4 mixture of glacial acetic acid:95% ethanol for 24 h. Following a rinse in 95% ethanol, neonates were macerated in 1% KOH solution overnight. The samples were counterstained for bones with 0.005% Alizarin Red S (Sigma) in 1% KOH for 1–3 h. Subsequently, skeletons were cleared in a series of glycerol washes containing decreasing concentrations of KOH (4:6, 6:4, 8:2 ratios of glycerol:0.1% KOH).
RESULTS
Strain Difference in RA-Induced Postaxial Ectrodactyly
Phenotype.
A previous study demonstrating the strain difference between C57 (Fig. 1A) and SWV (Fig. 1B) in the RA-induced forelimb malformation was reconfirmed (see Materials and Methods for the regimen selected for this study). As previously reported (Collins et al., 2006), C57 mice had postaxial ectrodactyly following IP administration of 50 mg/kg RA on GD 9.5 (Fig. 1C,D), whereas SWV mice were completely resistant to this teratogen-induced dysmorphogenesis (Fig. 1E,F). Skeletal analysis of GD 18 fetuses revealed that exogenous RA predominantly affected the morphogenesis of the digits, preferentially in the posterior aspect (Fig. 1C and Collins et al., 2006). This abnormal digit formation was visualized during gestation by WISH to type II collagen (Col II), which is expressed in osteochondroprogenitor cells and can be used as a marker of digit primordia (Kosher et al., 1986), demonstrating that this malformation becomes clearly discernible by 72 h after RA injection (GD 12.5) (Fig. 1G–J).
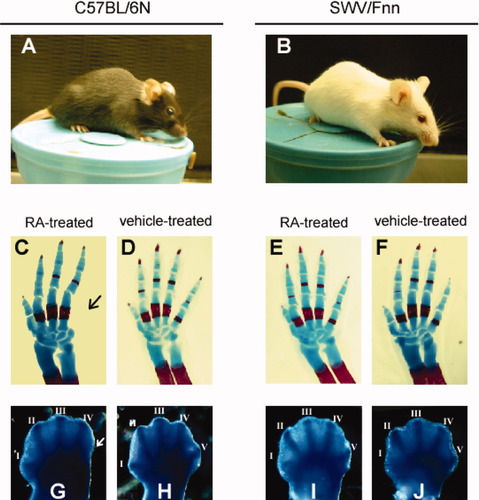
Differential sensitivity to RA-induced forelimb ectrodactyly of the C57 and SWV mouse strains. The C57 strain was sensitive to RA-induced forelimb postaxial ectrodactyly, whereas the SWV strain was resistant. (A,B) Adult mice (∼2 months old) of the C57BL/6N (A) and SWV/Fnn strains (B). (C–F) Skeletal preparations of the right forelimb autopod of GD 18.0 C57 mouse fetuses (C,D) and SWV mouse fetuses (E,F) following RA or vehicle treatment at GD 9.5. Fetal limbs are viewed dorsally with anterior to the left. The arrow in (C) indicates a missing digit V. (G–J) Chondrocyte condensation revealed by whole-mount in situ hybridization for Col II RNA in the forelimb autopod of the GD 12.5 embryo. Limb buds are viewed from the dorsal side with anterior to the left. Roman numerals indicate the digit from anterior (I) to posterior (V). Note the missing digit primordium in the posterior-most domain of the RA-treated C57 autopod [(G), arrow].
To further characterize the malformation throughout limb development, the limb morphology was compared at several time points in RA- and vehicle-treated mice of both strains. Of interest, by 36 h after treatment (GD 11.0), the loss of postaxial limb structure in RA-treated C57 mice was visually recognizable by a small notch or a flattened margin on the posterior domain of the developing limb bud (Fig. 2E, asterisk). In contrast, this phenotype was found neither in vehicle-treated C57 nor SWV mice that were treated with either vehicle or RA. Thus, it is presumed that this phenotypic deformity is a morphological marker of postaxial ectrodactyly prior to the manifestation of the skeletal abnormality. This observation indicates that RA-induced posterior digital absence is determined by 36 h after treatment on GD 9.5, if not sooner. Hence, in an attempt to explore the mechanisms to explain the strain difference, the expression of genes in limb buds prior to GD 11.0 was of particular interest.
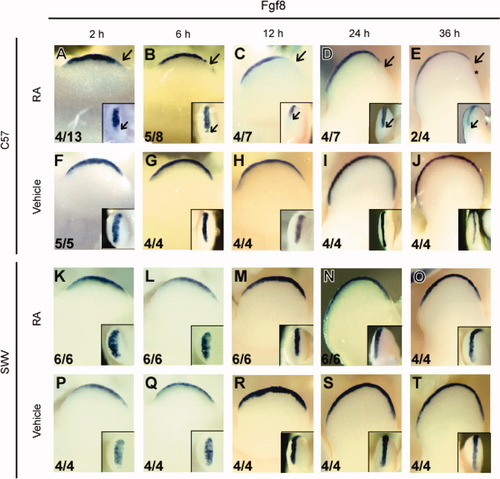
Absence of the postaxial Fgf8 expression in the postaxial pre-AER and the AER. Time-course localization of Fgf8 was visualized by WISH with antisense riboprobe. (A–J) C57 right forelimb buds treated with RA (A–E) or vehicle (F–J). (K–T) SWV right forelimb buds treated with RA (K–O) or vehicle (P–T). In all panels, limb buds are viewed from the dorsal side and oriented with the anterior side toward the left side of the panel and posterior side to the right. The inset in each panel represents the posterior [(C–E), (H–J), (M–O), and (R–T); distal to the top] and distal [(A,B), (F,G), (K,L), and (P,Q); anterior to the top] views of the right forelimb with a higher magnification. Severe reduction of the Fgf8 transcript was observed in the posterior margin of RA-treated C57 limb buds [arrows in (A–E)]. The numerical data in the lower left corner indicate the number of embryos with the photographed expression pattern/the total number of embryos examined. Note that the RA-induced postaxial malformation becomes recognizable by 36 h after RA treatment in C57 [asterisk in (E)].
AER morphology.
Previous studies have noted a truncation of the AER after administration of chemical agents that cause ectrodactyly (Sulik and Dehart, 1988; Bell et al., 1999; Chrisman et al., 2004; Scott et al., 2005). Based on this information, AER morphology was carefully observed under the microscope. Indeed, RA-treated C57 limbs (at 24 and 36 h after treatment) exhibited a shortening of the posterior AER in contrast to the normal AER morphology in SWV limb buds treated with either vehicle or RA and in vehicle-treated C57 limbs (data not shown, see Fig. 2). The AER truncation was located at the position of the postaxial digit loss and it was hypothesized that it might be causative for the defect.
AER
Because epithelial-mesenchymal signaling plays a pivotal role in limb bud morphogenesis (Zuniga et al., 2002), it was hypothesized that there was an association between the AER truncation and subsequent digit loss. Therefore, a subset of genes expressed in the AER and their putative downstream genes were examined to characterize the AER truncation. An approach to determining the causative gene product(s) in the dysmorphogenic process is to know the temporal sequence of altered gene expression in order to be able to identify the initial alteration. Thus, localized gene expression was determined at various developmental times following RA administration for a number of genes in this study.
Fgf 8.
Consistent with the microscopic observations, it was found that the expression of Fgf8 in the posterior AER was absent in a percentage of the RA-treated C57 forelimb buds (Fig. 2). It was intriguing that the posterior truncation of Fgf8 expression was observed in C57 limb buds at times that were 2–6 h after RA administration (Fig. 2A). At 2 h, Fgf8 truncation in the postaxial limb bud was detectable in some embryos, whereas it was frequently detectable in limb buds at 6 h after RA administration. At these earlier stages (2 and 6 h), the AER was not yet anatomically established, but Fgf8-expressing AER precursor cells (referred to as pre-AER) were broadly located in the ventral ectoderm.
Fgf10, Mkp3, Sef, Sprouty2.
To confirm that the loss of Fgf8 expression in the posterior margin of the limb bud affects signaling activity, the expression patterns of several presumptive Fgf8 downstream genes were examined, including Fgf10, Mkp3, Sef, and Sprouty2 (spry2).
Fgf10, which is normally expressed in the mesenchyme of the developing limb bud, was examined because its expression is known to be maintained by Fgf8 derived from the AER (Ohuchi et al., 1999). The expression of Fgf10 at 2 h was notably reduced in the RA-treated C57 limb buds as opposed to vehicle-treated C57 limb buds or SWV limb buds treated with vehicle or RA (Fig. 3A–D). In contrast, Fgf10 was expressed normally in both strains at 6 h following RA administration (data not shown).
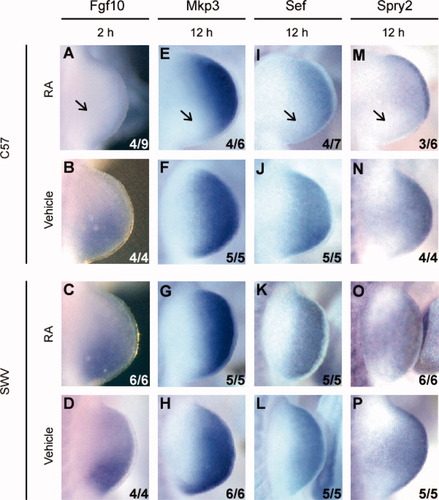
mRNA localization of the genes regulated by Fgf8 in RA- or vehicle-administered forelimb buds following injection at GD 9.5. Profile view of the right side of the embryo with anterior to the top. The data in the lower right corner indicate the number of embryos with the photographed expression pattern/the total number of embryos examined. (A–D) Fgf10 expression in C57 forelimb buds (A,B) and SWV limb buds (C,D). Fgf10 was expressed uniformly in limb mesenchyme at 2 h in vehicle-treated C57 forelimb buds (B) and RA- or vehicle-treated SWV forelimb buds (C,D), whereas Fgf10 expression was not detected in RA-treated C57 forelimb buds [arrow in (A)]. (E–H) mRNA of Mkp3 visualized by labeled riboprobe in the C57 (E,F) and SWV (G,H) forelimb buds. (I–L) Distribution of Sef message detected by WISH performed for the C57 (I,J) and SWV (K,L) forelimb buds. (M–P) Spry2 expression in the C57 (M,N) and SWV (O,P) forelimb buds in response to either RA or vehicle administration. Note the reduced expression of Mkp3, Sef, and Spry2 transcripts in the posterior mesenchyme of RA-treated C57 forelimb buds (arrows in [E,I,M]), whereas the RA-treated SWV forelimb bud (G,K,O) has the equivalent expression to the vehicle-treated forelimb buds (F,H,J,L,N,P).
Mkp3 (Kawakami et al., 2003; Dickinson et al., 2002), Sef (Harduf et al., 2005), and Spry2 (Minowada et al., 1999) are expressed in the distal limb bud and are predicted to be induced by Fgf8 signaling. Mkp3 and Sef were expressed normally in the distal limb bud excluding the AER in vehicle-treated limb buds of both strains (Fig. 3F,H,J,L) and the RA-treated SWV limb bud at 12 h after RA administration (Fig. 3G,K). However, the RA-treated C57 limb bud showed a diminution of the expression domain in the posterior mesenchyme at 12 h (Fig. 3E,I). Spry2 was expressed in the distal limb bud including the AER, as shown in vehicle-treated limb buds of both C57 and SWV (Fig. 3N,P) at 12 h after exposure. However, in response to the teratologic dose of RA, the C57 strain demonstrated a reduced expression of Spry2 in the posterior mesenchyme as well as the truncated expression in the posterior AER at 12 h, whereas the RA-treated SWV limb bud had a normal expression pattern of Spry2 (Fig. 3M,O). The differential expression of Mkp3, Sef, and Spry2 between the limb buds of the two strains found at 12 h after RA administration was not detectable at 6 h.
Bone morphogenetic protein 4 (Bmp4), Dlx2, Dlx3, p63, Sp8.
In addition to Fgf8, the AER precursor cells express a variety of genes such as Bmp4 (Ahn et al., 2001), Dlx2 (Grivodsky and Lonai, 2003), Dlx3, p63 (Yang et al., 1999), and Sp8 (Bell et al., 2003). Thus, the expression patterns of these additional marker genes were analyzed to determine if the decreased expression of Fgf8 was due to total loss of the pre-AER cells or alternatively to a gene-specific reduction in expression. WISH analyses revealed that, in C57 limbs at 2 and 6 h after RA administration, Dlx2 and p63 were expressed at normal levels in the appropriate position, including the posterior margin of the limb bud where Fgf8 was not expressed (Fig. 4A–H and data not shown). On the other hand, Bmp4, Dlx3, and Sp8 were not expressed, similar to Fgf8, in the postaxial pre-AER, particularly at 6 h after RA treatment (Fig. 4I–T). However, the loss of Bmp4 expression in the posterior pre-AER was found in all (8/8) the RA-treated C57 limb buds examined, whereas the misexpression of Dlx3 and Sp8 was observed with comparable frequency to the incidence of postaxial digit loss.
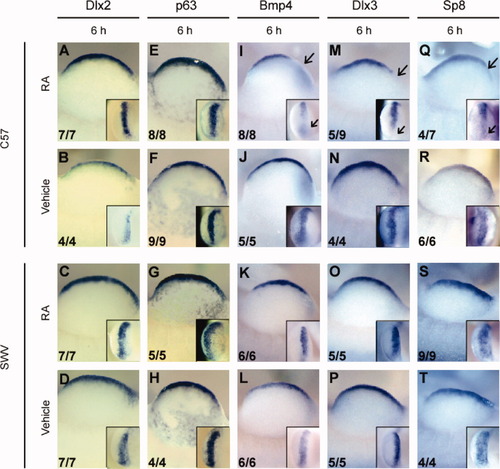
Gene-specific effect of RA on the posterior AER precursor revealed by the expressions of marker genes. Gene expression in the pre-AER (2 and 6 h) was examined by WISH assays following the maternal exposure to either RA or vehicle at GD 9.5. The numerical data presented in the lower left corner denote the number of embryos with the photographed expression pattern/the total number of embryos examined. (A–H) WISH to Dlx2 (A–D) and p63 (E–H) messages in C57 forelimb buds (A,B,E,F) and SWV forelimb bud (C,D,G,H). Unlike Fgf8 expression, these genes were normally expressed in the whole pre-AER including the postaxial region. (I–T) WISH to Bmp4 (I–L), Dlx3 (M–P), and Sp8 (Q–T) messages in vehicle-treated C57 forelimb buds (I,J,M,N,Q,R) and SWV forelimb buds (K,L,O,P,S,T). As opposed to Dlx2 and p63, these genes exhibited perturbation in the posterior expression (arrows in [I,M,Q]). In each panel, a limb is viewed dorsally with distal to the top and proximal to the bottom, and anterior to the left and posterior to the right. The inset in each panel demonstrates an enlarged image of a limb bud viewed distally with anterior to the top.
ZPA
Besides the pre-AER/AER signals, it was hypothesized that a functional difference in Shh signaling between the C57 and the SWV strains of mice could explain the distinct sensitivity to RA-induced postaxial ectrodactyly based on the following evidence: (1) attenuation of Shh results in preferential loss of the most posterior digits (Parr and McMahon, 1995; Lewis et al., 2001); (2) Fgf8 in the AER is necessary for the initiation and maintenance of Shh (Lewandoski et al., 2000; Moon and Capecchi, 2000); and (3) postaxial ectrodactyly caused by other teratogens has been shown to be associated with the activity of Shh but not the levels of Shh mRNA or protein. (Bell et al., 1999; Scott et al., 2005). To examine the role of Shh in the strain difference to RA, expression analyses of Shh and some representative downstream genes in the Shh pathway were conducted using WISH.
Shh, Hand2.
WISH assays showed that there was no strain difference in the Shh expression at 6, 12, 24, and 36 h in vehicle-treated limb buds (Fig. 5B,D,F,H,J,L). The Shh expression domain was severely reduced at the 12 h time point in RA-treated forelimb buds of both strains (Fig. 5E,G), whereas it was not apparently different from controls in both strains at 6 h (Fig. 5A,C). In the RA-treated C57 limb bud, this attenuation of Shh mRNA was maintained at 24 and 36 h (Fig. 5I and data not shown), whereas the RA-treated SWV limb bud exhibited recovery from the diminution of the message at 24 and 36 h (Fig. 5K and data not shown). Thus, the duration of the reduced expression of Shh is greater in the C57 than in the SWV limb.
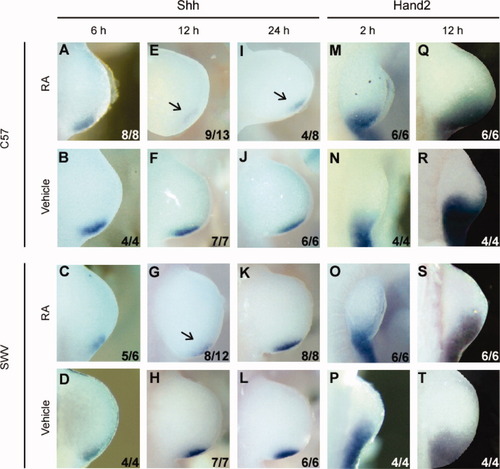
Shh and Hand2 expression in the forelimb buds after maternal exposure to a teratological level of RA. Shh expression in the forelimb bud was normal in both C57 and SWV strains at earlier stages (6 h), but became attenuated in both strains at 12 h. This reduction was maintained even at 24 h in the C57 limb bud, whereas the expression was recovered at 24 h in the SWV limb bud. On the other hand, the upstream gene of Shh, Hand2 was expressed normally in both strains at all the stages examined. (A–H) Localization of the Shh message at 6 (A–D), 12 (E–H), and 24 h (I–L). Note that the expression domain of Shh was clearly narrowed in the RA-treated C57 limb bud at 12 (E) and 24 h (I), whereas normal expression was observed at 6 h. Also note that the expression was reduced in the RA-treated SWV limb bud at 12 h as opposed to the normal expression at 6 and 24 h. (M–T) Hand2 expression at 2 (M–P) and 12 h (Q–T). Contrary to Shh expression, Hand2 was normally expressed both at 2 and at 12 h. All panels show dorsal views of the forelimb buds with anterior to the top and proximal to the left. Arrows in (E), (G), and (I) indicate reduction of Shh expression and the numerical data in the lower right corner demonstrate the number of embryos with the photographed expression pattern/the total number of embryos examined.
Hand2, which encodes a basic helix-loop-helix transcription factor, is required for the establishment of the ZPA and regulation of Shh expression (Fernandez-Teran et al., 2000; Charite et al., 2000). To assess whether Hand2 was responsible for the differential expression of Shh between the two murine strains, the expression patterns were examined at 2, 6, 12, 24, and 36 h using WISH (Fig. 5M–T and data not shown). However, no difference in expression was evident in Hand2 at any time point between the two strains in either the vehicle- or RA-treated limb buds. Therefore, although Shh expression demonstrated a strain difference in response to RA treatment where the C57 limb bud had a longer duration of attenuation than that of the SWV limb bud, this was not reflected by the alteration of the upstream gene Hand2.
Gli1, Gli3, Patched (Ptc), Suppressor of fused (Su(fu)).
Several representative genes that are downstream of Shh were tested to evaluate if the activity of Shh was different between C57 versus SWV limb buds in response to exogenous RA. Gli1, Gli3, and Ptc are known to be regulated by the Shh signal transduction pathway and are expressed in a Shh-dependent manner (Goodrich et al., 1996; Marigo et al., 1996; reviewed by Cohen, 2003). Thus, these genes were used as indicators of Shh signaling activity.
Gli1 and Gli3 are members of a DNA-binding zinc-finger protein family that mediates transcriptional effects of Shh signaling. The expression of Gli1 was detected in the posterior portion of the developing limb bud that included the putative ZPA (Fig. 6B,D,F,H). Of importance, a strain difference in the expression of Gli1 was observed at 12 and 24 h, where the C57 limb bud showed a loss of proximal posterior expression domain compared to the SWV limb bud after RA administration (Fig. 6E,G and data not shown). This strain difference was not clearly observed at 6 h following RA administration (Fig. 6A,C). In contrast to Gli1, Gli3 expression was gradually restricted to the anterior domain of the limb bud via inhibition by Shh at the posterior region during unperturbed embryogenesis, thus showing a complementary domain of expression to that of Shh (Litingtung et al., 2002). The RA-treated limb buds of both strains exhibited normal expression patterns of Gli3 at 24 h after RA administration (data not shown).
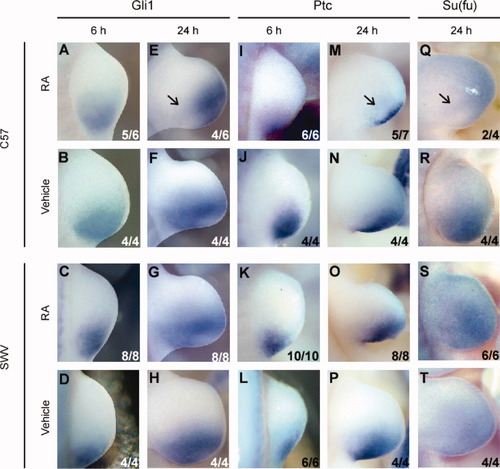
Attenuated expression of the genes in the Shh pathway observed in the RA-treated C57 forelimb bud at 24 h after RA treatment at GD 9.5. (A–H) Gli1 transcript visualized by labeled riboprobes in the C57 (A,B,E,F) and the SWV (C,D,G,H) forelimb buds at 6 (A–D) and 24 h (E–H) after injection. At 6 h, there was no strain difference in the expression of Gli1. At 24 h, however, the C57 forelimb bud exhibited diminution of Gli1 message (arrow in [E]), whereas SWV (G) exhibited comparable expression to the vehicle-administered limb bud. (I–P) Ptc transcript visualized by labeled riboprobes in the C57 (I,J,M,N) and the SWV (K,L,O,P) forelimb buds at 6 (I–L) and 24 h (M–P) after injection. Similar to Gli1, both strains had the same expression patterns of Ptc at 6 h, but the C57 forelimb bud showed reduced expression of Ptc (arrow in [M]) at 24 h, whereas SWV (O) showed the equivalent expression to the vehicle-administered limb bud. (Q–T) Su(fu) expression by WISH in the C57 (Q,R) and SWV (S,T) forelimb buds at 24 h after either RA or vehicle administration. Su(fu) expression was reduced after RA administration in the C57 forelimb bud (arrow in [Q]) compared with the SWV limb bud (S). All forelimb buds are viewed dorsally and oriented with anterior to the top and proximal to the left. The numerical data in the lower right corner indicate the number of embryos with the photographed expression pattern/the total number of embryos examined.
Ptc, which encodes the Shh receptor, was expressed in the same pattern as Gli1 in vehicle-treated limb buds (Fig. 6J,L,N,P). Similar to Gli1, the Ptc expression was attenuated in the C57 limb bud but not in the SWV limb bud at 12 and 24 h after RA administration (Fig. 6M,O and data not shown), whereas it was normally expressed in both strains irrespective of the treatment at 6 h after administration (Fig. 6I–L). These results indicate that although both strains had attenuation of the Shh message at 12 h after RA treatment, the SWV limb buds retained normal Shh signaling, whereas the C57 limb buds did not.
It is known that one of the members of the hedgehog family, Indian hedgehog (Ihh), shares potential functional redundancy with Shh (Yang et al., 1998). Thus, the localization of Ihh was also examined to determine whether it was increased when Shh was decreased. However, no ectopic expression of Ihh was detected by WISH at the relatively early gestational period in either of the strains with either treatment at 12 or 24 h (data not shown).
To further explore the functionality of the Shh signaling, the expression pattern of Su(fu) was examined. Su(fu) is postulated to tether Gli proteins in the cytoplasm and to function as a negative regulator of Hedgehog signaling (Ding et al., 1999); thus, it was possible that Shh signaling was altered even under the normal transcription status of Gli genes by the aberrant expression of Su(fu) following RA administration. As in vehicle-treated limb buds, the SWV limb bud exhibited the normal expression pattern of Su(fu) following RA administration (Fig. 6R–T), indicating that normal Shh signaling activity could occur. On the contrary, the C57 limb bud showed reduced expression of Su(fu) at 24 h, particularly in the posterior aspect of the limb bud (Fig. 6Q). Therefore, it is predicted that, in the C57 limb bud, the inhibitory activity of Su(fu) on the Shh pathway would be attenuated in response to RA treatment, but not in the SWV. This contrasts with the down-regulation of Shh expression in the RA-treated C57 limb bud.
Taken together, C57 and SWV limbs showed differential expression of downstream genes such as Gli1 and Ptc in response to a teratogenic dose of RA despite the reduction of the Shh message in both murine strains, indicating that Shh signaling activity is different in the two strains following RA administration. The strain difference in the Shh pathway cannot be explained by a strain-specific up-regulation of Ihh in the RA-treated SWV, although other unknown genes may have redundancy with Shh. However, the alteration of Su(fu) expression raises the possibility that numerous genes in the Shh signaling pathway may be involved in the polarizing activity in the ZPA. Further studies are needed to assay the functionality of Shh signaling.
Bmp2, Bmp4, Fgf4, Gremlin.
In addition to the bona fide target genes of Shh such as the Gli family and Ptc, a variety of genes or gene families have been identified as potential mediators of Shh signaling, including Bmp (Drossopoulou et al., 2000), Gremlin, Fgf (Zuniga et al., 1999), and the 5′ members of the HoxD complex (Chiang et al., 2001; Kraus et al., 2001). To further explore the differential activity of Shh-related genes between the two strains, the expression patterns of Bmp2, Bmp4, Fgf4, Gremlin, and 5′ HoxD genes at 12 and 24 h were investigated (for 5′ HoxD genes, see the next section).
Bmps are a family of genes negatively regulated by the antagonist Gremlin that serve as an intermediate between Shh in the ZPA and Fgf4 in the AER to maintain the proper patterning of the developing limb bud (Zuniga et al., 1999). Bmp4 is normally expressed throughout the length of the AER and in the underlying mesenchyme (Fig. 7F,H). There was a strain-specific absence of Bmp4 in the posterior pre-AER and AER at 6, 12, and 24 h in the C57 limb bud. This is consistent with the fact that the posterior AER is structurally truncated in the RA-treated C57 limb bud as demonstrated earlier in this study, thus this abnormality was not caused by the Shh reduction. Fgf4 and Gremlin, a member of the differential screening-selected gene aberrant in neuroblastoma/cerberus gene family and an inhibitor of Bmps, are other components of the AER/ZPA feedback loop. Unlike Fgf8, Fgf4 begins to be expressed only after the definitive AER is established (Sun et al., 2002). Thus, as expected, posterior Fgf4 expression was absent in the RA-treated C57 limb bud because there was a structural absence of the posterior AER, but was unaltered in the RA-treated SWV limb bud (Fig. 7I–L). Gremlin was expressed in the distal mesenchyme of the limb bud (Fig. 7N,P). There was no definitive strain difference induced by exogenous RA in the expression pattern of this gene in the limb (Fig. 7M,O).
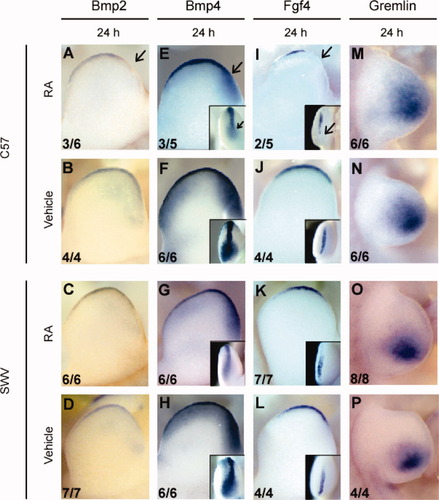
mRNA localization of some representative genes regulated by Shh in C57 and SWV forelimb buds in response to RA or vehicle administration at GD 9.5. The data in the lower left corner indicate the number of embryos with the photographed expression pattern/the total number of embryos examined. (A–D) Bmp2 expression in the C57 forelimb bud (A,B) and the SWV limb bud (C,D) at 24 h after RA administration. The mesenchymal expression was insufficient to recognize the differential expression pattern at this stage. (E–H) mRNA of Bmp4 visualized by WISH in the C57 forelimb bud (E,F) and the SWV limb bud (G,H) at 24 h. Other than the posterior AER, the expression pattern was not impaired in either strain with either treatment. (I–L) Transcript of Fgf4 detected by labeled riboprobe in the C57 forelimb bud (I,J) and the SWV limb bud (K,L) at 24 h. Note that, consistent with Fgf8 expression, expression patterns of Bmp2, Bmp4, and Fgf4 demonstrate that the RA-treated C57 forelimb has an absence of these genes in the postaxial AER in contrast to the normal AER in vehicle-treated C57 forelimb buds or the SWV forelimb buds with either treatment (arrows in [A,E,I]). (M–P) Distribution of Gremlin expression in the developing forelimb buds of C57 (M,N) and SWV (O,P) strains. There was no considerable difference in the expression pattern between the two strains. Each limb is dorsally viewed with anterior to the top and proximal to the left. Each inset shown in (E–L) demonstrates a posterior view of the forelimb bud (distal to the top). All arrows indicate loss of gene expression in the posterior AER.
Bmp2 has been hypothesized to be a downstream gene of Shh in chick (Yang et al., 1997; Drossopoulou et al., 2000). WISH analyses revealed that the posterior mesenchyme, where Shh is expressed, showed detectable Bmp2 expression in both strains at GD 11.0 with either treatment (36 h; data not shown) but not in the stages prior to the manifestation of the truncation in the posterior mesenchyme (Fig. 7A–D). For the AER expression of Bmp2, a strain difference was observed for the postaxial region of AER that was reduced in RA-treated C57 but not in RA-treated SWV (Fig. 7A–D and data not shown).
Genes Expressed in Response to Positional Signals
In the present study, WISH assays were performed to find critical genes associated with the strain difference in the sensitivity to RA-induced postaxial ectrodactyly. Genes of interest included not only factors involved in the onset of postaxial ectrodactyly, but also the components of the downstream events that were associated with the pathogenesis leading to teratogenesis. Because some genes involved in conveying positional information in the limb field were disturbed in this study, it was speculated that the subsequent events in limb development would also be affected. To further explore the downstream factors leading to postaxial ectrodactyly, selected genes functioning secondary to the positional information were examined with the focus of delineating the pathogenic pathway of the strain difference in ectrodactyly.
Hox cluster genes.
Hox genes, which are a subset of homeobox genes, encode a family of transcription factors that are predicted to be crucial in patterning the body axis. Here, the distribution of the transcripts of Hoxa-13, Hoxd-11, -12, and -13, which have long been implicated in autopod formation (Zákány et al., 1997; Fromental-Ramain et al., 1996; Davis and Capecchi, 1996), was examined.
WISH assays for Hoxa-13 mRNA showed localization in the distal region of the limb bud in vehicle-treated embryos at GD 10.5 in C57 and GD 10.67 in SWV (24 h postadministration). Following RA administration, the C57 limb bud demonstrated diminished expression of Hoxa-1, whereas the SWV limb bud showed relatively normal expression (Fig. 8A–D). The RA-treated C57 limb buds showed similar expression patterns to RA-treated SWV limb buds for Hoxd-11 and -12 (data not shown). However, the Hoxd-13 messages demonstrated a posterior reduction of the expression domains following RA administration in the C57 but not the SWV strain compared to the controls at 24 h (Fig. 8E–H). This is presumed to be due to an alteration in the positional information of the posterior domain. In addition, it should be noted that posterior Hoxd genes are partially regulated by Shh (Zákány et al., 2004). Therefore, the strain differences in Hox expression may reflect a differential activity of Shh between the two strains.
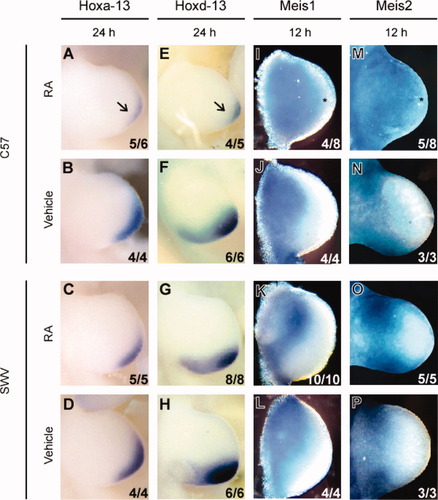
Whole-mount detection of Hox (A–H) and Meis (I–P) transcripts by labeled riboprobes revealed the differential expression patterns between the two strains in response to RA. (A–D) Distribution of Hoxa-13 message in C57 (A,B) and SWV (C,D) forelimb buds. (E–H) Localization of Hoxd-13 transcript in C57 (E,F) and SWV (G,H) forelimb buds. Note the reduced expression of both Hoxa-13 and Hoxd-13 in the RA-treated C57 forelimb buds (arrows in [A,E]) as opposed to the RA-treated SWV forelimb buds. (I–L) Meis1 expression in C57 (I,J) and SWV (K,L) forelimb buds. (M–P) Expression of Meis2 mRNA in C57 (M,N) and SWV (O,P) forelimb buds. Note that both Meis1 and Meis2 expression was distally expanded in response to RA administration in C57 forelimb buds (asterisks in [I,M]), whereas the distal expansion was less in SWV forelimb buds. The numerical data in the lower right corner denote the number of embryos with the photographed expression pattern/the total number of embryos examined. All panels are of dorsally viewed forelimb buds (anterior to the top and proximal to the left).
Meis genes.
Two closely related Drosophila homothorax orthologs containing homeobox domains, Meis1 and Meis2, are thought to play an important role in determining the limb proximodistal identity (Mercader et al., 2000). The normal expression patterns of these genes are localized in the proximal region in the developing limb bud (Fig. 8J,L,N,P). Notably, at 12 and 24 h after RA administration, C57 limb buds demonstrated an aberrant distal expression of Meis1 and Meis2, whereas the RA-treated SWV limb bud showed normal expression patterns (Fig. 8I,K,M,O). Therefore, the differential localization of Meis transcripts between C57 and SWV limb buds in response to RA treatment does reflect the strain difference with regard to RA-induced ectrodactyly.
Retinoid Metabolism Genes
The posterior digit may be more sensitive to RA-induced perturbation because the region of the limb bud that is fated to develop the most posterior digits has the highest endogenous level of RA (Maden et al., 1998). Thus, assuming that the endogenous RA distribution is not altered by exogenous RA, the posterior region may reach a threshold for perturbation prior to other regions of the limb bud. To examine if the administration of exogenous RA had a strain-specific effect on the production of endogenous RA, transcripts involved in endogenous RA production and destruction were localized.
In the developing limb bud, retinaldehyde dehydrogenase 2 (Raldh2) and the cytochrome P450 enzyme, Cyp26b1, play major roles in the quantitative control of endogenous RA metabolism by regulating RA synthesis and catabolism, respectively (Mic et al., 2004; Yashiro et al., 2004). The administration of the teratogenic dose of RA led to the decrease of Raldh2 and an increase of Cyp26b1 (data not shown). Raldh2 expression, which was observed in the lateral plate mesoderm and the somites, was slightly attenuated in the RA-treated limb buds at 2 and 6 h (data not shown). Cyp26b1 was located in the distal region of the limb bud in both ectoderm and mesoderm and decreased in a distal to proximal gradient and was increased uniformly in intensity (data not shown). These effects in Raldh2 and Cyp26b1 were not different between the strains. Thus, the endogenous RA homeostasis in response to the exogenous RA was not different between the two strains at the transcriptional level.
DISCUSSION
This study was predicated on the fact that RA administration on GD 9.5 to C57 mice causes forelimb ectrodactyly, whereas the same treatment in SWV mice does not cause this malformation. The dysmorphogenesis that results in the limb defect can be detected in mouse embryos at ∼GD 11, where it manifests as a notch in the posterior aspect of the developing limb bud. The goal of this study was to use a WISH assay in order to find genes involved in limb development that differed between the two mouse strains prior to the time of morphologic manifestation of the malformation. By delineating the differences in gene expression between the strains and by determining the temporal sequence of such events, it was hoped that the pathogenesis of the malformation could be described temporally and that early events in the pathogenesis would yield clues as to the mechanism of this malformation.
Effects of RA on the AER
Perturbation of postaxial pre-AER revealed byFgf8 expression.
The whole process of AER formation begins with induction of a population of cells in the ventral ectoderm (termed pre-AER before GD 10.0, corresponding to 12 h after treatment). The pre-AER cells then migrate towards the apex of the bud, where they undergo compaction to form a protruding ridge (the definitive AER after GD 10.0) that separates dorsal from ventral ectoderm (Loomis et al., 1998).
Based on the microscopic observation of a morphological truncation of the postaxial AER at 24 and 36 h in the RA-treated C57 limb bud, the expression of the pre-AER/AER marker gene, Fgf8 (Crossley and Martin, 1995), was examined. Indeed, the transcript for Fgf8 was diminished in the posterior domain of the AER in the RA-treated C57 limb bud at 24 and 36 h as found under microscopic observation, whereas the RA-treated SWV limb bud exhibited the same expression pattern as the vehicle-treated limb bud. This result is not unprecedented because truncation of the AER had been demonstrated in the induction of the same malformation caused by midgestational exposure to several teratogens, including ethanol (Chrisman et al., 2004), 13-cis-RA (Sulik and Dehart, 1988), cadmium (Scott et al., 2005), and acetazolamide/benzamil (Bell et al., 1999).
However, importantly, the present study has revealed that this AER disruption unveiled in the posterior margin of the RA-treated C57 limb bud was detectable even in AER precursor cells, which was manifested by the posterior loss of Fgf8 expression as early as 2 and 6 h after treatment. The early disruption of Fgf8 expression in pre-AER cells supports the concept that this reduction may be the initiating event in the AER truncation that could cause postaxial ectrodactyly. In contrast, other AER marker genes, Dlx2 and p63, were normally expressed in pre-AER cells in the RA-treated C57 limb bud, suggesting that the posterior insult to the pre-AER was gene-specific and not a total destruction of the pre-AER cells. Thus, it is hypothesized that the process by which AER precursor cells (at 2 and 6 h post-treatment) form the definitive AER (by 12 h post-treatment) was impeded by exogenous RA due to a lack of gene expression in the postaxial aspect of the C57 limb bud.
Perturbation of other pre-AER marker genes.
As discussed earlier, perturbation of Fgf8 expression in the postaxial ectoderm is a precursor to postaxial ectrodactyly in the RA-treated C57 limb bud. Indeed, as demonstrated by the diminished expression of several Fgf-induced genes including Mkp3, Sef, and Spry2, not only the message but also the functionality of the Fgf8 signaling in the posterior limb bud seems to be affected in the C57 but not in the SWV murine strain in a way that may be associated with the strain difference in ectrodactyly. In contrast to the expression patterns of Mkp3, Sef, and Spry2, which were altered at 12 h, the Fgf10 expression was perturbed at 2 h and this perturbation recovered at 6 h. Because Fgf10 induces the Fgf8 expression in the AER prior to GD 9.5 followed by formation of the feedback loop with Fgf8 to reciprocally maintain the expression, it is highly possible that the reduced expression of Fgf10 in the RA-treated C57 limb bud was a transient alteration unrelated to the perturbation of the Fgf8 expression.
Although the expression and functionality of Fgf8 is affected by exogenous RA in the C57 limb bud, loss of only Fgf8 is not sufficient to explain the failure in the AER formation because it has been demonstrated that the limb bud of an Fgf8 conditional knockout mouse with the gene specifically inactivated in the limb can form a normal AER (Moon and Capecchi, 2000; Lewandoski et al., 2000). Moreover, because Fgf8 is a member of a large family of genes, and there is a possibility of functional redundancy, it may be that mice that are Fgf8-null have an up-regulation of other Fgfs (Lu et al., 2006; Sun et al., 2000). Other Fgfs that are located in the AER include Fgf4, Fgf9, and Fgf17 (Lewandoski et al., 2000; Moon and Cappechi, 2000; Vogel et al., 1996).
The current study has demonstrated the expression of Bmp4, Sp8, and Dlx3 genes is also perturbed in the postaxial pre-AER of the limb of the C57 mouse but not the SWV mouse. Bmp4 is a good candidate gene for having differential expression between the strains because Bmps are the only genes, besides Fgfs, that have been shown to function in AER formation. Experiments in both chick and mouse have shown that in the absence of Bmp signaling, the AER was not formed and Fgf8 expression was not induced (Pizette et al., 2001; Ahn et al., 2001). Additionally, it has been demonstrated that Bmps expressed in the pre-AER might have a role in delimiting the boundaries of the AER by precluding adjacent nonridge ectodermal cells from turning into AER cells (Wang et al., 2004). However, it has been shown that the conditional Bmp4 mutant mouse can form delayed but normal AER (Selever et al., 2004). Moreover, the postaxial truncation of Bmp4 in the pre-AER was observed in both right and left limb buds of all the embryos examined. Because the ectrodactyly only occurred in ∼60% of the embryos with a preference for the right side (Collins et al., 2006), the Bmp4 truncation might not correlate with the malformation. Thus, it appeared that the loss of Bmp4 expression was not sufficient to induce ectrodactyly although it might be necessary. In addition, Sp8 was also a potential candidate to explain AER dysmorphogenesis because Sp8 mutant mice exhibit normal pre-AER marker genes initially, but they were eventually extirpated and AER formation was inhibited (Bell et al., 2003), indicating that Sp8 plays a crucial role in establishment of the definitive AER. Regarding Dlx3, the detailed molecular pathways in the developing limb bud have not been elucidated and the function is not completely understood at this time. However, it is located in the region of chromosome 11 where significant linkage to the strain difference in postaxial ectrodactyly was demonstrated by a QTL analysis (Lee et al., 2005). Hence, it is plausible that Dlx3 is associated with the strain difference. In addition to the selected genes examined in the current study, other pre-AER/AER localized genes purportedly involved in AER progression should be evaluated to more completely characterize the AER disruption, including p21, Msx1/2, CD44, Sp9, and Cx43.
Effects of RA on the ZPA
Differential Shh mRNA in the two strains.
A primary focus in the present study was to examine the effect of RA on the ZPA, which is located in the posterior margin of the developing limb bud and believed to play a pivotal role in anteroposterior specification of this structure. Importantly, altered ZPA function has been associated with teratogen-induced postaxial ectrodactyly due to its anatomical position as well as other factors (Hayes and Morriss-Kay, 2001; Chrisman et al., 2004; Bell et al., 2005; Scott et al., 2005). The function of the ZPA is mediated, at least partially if not exclusively, by Shh (Riddle et al., 1993). Furthermore, the accumulated evidence has suggested that a physiological level of RA is required for the induction of Shh in the developing limb bud (Riddle et al., 1993). On the other hand, the down-regulation of Shh by RA has been reported only in craniofacial dysmorphogenesis when embryos were exposed to teratogenic doses of RA (Helms et al., 1997). Although the predominant view of Shh expression is that it is downstream of the retinoid pathway, it has been demonstrated that Shh and RA act synergistically in differentiation of mesenchymal stem cells into glutaminergic neurons (Kondo et al., 2005). Perhaps RA and Shh are both involved in the polarizing activity of the ZPA.
The exposure to the embryotoxic dose of RA at GD 9.5 for C57 mice or GD 9.67 for SWV mice resulted in a severe reduction of Shh at the transcription level in limb buds prior to the manifestation of the malformation, although the upstream Hand2 gene was expressed normally in limb buds from both strains. Of interest, however, the diminution of the Shh expression was maintained longer in the ZPA of C57 (for ≥24 h) compared with the ZPA of SWV (for 12 h) following RA administration. The longer duration of Shh down-regulation in the C57 ZPA than in SWV may cause the preferential loss of the fifth digit in the sensitive strain because the patterning of this digit has been proposed to require both a high concentration and a long duration of Shh activity (Harfe et al., 2004). Alternatively, the loss of Shh expression at the longer time points may reflect the loss of postaxial mesenchymal cells as a prelude to the malformation, in which case the reduced Shh is a result of the malformation as opposed to a cause.
Differential Shh signaling activity in the two strains.
Previous work had suggested that cadmium- or acetazolamide-induced postaxial ectrodactyly resulted from reduced Shh signaling activity without decreased Shh transcription or translation (Scott et al., 2005; Bell et al., 2005). Thus, to evaluate the Shh activity in response to RA, the expression of several downstream genes was examined. In fact, Shh activity after administration of RA was presumed different between the C57 and SWV limb buds as indicated by the reduced expression of Gli1 and Ptc in the RA-treated C57 as opposed to the normal expression in the RA-treated SWV. Likewise, Hoxd-13, which is partially regulated by Shh in the developing limb bud (Zákány et al., 2004), demonstrated differential expression in limb mesenchyme between the two strains after maternal treatment with a teratogenic dose of RA. Because Hoxd-13 expression was diminished in response to RA in the C57 limb bud but normal in the SWV limb bud, the anteroposterior polarity established by the ZPA may be somewhat reduced in the C57. To support this conclusion, previous studies have demonstrated, using a polarizing activity assay, an abrogated polarizing activity of ZPA by teratogen exposures that specifically produce forelimb ectrodactyly (Bell et al., 1999; Scott et al., 2005). However, Shh activity manifested by the expression of downstream genes does not necessarily mirror polarizing activity because there has been no evidence so far suggesting that downstream gene products exhibit polarizing activity (Capdevila and Izpisúa Belmonte, 2001). Thus, it would be valuable to know if forelimb ectrodactyly caused by RA is associated with an alteration of polarizing activity. Moreover, future studies should focus on the mechanisms by which the Shh activity is reduced, as well as how the loss or attenuation of polarizing activity results in ectrodactyly.
Intriguingly, it was demonstrated that Su(fu), which negatively regulates Shh signaling by modulating Gli activity (Barnfield et al., 2005), was attenuated in the C57 limb bud but not in the SWV limb bud following RA administration. This finding contrasts with the concept that Shh signaling was reduced in the C57 but not in the SWV limb bud. In fact, emerging bodies of evidence have demonstrated that, in Drosophila, several factors in the Hh signaling pathway have been implicated in Gli or Ci (ortholog of vertebrate Gli) modulation (reviewed by Cohen, 2003). This raises the possibility that Gli transcription is determined by a variety of Shh-related factors. Further studies are required to dissect the Shh signal transduction pathway altered by toxicological levels of RA.
Altered Proximodistal Patterning
The foregoing discussion focuses on the alterations of gene expression in the AER and the ZPA that lead to the hypothesis that ectrodactyly results from a strain-specific effect on limb bud outgrowth or anteroposterior specification, respectively. However, it could be hypothesized that the data support a strain-specific perturbation of proximodistal patterning. Previous research has suggested that RA administration causes a proximalization of limb patterning, which results in a phenotype including ectrodactyly (reviewed in Lee et al., 2004). The results of the current study, where the expression domains of Meis1 and -2 were distally expanded in the limb bud and the expression of Hoxa-13 was significantly reduced in the RA-treated C57 mouse, indicate that the C57 mouse is susceptible to the proximalization, whereas the SWV strain is not. Moreover, the distal expansion of the Meis expression in the RA-treated C57 limb bud is consistent with the previous finding that Meis is negatively regulated by Fgf signals from the AER (Mercader et al., 2000), because down-regulation of Fgf8 in the posterior region could lead to up-regulation of Meis.
Morphogenic Interactions between the AER and the ZPA
The present study has led to the hypothesis that alterations of AER signaling and/or ZPA signaling may explain the strain difference in sensitivity to RA-induced forelimb ectrodactyly and has suggested a sequence of events in the pathogenesis of the digital malformation. Given that perturbation of the pre-AER was detected in a strain-specific manner at early stages (2–6 h postadministration) in the putative domain where the malformation was formed, one potential hypothesis is that this perturbation was causative for posterior ectrodactyly. However, the mechanism of the perturbation in the pre-AER remains elusive.
One possibility is that the exogenous RA directly inhibits a subset of genes expressed in the postaxial pre-AER of the C57 limb bud. This is consistent with the fact that pre-AER perturbation was caused relatively soon after the compound administration (2 or 6 h). In fact, it has been reported that the embryonic concentration of RA administered IP reaches a maximum during this period (Collins et al., 2006), which indicates that expression effects at 2–6 h in vivo are relatively rapid. To explain why the malformation occurs almost exclusively in the posterior aspect, it can be hypothesized that there are interactions with other genes that are expressed exclusively in the postaxial pre-AER. However, although there are genes that are expressed predominantly in the postaxial AER, for example Fgf4, Fgf9, and Fgf17 (Lewandoski et al., 2000), there are no known genes expressed exclusively in the equivalent domain in the pre-AER. The present study also tested the possibility that the endogenous enzymes responsible for RA metabolism were differentially expressed between the strains. A strain difference was not observed in the expression patterns of these genes following exogenous RA administration although the observed transcriptional down-regulation of the RA synthesizing gene and the up-regulation of the RA oxidizing gene by exogenous RA were logical homeostatic responses.
Another possibility is that the Shh signaling is perturbed immediately after RA treatment in the C57 limb bud by a process that is independent of Shh transcription, resulting in an insult to a subset of genes in the postaxial pre-AER. Indeed, it was suggested that, in contrast to the conventional concept that Shh signaling occurs within the limb bud mesenchyme, Shh signaling could occur in the non-AER ectoderm overlying the ZPA to maintain the posterior AER (Bell et al., 2005). This pathway of events could explain the posterior nature of the digit defect because Shh signaling is presumed to be most prominent in the posterior domain due to the localization of Shh mRNA. However, the nature of the Shh signaling to the ectoderm remains enigmatic, and Shh signaling cannot be effectively determined by gene expression patterns. Therefore, the molecular basis of the presumptive Shh signaling from the ZPA to the ectoderm requires further studies.
It is hypothesized that the insult to the pre-AER leads to the failure in the formation of the postaxial AER. Because substantial evidence has delineated that the AER signal is indispensable for the maintenance of Shh (Moon and Capecchi, 2000; Lewandoski et al., 2000; Ros et al., 1996; Crossley et al., 1996; Grieshammer et al., 1996), it is presumed that the RA-induced loss of the posterior AER might subsequently result in the diminution of Shh expression and downstream genes such as Gli1 and Ptc in the RA-treated C57 limb bud (at 24 h). Alternatively, it could be interpreted that, in the RA-treated SWV limb bud, the transient attenuation of the Shh expression observed at 12 h was rescued due to the intact posterior AER activity, but in the RA-treated C57 limb bud, such a recovery does not occur due to the absence of the posterior AER activity.
In summary, it could be hypothesized that there are two potential targets for the pathogenesis of postaxial ectrodactyly. One target is the pre-AER (Fig. 9BI-b) and the other is the ZPA (Fig. 9BI-a).
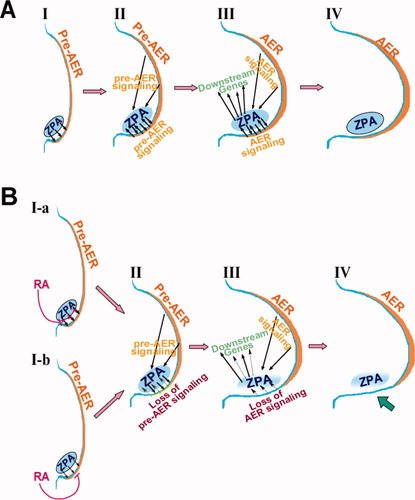
A schematic model depicting the temporal molecular process of postaxial ectrodactyly with emphasis on the pre-AER/AER signal and the ZPA signal. (A) In normal limb development, the ZPA signaling and the pre-AER/AER signaling reciprocally mediate each other (I, II). Shh in the ZPA regulates several downstream genes (III) and modulates proper limb development (IV). RA-treated SWV may have some impaired Shh transcription but this effect is presumed marginal based on the fact that the RA-treated SWV limb has normal morphology. (B) The two possibilities explaining the perturbation of the posterior pre-AER in the RA-treated C57 limb bud are discussed in the current text. One possibility is that exogenous RA directly affects the posterior pre-AER, which results in the gene-specific absence of several genes such as Fgf8, Bmp4, Sp8, and Dlx3 but not Dlx2 or p63 (at 2–6 h) (I-a). Alternatively, exogenously administered RA perturbs the Shh signal from the ZPA by a process that is independent of Shh transcription, causing the insult to a subset of genes in the postaxial pre-AER (at 26 h) (I-b). The perturbation in the postaxial pre-AER leads to the perturbation in the formation of the definitive AER posteriorly, which, in turn, affects the ZPA (Shh) due to the loss of the signal from the posterior AER (at 12–24 h) (II). As a result, the expression patterns of Shh-downstream genes including Gli1, Ptc, and 5′Hoxd genes are altered, whereas some genes including Bmp and Gremlin are unaltered (at 12–24 h) (III). Subsequently, perturbations of the AER and the ZPA cause the posterior limb truncation (IV).
Acknowledgements
The authors acknowledge with much appreciation the generous gifts of cDNA probes from the following individuals: Dr. Neal Copeland (Meis1, -2: Frederick Cancer Research and Development Center, Frederick, MD), Dr. Gregg Duester (Raldh2: Burnham Institute, San Diego, CA), Dr. Hiroshi Hamada (Cyp26b1: Osaka University, Osaka, Japan), Dr. Richard Harland (Gremlin: UC Berkeley, Berkeley, CA), Dr. Jeffrey Innis (Hoxa-13: University of Michigan, Ann Arbor, MI), Dr. Alexandra Joyner (Gli1, Gli3: NYU, New York, NY), Dr. Marie Kmita (Hoxd-11, Hoxd-12, Hoxd-13: University of Geneva, Geneva, Switzerland), Dr. Toshihisa Komori (Ihh: Nagasaki University, Nagasaki, Japan), Dr. Karen Lyons (Bmp2, Bmp4: UCLA, Los Angeles, CA), Dr. Gail Martin (Fgf4, Fgf8, Fgf10: UC San Francisco, San Francisco, CA), Dr. Frank McKeon (p63: Harvard University, Boston, MA), Dr. Andrew McMahon (Shh: Harvard University, Cambridge, MA), Dr. John Rubenstein (Dlx2: UC San Francisco, San Francisco, CA), Dr. Matthew Scott (Ptc: Stanford University, Palo Alto, CA), Dr. Deepak Srivastava (Hand2: University of Texas Southwestern Medical Center, Dallas, TX), and Dr. Yingzi Yang (Col II: NIH, Bethesda, MD).




