Factor Analysis of the Missed Diagnosis of Total Anomalous Pulmonary Venous Connection in Prenatal Echocardiography
Funding: This work was supported by National Science Foundation of China (Grant 81271593) and the Science and Technology Department of Hunan Province (Grant 1013-70).
Qichang Zhou and Dongmei Liu contributed equally to this study.
ABSTRACT
Aim
This study investigated the major factors contributing to the missed diagnosis of total anomalous pulmonary venous connection (TAPVC) in fetal echocardiography.
Methods
We retrospectively analyzed the prenatal ultrasonic images of 32 fetuses with missed diagnoses of TAPVC, compared them with autopsy and postnatal surgical records, and summarized the most likely reasons leading to the missed diagnoses.
Results
We studied a total of 157 fetuses with TAPVC, 32 (20.3%) of whom were missed in prenatal echocardiography. The main factors for the missed diagnoses of TAPVC in the 32 fetuses were anatomic variants leading to the formation of a false pulmonary venous horn-like structure, the combination of TAPVC with other intracardiac anomalies, difficulty or inability to show the course and abouchement of TAPVC on conventional color Doppler flow imaging (CDFI), and excessive color flow gain, with a rate of approximately 53.1% (17/32). A decreased left atrial size and augmentation of the PLAS index may be indicators of false pulmonary venous horn-like structure.
Conclusion
False pulmonary venous horn-like structures due to anatomic variants are a major factor in the missed diagnosis of fetal TAPVC. The presence of pulmonary venous horn-like structure in a four-chamber view does not completely exclude TAPVC.
1 Introduction
Total anomalous pulmonary venous connection (TAPVC) is a rare and severe congenital heart disease (CHD), accounting for approximately 1%–3% (Hoffman and Kaplan 2002; Michielon et al. 2002) of congenital heart disease cases, and is the fifth largest cause of cyanotic CHD. The prognosis is extremely poor, with 80% of babies dying in infancy without surgical treatment. If an accurate diagnosis can be obtained in utero or in the neonatal period, it is of great clinical significance for early intervention, the formulation of surgical plans, and the improvement of the prognosis. (Kao et al. 2017; Olsen et al. 2016) However, the small heart of the intrauterine fetus, thin diameter of the pulmonary veins, the lower blood flow velocity, and the limitations of the ultrasound probe's resolution and blood flow sensitivity make the prenatal diagnosis of TAPVC difficult and challenging. In the past 10 years, with the increase in the probe resolution and low-speed blood flow sensitivity of ultrasound instrument, the number of cases of correct prenatal diagnosis, timely surgical treatment of newborns, number of newborns with a good prognosis has increased (Olsen and others, 2016; Vanderlaan and Caldarone 2018). These results show good prospects for the treatment of the fetuses TAPVC. However, it is important to be aware that TAPVC is still one of the most commonly missed CHD diagnoses in prenatal echocardiography. Large sample analyses of the causes of the missed diagnosis of TAPVC in prenatal ultrasound have been reported less frequently. In this study, we collected data for 32 cases of missed diagnosis in prenatal ultrasound confirmed by autopsy, postnatal echocardiography, or computed tomographic angiography (CTA) and surgery for analysis. Our aim was to explore the main factors contributing to missed diagnosis and to provide a scientific and objective basis for the development of strategies and methods to reduce the missed prenatal diagnosis rate for fetuses with TAPVC.
2 Methods
2.1 Study Population
This study was conducted retrospectively at the Second Xiangya Hospital of Central South University, Xiangya Hospital, Hunan Maternal and Child Health Care Hospital, Changsha Maternal and Child Health Care Hospital, Changde Maternal and Child Health Care Hospital, and Zhuzhou Maternal and Child Health Care Hospital, all of which are tertiary hospitals, from January 2015 to December 2022. We reviewed all the conventional prenatal ultrasound databases of these hospitals and concluded that there were 436,054 singleton fetuses delivered at 15 to 40 gestational weeks. Then we collected information for the mothers and newborns (names, ID card numbers and home addresses) and the neonatal echocardiography, surgery, and autopsy records.
2.2 Inclusion Criteria for the Fetal TAPVC Group Were as Follows
All examinations were carried out by trained operators, who were blinded to the group status.
(1) singleton pregnancy; (2) fetuses with accurate gestational weeks (gestational age corrected for estimated fetal weight); (3) fetuses with complete data and complete ultrasound biological parameters; (4) fetuses with autopsy, postnatal echocardiography, or CTA examinations and surgical confirmation; and (5) fetuses with follow-up records. In addition, 32 nonconsecutive, same year examination, same gestational age (GA) pairs with normal postnatal examinations were randomly selected from the database as normal controls.
2.3 Exclusion Criteria for the Fetal TAPVC Group Were as Follows
(1) twin pregnancy; (2) fetuses with incomplete data and missing fetal measurement parameters; (3) fetuses with incomplete echocardiography, CTA examination or surgical results after birth; and (4) fetuses lost to follow-up.
The study was approved by the Institutional Ethics Committees of the Second Xiangya Hospital, Xiangya Hospital, Hunan Maternal and Child Health Care Hospital, Changsha Maternal and Child Health Care Hospital, Changde Maternal and Child Health Care Hospital, and Zhuzhou Maternal and Child Health Care Hospital. All pregnant women signed the prenatal diagnosis informed consent form before undergoing the examination and provided valid contact information to facilitate follow-up and tracking.
2.4 Ultrasound Methods
GE Voluson E10 and E8 color Doppler ultrasound diagnostic instruments with probe models C2-9-D and C1-5 were used. Fetal obstetric examination procedures were selected following the As Low As Reasonably Achievable (ALARA) principle. First, obstetric sonographers conducted conventional obstetric ultrasonography and biological measurements to evaluate fetal growth and development as a whole and to screen for structural malformations of the extracardiac system. Subsequently, fetal cardiac examination was performed according to the practice guidelines for fetal cardiac ultrasound screening developed by the International Society of Obstetrics and Gynecology Ultrasound (ISUOG) (International Society of Ultrasound in and others 2013). The atrioventricular size, aortic and pulmonary artery diameters, foramen ovale diameter, and PLAS index (the ratio of the left atrium-descending aorta distance divided by the descending aorta diameter) were routinely measured. Pulmonary venous examination and measurements were performed by obtaining a standard four-chamber view, magnifying the image until the heart occupied at least one-third to one-half of the screen, and lowering the color Doppler velocity scale to 20 cm/s, showing that at least two pulmonary veins drained into the left atrium (LA) (Chaoui, Heling, and Karl 2014).
All static and dynamic antenatal ultrasound images of the fetuses with missed TAPVC diagnoses were retrieved from the database. Three prenatal diagnostic experts and one pediatric cardiac surgeon in the research group analyzed each case frame by frame to identify and summarize the main reasons for the missed diagnosis.
2.5 Statistical Analysis
SPSS 25.0 was used for statistical analysis. Continuous variables that conformed to the normal distribution are presented as the mean ± standard deviation (mean ± SD). The paired-sample t-test was used for comparison between the two groups. If p < 0.05, statistical significance was assumed.
3 Results
3.1 General Condition
By searching the fetal and neonatal databases of the above hospitals, 32 fetuses were shown to have missed TAPVC diagnoses, and 125 fetuses with TAPVC were correctly diagnosed. According to the TAPVC classification, among the 32 fetuses with missed TAPVC diagnoses, 12 had intracardiac TAPVC (37.5%), 9 had supracardiac TAPVC (28.1%), 6 had infracardiac TAPVC (18.7%), and 5 had mixed TAPVC (15.6%). Detailed information on fetuses with missed diagnoses was shown in Table 1.
| Case | Type of TAPVC | GA(W) | Concomitant deformity | Factors missed by ultrasound examination | Outcome |
|---|---|---|---|---|---|
| 1 | Intracardiac | 26 | No | The 4CV was not standard, no CPV, false pulmonary venous horn-like structure, four pulmonary veins directly drained into the RA. | Surgery |
| 2 | Intracardiac | 25 | DORV | The CPV was located below the posterior LA and could not be visualized in a 4CV creating false images of a pulmonary venous horn-like structure. | Autopsy |
| 3 | Supracardiac | 20 | No | The 4CV was not clear, the CPV adjacent to the atrial wall was mistaken for a pulmonary vein horn-like structure, the scale was too high. | Surgery |
| 4 | Intracardiac | 21 | No | The 4CV was unclear, the scale was too high, and a suspicious CPV was ignored. | Surgery |
| 5 | Intracardiac | 22 | PS, LSVC | The instrument was not set properly, the scale was too high, and the pulmonary vein course and abouchement were not shown. | Surgery |
| 6 | Intracardiac | 25 | F4 | Decreased size of the LA and increased PLAS index, false pulmonary venous horn-like structure, disregarded the PV blood flowing into the CS. | Autopsy |
| 7 | Intracardiac | 26 | TGA, PS | Complex intracardiac anomalies affecting the 4CV were unclear, the pulmonary vein horn-like structure was poorly displayed, and the PLAS index was augmented. | Autopsy |
| 8 | Supracardiac | 28 | No | Color blood flow outflow, false pulmonary venous horn-like structure, and anatomic variation. | Surgery |
| 9 | Mixed | 18 | No | Low gestational week, insignificant PLAS augmentation, finer CPV showing poor, anatomical variations. | Surgery |
| 10 | Mixed | 18 | No | The 4CV was not standard; oligohydramnios, the scale was too high, and the PV blood flow course was not shown. | Surgery |
| 11 | Supracardiac | 21 | HLHS, AS | No CPV, dilated CS, neglected pulmonary vein into the CS, false pulmonary venous horn-like structure. | Autopsy |
| 12 | Mixed | 25 | ASD, VSD, AS | The 4CV was not standard, with a pulmonary venous horn-like structure and poorly displayed post-left atrium region. | Autopsy |
| 13 | Intracardiac | 17 | AVSD, PS | Complex intracardiac anomalies, and ignored the decreased size of the LA and augmentation of the PLAS index, loss of the pulmonary vein horn-like structure, and CDFI color flow spillover. | Autopsy |
| 14 | Supracardiac | 20 | No | CDFI color flow spillover, LA flow mixed with the CPV, false pulmonary venous horn-like structure. | Surgery |
| 15 | Mixed | 25 | No | Mistakenly identified the posterior right atrial CPV as the right atrial blood flow color due to blood flow overflow. Ignored the CPV at which the post-left atrial CPV confluences to the SVC and two pulmonary veins drained into the RA. | Surgery |
| 16 | Supracardiac | 27 | No | Improper instrument settings, the scale was too high and the PV was not displayed. | Surgery |
| 17 | Supracardiac | 23 | Pulmonary artery atresia | Lack of TAPVC awareness, ignored the smooth posterior LA wall and the augmentation of the PLAS index. Mistook the curved CPV for the pulmonary venous horn-like structure (false pulmonary venous horn-like structure). | Autopsy |
| 18 | Supracardiac | 25 | TA, VSD | Complex intracardiac anomalies affected observation of the 4CV, the decreased size of the LA and augmentation of the PLAS index, and CDFI did not show pulmonary venous blood flow course or abouchement. | Autopsy |
| 19 | Infracardiac | 34 | No | Anatomic variant of the CPV had a branch into the LA, ignored the presence of CPV. | Surgery |
| 20 | Intracardiac | 21 | No | The 4CV was not standard, CDFI was not properly prepped, pulmonary venous flow was unclear, and the CS was dilated. | Surgery |
| 21 | Intracardiac | 24 | No | False pulmonary venous horn-like structure, mistook the blood flow from the pulmonary veins into the CS as entering the LA. | Surgery |
| 22 | Supracardiac | 26 | SV, AVSD, TGA | Complex intracardiac anomalies affected the 4CV on PV reflux observation, focused solely on other cardiac anomalies. | Autopsy |
| 23 | Intracardiac | 26 | LSVC | CS dilatation, false pulmonary venous horn-like structure, LSVC | Surgery |
| 24 | Infracardiac | 31 | No | Color blood flow spillover, false pulmonary venous horn-like structure. | Surgery |
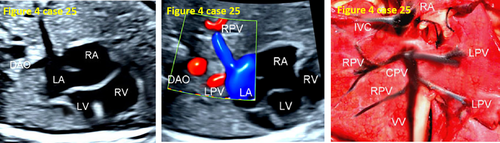
| 25 | Infracardiac | 28 | AVSD | False pulmonary venous horn-like structure, CDFI did not show pulmonary vein course or abouchement. | Autopsy |
| 26 | Intracardiac | 19 | VSD, PA | The 4CV was not standard, no CPV, simply focused on other cardiac anomalies, CDFI did not show the pulmonary vein course or abouchement. | Autopsy |
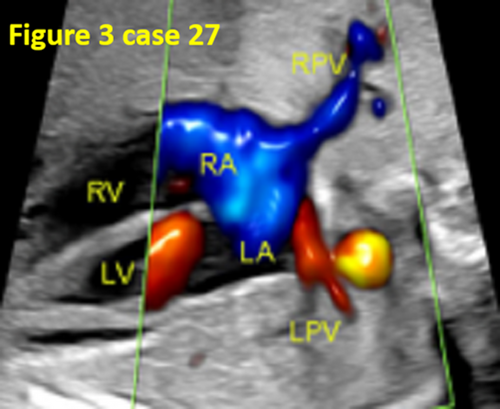
| 27 | Infracardiac | 27 | No | False pulmonary venous horn-like structure, CDFI did not show the pulmonary vein course or abouchement. | Surgery |
| 28 | Infracardiac | 37 | No | CDFI did not show the pulmonary vein course or abouchement, ignored the decreased size of the LA and augmentation of the PLAS index. After the four pulmonary veins converged into the CPV, a small branch was sent from the CPV into the LA, and then most of the pulmonary venous blood entered the IVC through the VV and re-entered the RA. | Surgery |
| 29 | Intracardiac | 25 | No | False pulmonary venous horn-like structure, neglected the decreased size of the LA and augmentation of the PLAS index, CDFI did not show pulmonary vein course or abouchement, excessive color flow gain. | Surgery |
| 30 | Supracardiac | 26 | DORV, PS | The 4CV was not standard, did not focus on observation of pulmonary veins, and CDFI did not show the pulmonary vein course. | Autopsy |
| 31 | Mixed | 32 | No | False pulmonary venous horn-like structure, RPV directly into the RA, LPV into the CS. | Surgery |
| 32 | Infracardiac | 34 | No | False pulmonary venous horn-like structure, neglected the decreased size of the LA and augmentation of the PLAS index, CDFI did not show the pulmonary vein course. | Surgery |
- Abbreviations: 4CV, four-chamber view; AVSD, atrioventricular septal defect; CPV, pulmonary venous confluence; CS, coronary sinus; DORV, right ventricular double outlet; F4, tetralogy of Fallot; GA, gestational age (weeks); LSVC, left superior vena cava; PA, pulmonary atresia; PS, pulmonary stenosis; SV, single ventricle; TA, tricuspid valve atresia; TGA, transposition of the great arteries; VSD, ventricular septal defect.
3.2 Factors Contributing to Missed Diagnosis
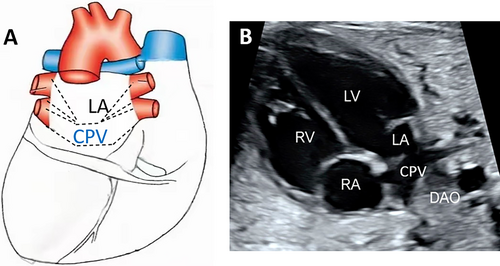
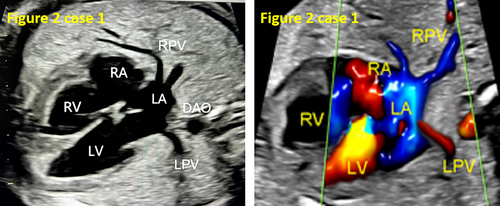
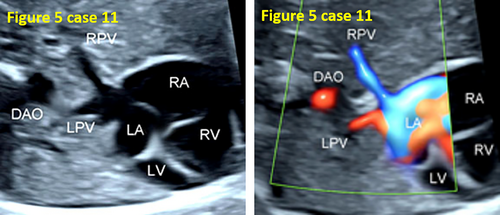
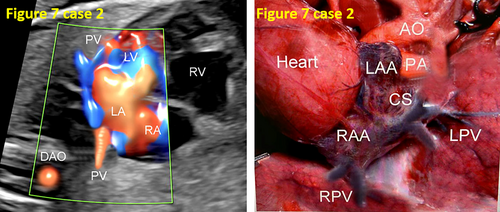
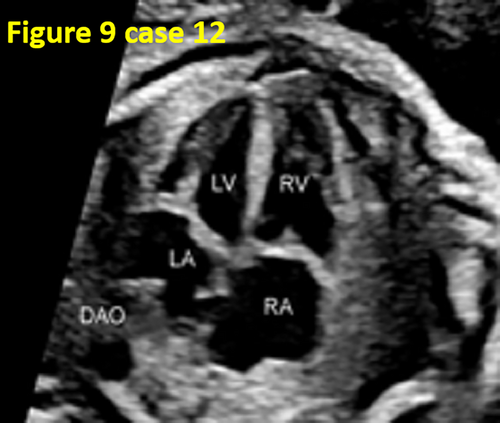
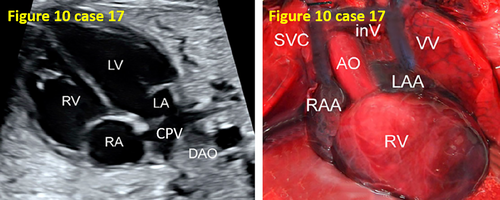
- False pulmonary venous horn-like structures (as shown in Figure 1) were a major factor contributing to missed diagnoses, with a rate of approximately 53.1% (17/32). Seventeen fetuses with TAPVC showed false image a pulmonary venous horn-like structure that were almost indistinguishable from normal pulmonary venous horn-like structures. In the four-chamber view of a fetus with a normal image, pulmonary venous horn-like structures showed a left and right pulmonary vein draining into the LA, showing a “horn-like” change. In some fetuses with TAPVC, the four pulmonary veins formed the common pulmonary vein at the posterior lower part of the LA (not returning to the LA), which showed a false image of a positional pulmonary venous horn-like structure. These cases were missed by the examining physician because they mistakenly thought that the pulmonary venous horn-like structure was normal (as shown in Figures 2-5, 7, 8, and 10).
- TAPVC combined with other CHDs also contributed to missed diagnoses. The rate of missed diagnoses was approximately 40.6% (13/32). Standard chamber views could not be obtained for 13 fetuses with TAPVC with other CHDs, which affected the display of TAPVC features. In addition, ultrasonographers focused on showing intracardiac anomalies and ignored the presence of pulmonary vein abnormalities (as shown in Figures 4, 5, 7, and 10).
- Conventional ultrasound CDFI did not accurately show low-velocity pulmonary venous blood flow. This represented the lack of the normal influence of the left atrial hemodynamics and may be due to a distant or dampened connection to a systemic vein. In 11 fetuses (34.3%, 11/32) with TAPVC, the course and abouchement of an anomalous pulmonary venous connection were difficult to display.
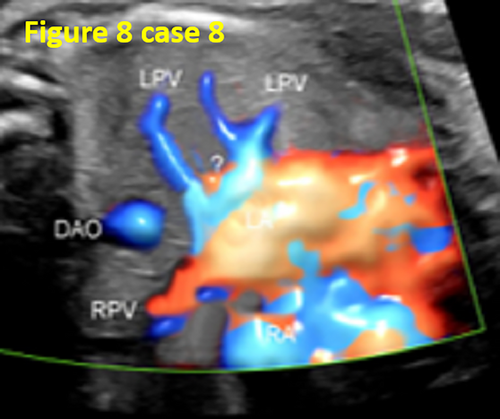
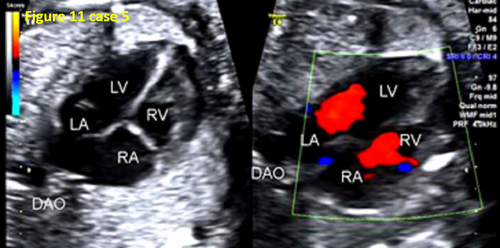
- The instruments were not properly preset. In 7 fetuses (21.8%, 7/32) with TAPVC, during examination, a higher scale setting or lower color flow gain resulted in an unclear display of the pulmonary vein and pulmonary venous confluence (CPV). In contrast, a lower scale setting or higher color flow gain led to visual confusion between the blood flow of the CPV posterior the LA and the blood flow of the LA. This led to a false image of the blood flow pulmonary venous horn-like structure. Thus, operators mistakenly believed that the pulmonary vein drained into the LA, forming a pulmonary venous horn-like structure, resulting in a missed diagnosis (as shown in Figures 8 and 11).
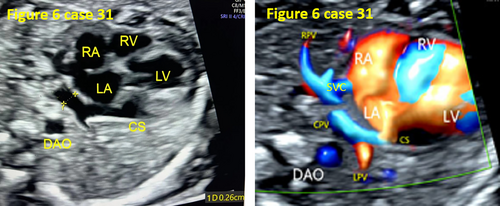
- In 13 cases (40.6%, 13/32), the four-chamber view was not standard, and the pulmonary venous horn-like structure, decreased left atrial size, and the PLAS index were ignored, resulting in missed diagnosis (as shown in Figures 6, 9, and 11).
- In case 17, one diagnosis was missed due to insufficient subjective awareness of an anomalous pulmonary venous connection and ignorance of the displayed ultrasound features (as shown in Figure 10).
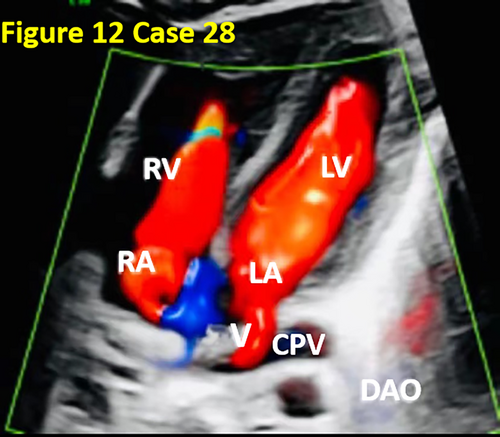
- In case 28, CDFI did not reveal the pulmonary vein course or abouchement, ignoring the decreased size of the LA and augmentation of the PLAS index. After the four pulmonary veins converged into the CPV, a small branch was sent from the CPV into the LA, and most of the pulmonary venous blood entered the inferior vena cava (IVC) through the vertical vein (VV) and re-entered the RA. However, during the prenatal ultrasound examination, the CPV was mistakenly considered part of the LA, and the small branch veins emitted by the CPV were considered normal returning pulmonary veins, so the diagnosis was missed (as shown in Figure 12).
- In case 10, oligohydramnios and spinal acoustic shadow occlusion caused unclear standard views and cardiac structure. Thus, the anomalous pulmonary venous connection was ignored.
Among the 32 cases of missed diagnoses, only 12.5% (4/32) were missed due to one influencing factor, 50.0% (16/32) were missed due to two influencing factors, and 37.5% (12/32) were missed due to three or more influencing factors.
4 Comparison of Echocardiographic Parameters Between Fetuses With Missed Diagnoses and Normal Fetuses
The group with a missed TAPVC diagnosis showed decreased sizes of the LA and the left ventricle (LV) and augmentation of the ratio of the right atrium (RA) and LA width diameter, ratio of the right ventricle (RV) and LV width diameter, the left atrium-descending aorta distance (LD), and PLAS index. The P values were all less than 0.001(Tables 2 and 3).
| Parameters | Control group | TAPVC missed diagnosis group | P |
|---|---|---|---|
| LAD (mm) | 10.15 ± 3.76 | 7.58 ± 2.76 | < 0.001 |
| RAD (mm) | 11.19 ± 3.85 | 11.28 ± 3.96 | 0.541 |
| LVD (mm) | 11.22 ± 3.94 | 8.53 ± 3.17 | < 0.001 |
| RAD/LAD | 1.10 ± 0.20 | 1.48 ± 0.32 | < 0.001 |
| RVD (mm) | 11.91 ± 4.06 | 12.09 ± 4.10 | 0.213 |
| RVD/LVD | 1.06 ± 0.22 | 1.42 ± 0.33 | < 0.001 |
| PA (mm) | 6.77 ± 2.62 | 6.55 ± 2.53 | 0.565 |
| AO (mm) | 5.52 ± 2.49 | 5.44 ± 2.45 | 0.167 |
| PA/AO | 1.19 ± 0.13 | 1.20 ± 0.14 | 0.124 |
| LD (mm) | 2.75 ± 1.04 | 6.52 ± 1.60 | < 0.001 |
| DA (mm) | 4.60 ± 1.69 | 4.48 ± 1.45 | 0.406 |
| PLAS index | 0.59 ± 0.15 | 1.50 ± 0.24 | < 0.001 |
- Abbreviations: AO, aorta diameter; DA, descending aorta diameter; LAD, left atrial diameter; LD, left atrium-descending aorta distance; LVD, left ventricular diameter; PA, pulmonary artery diameter; PLAS index, post-left atrium space index; RAD, right atrial diameter; RVD, right ventricular diameter.
| Control group (n = 32) | TAPVC missed diagnosis group (n = 32) | |
|---|---|---|
| Maternal age, years | 25.4 ± 1.6 | 28.1 ± 6.3 |
| GA at initial examination, weeks | 25.1 ± 3.7 | 25.1 ± 4.8 |
| GA at delivery, weeks | 39.2 ± 2.1 | 31.1 ± 2.1 |
| Delivery mode |
27(84.4%) Spontaneous labor 5(15.6%) cesarean section |
12 (37.5%) induced labor 11(34.3%) Spontaneous labor 9(28.1%) cesarean section |
| Birth weight, Kg | 3.31 ± 0.66 | 2.39 ± 0.53 |
| Apgar scores for 1 min | 10.00 ± 0.00 | 8.00 ± 0.71 |
| Apgar scores for 5 min | 10.00 ± 0.0 | 5.75 ± 0.97 |
5 Discussion
Among cyanotic heart diseases, TAPVC is the only one involving venous system malformations. Approximately one-third of these cases are combined with other severe intracardiac anomalies such as triatriatum, single ventricle, tetralogy of Fallot, transposition of the great arteries, persistent truncus arteriosus, aortic coarctation, and left ventricular dysplasia syndrome, as well as with extracardiac anomalies such as heterotaxy syndrome. TAPVC is easily missed and misdiagnosed by prenatal ultrasonography because of the small size of the fetal heart, thin pulmonary veins, and low blood flow velocity. There are more factors reported in the literature for missed diagnoses with varying causes, but fewer analyses of missed diagnoses have been reported in large samples. A systematic study of 424 infants with postnatal TAPVC diagnoses at 19 children's heart centers in the United Kingdom, Ireland, and Sweden from 1998 to 2004 showed a prenatal ultrasound detection rate of only 1.9% (Seale et al. 2012). France reported a 10% prenatal diagnosis rate for 95 newborns with isolated TAPVC born between 2001 and 2011 (Laux et al. 2013). Olsen R reported 5 cases of fetuses with missed TAPVC diagnoses, 3 of which were isolated cases and 2 of which were combined with complex cardiac anomalies and heterogeneous syndromes. None of the 5 missed cases could be visualized by conventional CDFI with color flow Doppler in any of the pulmonary veins (Olsen and others, 2016).
In this study, we retrospectively analyzed the data of 32 fetuses with missed TAPVC diagnoses in a larger sample to comprehensively summarize the main subjective, objective, and technical factors contributing to missed prenatal ultrasound diagnosis; this study provides more comprehensive and detailed information than previous studies reporting individual cases. First, we found that false pulmonary venous horn-like structures were the primary factor contributing to missed diagnosis, with the intracardiac type being the most likely to be missed (12/32, 37.5%). The main reason was because that there are multiple TAPVC subtypes that are prone to anatomic variation. When the CPV was located behind posterior and inferior to the LA rather than posterior to the LA, it was obscured by the left atrial image in the four-chamber view, so it could not be shown in the conventional four-chamber view. When the four pulmonary veins drained into the RA, the course of the left pulmonary vein was behind the posterior and inferior to the LA with an acoustic image similar to that of a normal pulmonary vein draining into the LA, and the CDFI showed a false pulmonary venous horn-like structure, which was almost always missed. The second major factor was the combination of intracardiac anomalies in TAPVC patients, mainly due to the inability to obtain a standard four-chamber view in most cases combined with complex intracardiac anomalies. This affected the observation and identification of the left atrial size, PLAS index, pulmonary venous horn-like structure, and CPV. Missed diagnosis was more likely when the operator had insufficient subjective awareness of TAPVC, the focus was on the observation of intracardiac anomalies, and the partially displayed sonographic features of TAPVC were ignored. The third major factor was the small size of the fetal pulmonary veins and their low flow velocity, which made it difficult or impossible to show the ectopic pulmonary vein course or abouchement due to the low sensitivity of conventional CDFI and low velocity flow. The four-chamber view was not standard during conventional echocardiography, and the instrument settings were not to be adjusted when examining the pulmonary veins. Either the scale was too high to show low velocity flow in the pulmonary veins, or the color flow gain was too high and the flow from the CPV merged with the left atrial flow, creating false pulmonary venous horn-like structure and thus leading to a missed diagnosis. Moreover, multiple factors of underdiagnosis can coexist, which makes underdiagnosis more likely. Therefore, to improve the detection rate of TAPVC and reduce the number of missed diagnoses, there are some points to consider: the standard four-chamber view, individualized adjustment of instrument parameter presets, the combination of complex intracardiac anomalies and TAPVC, the use of new ultrasound views and techniques to show the anatomy of the posterior inferior LA variant, and paying attention to the low velocity pulmonary venous flow stroke and confluence. The study by James C Huhta concluded that the missed factors could include (a) right atrial isomerism, (b) mixed total anomalous pulmonary venous connection (most commonly to the coronary sinus and the left vertical vein), and (c) common pulmonary vein atresia (Huhta, Gutgesell, and Nihill 1985). Guocheng Shi's study of supracardiac anomalous pulmonary venous connection (SAPVC) concluded that the complex anatomical variations in the branches or the common trunk branches of the veins in SAPVC could be missed due to the lack of typical echocardiography signs (Shi et al. 2017). In addition, Nikunj Vaidhya reported that the isolated SAPVC was easily missed because the hemodynamics generally did not cause significant changes in the right heart system, the chambers were essentially normal in size, and the inner diameter of the VV was usually very small, and it was less likely to be combined with pulmonary hypertension or other intracardiac malformations (Vaidhya, Mishra, and Agrawal 2020). The results of other researchers regarding the factors underlying missed diagnoses caused by complex anatomical variations in venous branches and other complex cardiac malformations are similar to our findings.
In academic fetal echocardiography, it has long been a common understanding that a four-chamber view showing two pulmonary veins on the right and left sides draining into the LA to form a pulmonary vein horn-like structure could exclude TAPVC (International Society of Ultrasound in and others 2013; Paladini et al. 2018). However, our study showed that 17 of the fetuses with missed TAPVC diagnoses had pulmonary vein horn-like structures that were almost indistinguishable from the parameters of normal fetuses, and false pulmonary venous horn-like structures were the main reason for the missed diagnoses. It is suggested that the presence of pulmonary vein horn-like structures during conventional fetal echocardiography does not completely exclude TAPVC. Compared to normal fetal parameters, the decreased size of LA and augmentation of the PLAS index can be important indicators of false pulmonary venous horn-like structures (Kawazu et al. 2014; Mao et al. 2017). We propose a new concept of false pulmonary venous horn-like structures and a new view that the presence of pulmonary vein horn-like structures in a four-chamber view does not completely exclude TAPVC, and we look forward to discussing it in-depth with our colleagues.
A limitation of this study is that it was a multi-center retrospective study with a long study period, a large number of participants, and varying skill levels, and there may be bias in some of the results. Standardization and normalization of ultrasound views and operative techniques will be performed as much as possible in future studies.
Author Contributions
Qichang Zhou, Dongmei Liu, and Jiawei Zhou contributed to the conception and design of the study. Dongmei Liu completed the data collection and wrote the first draft of the manuscript. Qichang Zhou contributed to manuscript revision and review. All authors approved the submitted version.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




