Maternal Exposure to Tap Water Disinfection By-Products and Risk of Selected Congenital Heart Defects
Funding: This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposures (BD-STEPS).
The findings, conclusions, and views expressed in this manuscript are those of the authors and do not necessarily represent the views, policies, or official position of the U.S. Environmental Protection Agency, the Centers for Disease Control and Prevention, North Carolina Department of Health and Human Services, Division of Public Health, or the New York State Department of Health.
This analysis has been replicated by Thomas J. Luben, PhD, who is also a coauthor on the project.
ABSTRACT
Background
The use of chlorine to treat drinking water produces disinfection by-products (DBPs), which have been associated with congenital heart defects (CHDs) in some studies.
Methods
Using National Birth Defects Prevention Study data, we linked geocoded residential addresses to public water supply measurement data for DBPs. Self-reported water consumption and filtration methods were used to estimate maternal ingestion of DBPs. We estimated adjusted odds ratios and 95% confidence intervals using logistic regression controlling for maternal age, education, body mass index (BMI), race/ethnicity, and study site to examine associations between CHDs and both household DBP level and estimated ingestion of DBPs.
Results
Household DBP exposure was assessed for 2717 participants (1495 cases and 1222 controls). We observed a broad range of positive, null, and negative estimates across eight specific CHDs and two summary exposures (trihalomethanes and haloacetic acids) plus nine individual DBP species. Examining ingestion exposure among 2488 participants (1347 cases, 1141 controls) produced similarly inconsistent results.
Conclusions
Assessing both household DBP level and estimated ingestion of DBPs, we did not find strong evidence of an association between CHDs and DBPs. Despite a large study population, DBP measurements were available for less than half of participant addresses, limiting study power.
1 Introduction
The benefits of disinfection of drinking water in greatly reducing morbidity and mortality due to waterborne pathogenic organisms are well-known (Cutler and Miller 2005). Chlorine is an effective and inexpensive disinfectant; however, chlorine and other chemical disinfectants used to treat drinking water react with inorganic and organic matter, resulting in the formation of a complex mixture of disinfection by-products (DBPs) including trihalomethanes (THMs) and haloacetic acids (HAAs). Toxicological studies involving animals and cell cultures have found evidence of developmental and reproductive effects of exposure to DBPs, however, often only at high doses not commonly encountered in human populations (Tardiff, Carson, and Ginevan 2006). Nevertheless, special attention has been paid to HAAs and brominated DBPs as being potentially more harmful to human health (reviewed in [Richardson et al. 2007; Tardiff, Carson, and Ginevan 2006]).
Local public water supply (PWS) reporting requirements, including the types and frequency of DBPs reported, influence or dictate the data available for investigating DBP exposure (Kolb, Francis, and VanBriesen 2017; Parvez, Frost, and Sundararajan 2017; Rupal, Ashok Kumar, and Partha Sarathi 2021). Both the type of water consumed (unfiltered tap, filtered tap, and bottled) and the quantity consumed affect DBP exposure. Although the most recent studies involving birth defects have used exposure assessments based on periconceptional address and detailed PWS levels of specific THMs and HAAs to estimate prenatal exposure during the critical period of development (Säve-Söderbergh et al. 2021; Wright et al. 2017), few have used exposure metrics incorporating self-reported water consumption information (Grazuleviciene et al. 2013; Luben et al. 2008; Weyer et al. 2018; Zaganjor et al. 2020). Accounting for individual water consumption patterns can allow for more accurate estimates of DBP exposure especially for nonvolatile DBPs such as HAAs.
Epidemiologic studies provide some evidence of an association between exposure to DBPs and adverse developmental outcomes including birth defects (Nieuwenhuijsen et al. 2009; Säve-Söderbergh et al. 2021; Waller et al. 1998; Wright and Rivera-Nunez 2011). Several studies have noted associations between DPBs and congenital heart defects (CHDs) (Grazuleviciene et al. 2013; Nieuwenhuijsen et al. 2009, 2008; Righi et al. 2012); however, few have examined associations with specific CHD subtypes. Reported findings include some indication of an association with all CHDs as a group and ventricular septal defects (VSDs) as the main subtype, but with inconsistent results across studies (Cedergren et al. 2002; Hwang, Jaakkola, and Guo 2008; Shaw et al. 2003; Wright et al. 2017). Most of these studies involved small numbers of cases and included a limited set of DBP exposures (rarely both THMs and HAAs) and few early studies had individual level water use data to examine more direct exposure measures.
In the present analysis, we used data from the National Birth Defects Prevention Study (NBDPS), a large multisite case–control study of birth defects. By utilizing the NBDPS, the present study uniquely contributes by assessing DBP exposure and the risk of a range of specific, well-defined CHDs. In addition, we were able to incorporate detailed water consumption data into the exposure assessment and adjust for a variety of potential confounding variables.
2 Methods
2.1 Study Design
The NBDPS was a multisite population-based case–control study that included deliveries ending on or after October 1, 1997 through estimated dates of delivery (EDD) on or before December 31, 2011 and has been detailed elsewhere (Reefhuis et al. 2015). Briefly, participating study sites included states with active population-based surveillance systems (Arkansas [AR], California [CA], Georgia [GA], Iowa [IA], Massachusetts [MA], North Carolina [NC], New Jersey [NJ], New York [NY], Utah [UT], and Texas [TX]; some sites participated only for a portion of the study years [NJ 1998–2002, NC 2003–2011, and UT 2003–2011]). Cases included livebirths, stillbirths, or induced abortions with one or more of over 30 different categories of major structural defects, excluding those attributed to a known chromosomal abnormality or single-gene condition. Controls were live-born infants without birth defects randomly selected from hospital records or birth certificates in the same time period and geographic areas as the cases. The enrolled mothers of case and control children provided informed consent and took part in a detailed telephone interview, in English or Spanish, between 6 weeks and 2 years after the child's estimated date of delivery. The interview collected information including, but not limited to, the following topics: pregnancy history, health conditions, medication use, diet, occupation, family history of birth defects, tap water use, and demographic factors. For the entirety of the NBDPS, the participation rate was 67% for cases and 65% for controls. The Centers for Disease Control and Prevention (CDC), which funded the study, as well as each of the participating study sites, obtained institutional review board approval from their respective institutions.
2.2 Analytic Sample
The present analysis was limited to CHD cases and controls with EDD between 2000 and 2005, the period during which the telephone interview included a detailed module about water use and consumption. Our analytic sample includes data from all NBDPS sites for this period except two sites that did not contribute PWS data (CA, NJ). Also, there were two sites that only contributed cases to the NBDPS for EDDs beginning in 2003 (NC, UT). The NBDPS included structural heart defects that were confirmed by echocardiography, cardiac catheterization, or autopsy. The details of the CHD classification method have been described elsewhere (Botto et al. 2007). Briefly, CHD cases were reviewed by clinical geneticists and classified as isolated (no extracardiac defects) or multiple (one or more major extracardiac defects) and additionally classified based on morphology to create homogeneous groups. We included only case groups with 30 or more cases for whom exposure data were available (detailed below). The resulting eight CHDs included in this analysis are tetralogy of Fallot (TOF), d-transposition of the great arteries (d-TGA), hypoplastic left heart syndrome (HLHS), coarctation of the aorta (CoA), aortic stenosis (AS), pulmonary valve stenosis (PVS), perimembranous VSD, and secundum type atrial septal defect (ASD). Because cases recorded as atrial septal defect not otherwise specified were likely secundum type, those cases were grouped with secundum atrial septal defects.
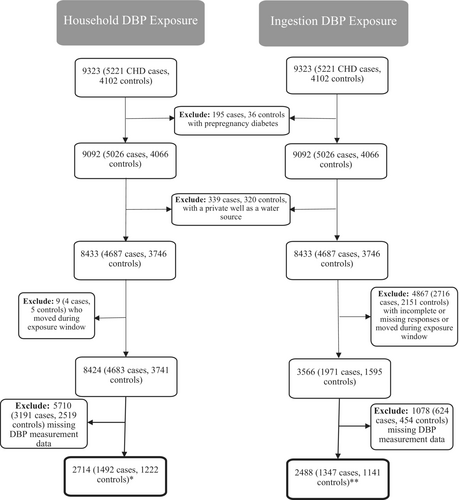
A total of 9323 participants (5221 CHD cases and 4102 controls) from eight sites participated in the interview and were eligible for this analysis. As described in Figure 1, we excluded 195 case and 36 control mothers who reported either pregestational type 1 or type 2 diabetes due to the established association with elevated risk of CHDs (Correa et al. 2008; Tinker et al. 2020). Next, we excluded those who indicated that their water source was from a private well (339 cases, 320 controls) to restrict our analysis to only mothers who were served by a PWS to allow for exposure assessment. The remaining mothers were eligible to be included in both arms of the exposure assessment, one characterizing household exposure and the other focused on ingestion. Each approach to the exposure assessment includes important and relevant inclusion and exclusion criteria, which are detailed below.
2.3 Exposure Assessment
2.3.1 DBP Measurement Data
To assess exposure to DBPs, we utilized the approach developed by colleagues at the University of Iowa (Weyer et al. 2018). While this method has already been described in detail, we provide an overview of the method as it was utilized in this analysis. The foundation of this exposure assessment relies on the linkage of PWS measurement data to the residences of the participants in the NBDPS. This was achieved through the NBDPS telephone interview, which collected residential information for all participants including street addresses and timing (month, year) of each residence before and during the pregnancy. The addresses were geocoded and then linked to PWS maps. In instances where an address was within a city that had multiple water districts and their boundaries could not be determined, the maternal residences were linked to the largest water district in the city.
Through the passage of the Safe Drinking Water Act, PWSs are required to routinely monitor for the presence of DBPs in the United States. We accessed these measurement data, which were then linked to each of the geocoded residences when possible. Depending on the size of the PWS, determined by the size of the population served, the testing and reporting requirements differ. Systems are required to monitor for exceedances of the maximum contaminant level (MCL) which, for total THMs (THM4), the MCL is 80 μg/L, and for the group of five most common HAAs in drinking water (HAA5s), the MCL is 60 μg/L (in the analytic sample, DBP measurements above the MCL were infrequent [THM4: 133 case and 104 control households; HAA5: 107 case and 69 control households]). Quarterly monitoring is required for larger systems serving more than 10,000 residents and annual monitoring is required for systems serving fewer than 10,000 residents (U.S. EPA Environmental Protection Agency 2010). Given these variations in measurement frequency, linkage of a residence to a water supply did not always provide DBP measurement values during the exposure window of interest. We defined the exposure window as the month before conception through the third month of pregnancy because it encompasses the critical period of heart development. As described by Weyer et al. (2018), when measurements taken on multiple days during the exposure window were available, adjustments were made to account for seasonal and spatial variations using an inverse-time weighted mean. If multiple measurements were taken throughout a system on a single day, the average of those measurements was used. Data collected from participating study sites included DBP concentrations (standardized to micrograms per liter [μg/L]), sampling date, and location. Two sites (MA, UT) were able to provide concentrations for THM4 and HAA5 in drinking water, while the rest of the sites were able to provide both THM4, HAA5, and individual THM and HAA species concentrations. Mothers from MA and UT were excluded from any analyses involving individual DBP species and included only in analyses of THM4 and HAA5. The individual species include the following THMs: chloroform, bromoform, bromodichloromethane (BDCM), dibromochloromethane (DBCM) and the following HAAs: monobromoacetic acid (MBAA), monochloroacetic acid (MCAA), dibromoacetic acid (DBAA), dichloroacetic acid (DCAA), and trichloroacetic acid (TCAA).
2.3.2 Household Exposure to DBPs
The first approach to characterizing DBP exposure was based on DBP concentrations measured in the PWS serving the mother's residence between 1999 and 2005. Mothers who moved during the exposure window were excluded from the analysis. For the purposes of this analysis, we will refer to this as household DBP exposure. As depicted in Figure 1, there were 1492 cases and 1222 controls linked to a PWS for whom DBP measurements were available. To categorize DBP household exposure, we examined the distribution quantiles of measurement data among controls (in micrograms per day [μg/day]) within the household exposure sample to determine the cut points to use for categorizing exposure to each DBP category. Depending on the observed distribution, exposures were categorized into two or three categories to capture nonzero values in the “exposed” categories and divide exposure into higher and lower categories while also maintaining adequate counts in each category. Most DBP categories were divided into three exposure levels (THM4, CHLF, BDCM, DBCM, HAA5, DCAA, and TCAA) with the remainder divided into two (BFR, MBAA, MCAA, and DBAA).
2.3.3 Ingestion Exposure to DBPs
A second exposure assessment utilized in this analysis incorporated the responses mothers provided to questions about water use and consumption. This additional individual-level information allowed us to estimate exposure via ingestion, which we will refer to as DBP ingestion exposure. As described in Weyer et al. (2018), mothers were asked about water source (private well or public), chemical disinfectant, filtration, and how tap water was used (drinking, cooking, and both) for the mother's residence around the time of conception. For all residences, they were asked about the source (unfiltered tap, filtered tap, bottled, and other), how many 8-oz glasses were consumed each day, and changes in drinking water source during pregnancy. Similar information was gathered about any job locations during pregnancy. Mothers were also asked about other sources of water consumption. These descriptions were examined to classify the water consumed as unfiltered tap water, filtered tap water, or bottled water. Average total daily consumption was calculated for the 4-month exposure window (120 days) using all water sources and amounts reported through the interview. When water is heated, certain DBPs volatilize (Carrasco-Turigas et al. 2013). Sufficient detail to correctly account for the impacts of heating and boiling water were not collected therefore, hot drinks and water used for cooking were excluded from the calculation.
If changes in water consumption amounts during pregnancy were reported (queried by month), we used the responses to questions about water consumption from the various sources to estimate DBP ingestion exposure. Questions about changes in the amount of water consumed were not asked separately for each water source. Since some mothers reported both consuming water from multiple water sources and a change in amount consumed, different approaches were taken to estimate DBP ingestion exposure: an unweighted approach and two weighted approaches (for a detailed explanation and illustration of this algorithm see Weyer et al. (2018)). The unweighted approach, which distributed the total consumption amounts across all sources according to the proportion each represented prior to the change in consumption, was used in this analysis. As mentioned previously, mothers could report a change in water source, but the interview did not collect information on the timing of that change. In this analysis, we assumed that any change in consumption occurred after the exposure window.
2.3.4 Estimating Ingestion of DBPs
Additional assumptions were made related to assessing DBP ingestion exposure. Because work locations were not linked to DBP measurements in their public water supplies, the measurements were assumed to be the same as those for a mother's residence. If mothers indicated that they did not drink their tap water, their exposure was assumed to be 0 μg/day. Additionally, the interview allowed mothers to indicate if the water source (tap) was filtered, what type of filter was used, and the brand name. This information was used to determine if the filter could reduce the amount of DBPs ingested. A 90% reduction in DBP concentrations was assumed for filters known to remove DBPs. For other filters, or if the type of filter or its ability to remove DBPs was unknown, we assumed only a 10% reduction in DBPs. Final ingestion amounts were then calculated by multiplying the concentration measured in the linked PWS by the amount of water consumed.
As shown in Figure 1, the analytic sample for the ingestion analysis excludes mothers who moved during the exposure window or had incomplete or missing responses to relevant questionnaire data, such as water sources and amounts for home or work, or description of all water sources as unfiltered tap, filtered tap, or bottled. In addition, this sample was restricted to only those for whom a DBP measurement value was available (1347 cases and 1141 controls). In the same manner described above for household exposure, we categorized DBP ingestion exposure by examining the distribution quantiles of ingestion amounts among controls (in micrograms per day [μg/day]) within the ingestion sample to determine the cut points to use for categorizing exposure to each DBP category. Most DBP categories were divided into three exposure levels (THM4, CHLF, BDCM, DBCM, HAA5, DCAA, and TCAA) with referents based on median ingestion distributions with the remainder divided into two (BFR, MBAA, MCAA, and DBAA).
2.4 Statistical Analysis
Univariate analyses were conducted to examine characteristics of cases and controls and the DBP exposure profile. These included maternal age at delivery (<19, 20–34, ≥35 years), maternal race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other), maternal education (less than high school, high school, some college, and college degree), prepregnancy BMI (underweight <18.5 kg/m2, normal 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, and obese ≥30.0 kg/m2), alcohol consumption during pregnancy (yes, no), cigarette smoking during pregnancy (yes, no), study site (AR, GA, IA, MA, NC, NY, TX, and UT).
The same analytic method was used for the household and ingestion exposures. To assess associations of THM4, HAA5, and individual species on risk of each of the eight CHDs, we calculated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) using unconditional logistic regression adjusting for a list of covariates determined by a directed acyclic graph (DAG): maternal age (continuous), maternal education, race/ethnicity, prepregnancy BMI (categorical), and study site (Figure S1). We adjusted for race/ethnicity not as a biological factor, but rather as a crude proxy for exposure to structural racism during the mother's lifetime. We present crude odds ratio estimates for comparisons with three or four exposed cases, or in comparisons for which exposure was common, three or four nonexposed cases. We employed Firth's penalized likelihood method to account for convergence issues in the models due to small sample size and empty cells in the strata of covariates included in the model (Heinze and Schemper 2002). For our evaluation of the results, moderately elevated or reduced aOR estimates were defined as 2.0 or greater or 0.5 or less, respectively. Analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC USA).
3 Results
We present the distribution of maternal characteristics in Table 1 by categories of household exposure status (average per liter exposure to THM4 and HAA5 during the exposure window). The same covariates stratified by case and control status and by specific CHD phenotype are available in Table S1. Table S2 presents the correlations between THM4s, individual THM species, between HAA5, and individual HAA species. In Table 2, we provide summary statistics (mean, standard deviation, maximum value, 25th, 50th, and 75th percentile values) for the PWS measurement data in the household exposure sample. For most of the DBPs, the data were not normally distributed and are skewed to the right. The extent to which each distribution was skewed varied greatly with BRF, BDCM, MBAA, and DBAA being the most skewed.
| Covariable | Household exposure to disinfection by-products among the study populationa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THM4b (N = 2445) | HAA5b (N = 1848) | |||||||||||
| ≤50th | 50th–75th | >75th | ≤50th | 50th–75th | >75th | |||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Maternal age at delivery (years) | ||||||||||||
| <20 | 80 | (6.4) | 43 | (7.3) | 33 | (5.5) | 54 | (6.0) | 33 | (6.9) | 39 | (8.2) |
| 20–34 | 959 | (76.6) | 446 | (75.5) | 470 | (78.1) | 694 | (77.7) | 359 | (75.3) | 365 | (76.4) |
| ≥35 | 213 | (17.0) | 102 | (17.3) | 99 | (16.4) | 145 | (16.2) | 85 | (17.8) | 74 | (15.5) |
| Maternal race/ethnicity | ||||||||||||
| White NH | 767 | (61.3) | 334 | (56.5) | 343 | (57.0) | 559 | (62.6) | 240 | (50.3) | 232 | (48.5) |
| Black NH | 145 | (11.6) | 95 | (16.1) | 108 | (17.9) | 107 | (12.0) | 101 | (21.2) | 114 | (23.8) |
| Hispanic | 266 | (21.2) | 123 | (20.8) | 110 | (18.3) | 171 | (19.1) | 100 | (21.0) | 101 | (21.1) |
| Other | 67 | (5.4) | 36 | (6.1) | 39 | (6.5) | 51 | (5.7) | 34 | (7.1) | 29 | (6.1) |
| Maternal education | ||||||||||||
| Less than high school | 154 | (12.3) | 95 | (16.1) | 106 | (17.6) | 118 | (13.2) | 74 | (15.5) | 99 | (20.7) |
| High school | 286 | (22.8) | 134 | (22.7) | 135 | (22.4) | 210 | (23.5) | 114 | (23.9) | 112 | (23.4) |
| Some college | 359 | (28.7) | 159 | (26.9) | 132 | (21.9) | 250 | (28.0) | 121 | (25.4) | 113 | (23.6) |
| College degree | 451 | (36.0) | 203 | (34.3) | 228 | (37.9) | 314 | (35.2) | 167 | (35.0) | 153 | (32.0) |
| Prepregnancy BMI (kg/m2) | ||||||||||||
| Underweight (<18.5) | 56 | (4.5) | 23 | (3.9) | 28 | (4.7) | 44 | (4.9) | 16 | (3.4) | 19 | (4.0) |
| Normal (18.5–24.9) | 603 | (48.2) | 284 | (48.1) | 299 | (49.7) | 432 | (48.4) | 242 | (50.7) | 220 | (46.0) |
| Overweight (25.0–29.9) | 318 | (25.4) | 137 | (23.2) | 128 | (21.3) | 228 | (25.5) | 109 | (22.9) | 101 | (21.1) |
| Obese (≥30.0) | 233 | (18.6) | 117 | (19.8) | 118 | (19.6) | 162 | (18.1) | 87 | (18.2) | 108 | (22.6) |
| Alcohol consumption during pregnancy | ||||||||||||
| Yes | 423 | (33.8) | 200 | (33.8) | 184 | (30.6) | 309 | (34.6) | 142 | (29.8) | 159 | (33.3) |
| No | 822 | (65.7) | 390 | (66.0) | 414 | (68.8) | 580 | (64.9) | 333 | (69.8) | 316 | (66.1) |
| Cigarette smoking during pregnancy | ||||||||||||
| Yes | 201 | (16.1) | 94 | (15.9) | 99 | (16.4) | 141 | (15.8) | 75 | (15.7) | 93 | (19.5) |
| No | 1050 | (83.9) | 494 | (83.6) | 502 | (83.4) | 751 | (84.1) | 399 | (83.6) | 384 | (80.3) |
| Study site | ||||||||||||
| Arkansas | 135 | (10.8) | 103 | (17.4) | 138 | (22.9) | 124 | (13.9) | 112 | (23.5) | 144 | (30.1) |
| Iowa | 255 | (20.4) | 84 | (14.2) | 54 | (9.0) | 160 | (17.9) | 20 | (4.2) | 8 | (1.7) |
| Massachusetts | 205 | (16.4) | 92 | (15.6) | 93 | (15.4) | 190 | (21.3) | 39 | (8.2) | 33 | (6.9) |
| New York | 35 | (2.8) | 11 | (1.9) | 12 | (2.0) | 36 | (4.0) | 7 | (1.5) | 4 | (0.8) |
| Texas | 237 | (18.9) | 91 | (15.4) | 67 | (11.1) | 132 | (14.8) | 79 | (16.6) | 59 | (12.3) |
| Georgia | 196 | (15.7) | 104 | (17.6) | 87 | (14.5) | 123 | (13.8) | 109 | (22.9) | 116 | (24.3) |
| North Carolina | 62 | (5.0) | 81 | (13.7) | 142 | (23.6) | 45 | (5.0) | 93 | (19.5) | 111 | (23.2) |
| Utah | 127 | (10.1) | 25 | (4.2) | 9 | (1.5) | 83 | (9.3) | 18 | (3.8) | 3 | (0.6) |
- Abbreviations: HAA5 = group of five haloacetic acids most commonly found in drinking water, THM4 = total trihalomethanes.
- a Includes selected CHD cases and all controls classified as having maternal household exposure to DBPs, excludes private well water drinkers.
- b Exposure categories determined by assessing distribution of THM/HAAs exposure among controls, percentile cut points: THM4 50th 36.6 μg/L, 75th 52.6 μg/L, HAA5: 50th 22.9 μg/L, and 75th 37.1 μg/L.
| DBP | Mean | SD | Median | IQR | 25th % | 50th % | 75th % | Max. |
|---|---|---|---|---|---|---|---|---|
| THM4 | 41.2 | 36.4 | 35.9 | 32.9 | 19.4 | 35.9 | 52.3 | 400.1 |
| CHLF | 25.8 | 28.7 | 19.4 | 31.1 | 4.2 | 19.4 | 35.3 | 269.0 |
| BRF | 3.4 | 7.5 | 0.8 | 3.2 | 0.0 | 0.8 | 3.2 | 67.6 |
| BDCM | 9.0 | 9.0 | 7.6 | 6.6 | 4.4 | 7.6 | 11.0 | 124.7 |
| DBCM | 5.4 | 8.8 | 2.7 | 6.2 | 1.0 | 2.7 | 7.2 | 142.9 |
| HAA5 | 28.6 | 27.0 | 23.9 | 27.1 | 10.3 | 23.9 | 37.4 | 245.1 |
| MBAA | 0.8 | 1.6 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 23.1 |
| MCAA | 3.2 | 7.5 | 1.9 | 3.6 | 0.0 | 1.9 | 3.6 | 100.9 |
| DBAA | 2.7 | 4.8 | 1.0 | 2.4 | 0.0 | 1.0 | 2.4 | 38.3 |
| DCAA | 15.1 | 13.1 | 12.6 | 13.7 | 6.6 | 12.6 | 20.4 | 99.4 |
| TCAA | 11.5 | 12.3 | 9.1 | 14.0 | 2.0 | 9.1 | 16.0 | 108.4 |
- Abbreviations: BDCM = bromodichloromethane, BRF = bromoform, CHLF = chloroform, DBAA = dibromoacetic acid, DBCM = dibromochloromethane, DCAA = dichloroacetic acid, HAA5 = group of five haloacetic acids most commonly found in drinking water, MBAA = monobromoacetic acid, MCAA = monochloroacetic acid, TCAA = trichloroacetic acid, THM4 = total trihalomethanes.
Table 3 shows the aOR estimates and 95%CIs for the associations between specific CHDs and household exposure to DBPs among mothers who resided at a home with a linked public water system and a measurement value during the exposure window. Compared with the lower quantile of exposure, we observed moderately elevated aORs for the associations between the 2nd quantile of exposure to BDCM and HLHS and between the 2nd quantile of exposure to chloroform and AS, with 3rd quantile exposures to each associated with elevated aORs closer to the null. Moderately elevated associations were observed between the upper quantile of MCAA exposure and both HLHS and AS. We observe moderately elevated and reduced aORs for a few DBPs and HLHS and AS; however, HLHS and AS are the smallest case groups with the least precise estimates. Although the aORs did not reach our threshold for “moderate” elevations, elevated aORs reached statistical significance for associations between household BDCM, HAA5, and DCAA exposures and ASDs.
| C | TOF | d-TGA | HLHS | CoA | AS | PVS | VSD | ASD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBP exposurec | N | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) |
| THM4e | |||||||||||||||||
| ≤50th | 606 | 55 | REF | 42 | REF | 27 | REF | 68 | REF | 28 | REF | 95 | REF | 158 | REF | 249 | REF |
| 50th–75th | 303 | 38 | 1.3 (0.9, 2.1) | 15 | 0.8 (0.4, 1.4) | 19 | 1.5 (0.8, 2.8) | 30 | 0.9 (0.5, 1.4) | 8 | 0.8 (0.3, 1.6) | 35 | 0.8 (0.5, 1.2) | 67 | 0.8 (0.6, 1.1) | 101 | 0.8 (0.6, 1.1) |
| >75th | 303 | 33 | 1.2 (0.7, 1.9) | 17 | 0.9 (0.5, 1.6) | 15 | 1.3 (0.6, 2.5) | 30 | 0.8 (0.5, 1.4) | 7 | 0.7 (0.3, 1.6) | 49 | 0.9 (0.6, 1.4) | 63 | 0.7 (0.5, 1.0) | 113 | 1.0 (0.7, 1.3) |
| CHLFf | |||||||||||||||||
| ≤50th | 354 | 41 | REF | 20 | REF | 18 | REF | 42 | REF | 12 | REF | 53 | REF | 107 | REF | 223 | REF |
| 50th–75th | 167 | 21 | 1.0 (0.5, 1.9) | 9 | 1.2 (0.5, 3.0) | 9 | 1.2 (0.4, 3.4) | 23 | 1.0 (0.5, 1.9) | 9 | 2.2 (0.8, 6.0) | 29 | 0.9 (0.5, 1.6) | 49 | 0.8 (0.5, 1.2) | 49 | 0.9 (0.6, 1.4) |
| >75th | 174 | 26 | 1.2 (0.7, 2.3) | 11 | 1.4 (0.6, 3.6) | 11 | 1.5 (0.6, 4.3) | 22 | 1.0 (0.5, 2.0) | 7 | 1.9 (0.6, 5.6) | 40 | 1.1 (0.6, 1.9) | 46 | 0.6 (0.4, 1.0) | 80 | 1.3 (0.8, 2.0) |
| BRFg | |||||||||||||||||
| ≤75th | 663 | 68 | REF | 30 | REF | 26 | REF | 65 | REF | 22 | REF | 92 | REF | 157 | REF | 189 | REF |
| >75th | 221 | 20 | 0.7 (0.3, 1.5) | 10 | 1.1 (0.3, 3.2) | 12 | 1.5 (0.5, 4.7) | 22 | 1.0 (0.4, 2.1) | 6 | 0.9 (0.2, 3.0) | 30 | 1.3 (0.7, 2.4) | 45 | 0.8 (0.5, 1.4) | 163 | 0.8 (0.5, 1.2) |
| BDCMh | |||||||||||||||||
| ≤50th | 441 | 49 | REF | 19 | REF | 14 | REF | 47 | REF | 17 | REF | 60 | REF | 105 | REF | 150 | REF |
| 50th–75th | 222 | 18 | 0.8 (0.4, 1.3) | 15 | 1.4 (0.7, 3.0) | 16 | 2.5 (1.1, 5.5) | 22 | 0.9 (0.5, 1.5) | 4 | 0.5 (0.2, 1.3) d | 36 | 1.3 (0.8, 2.1) | 49 | 0.9 (0.6, 1.3) | 96 | 1.3 (0.9, 1.8) |
| >75th | 220 | 21 | 0.8 (0.4, 1.4) | 6 | 0.7 (0.2, 1.8) | 8 | 1.5 (0.6, 3.8) | 18 | 0.7 (0.4, 1.3) | 7 | 1.1 (0.4, 2.7) | 27 | 0.9 (0.5, 1.5) | 48 | 0.9 (0.6, 1.3) | 102 | 1.7 (1.2, 2.4) |
| DBCMi | |||||||||||||||||
| ≤50th | 441 | 44 | REF | 17 | REF | 17 | REF | 50 | REF | 16 | REF | 67 | REF | 114 | REF | 126 | REF |
| 50th–75th | 222 | 24 | 1.1 (0.6, 2.0) | 12 | 1.6 (0.7, 3.5) | 10 | 1.4 (0.6, 3.2) | 18 | 0.7 (0.4, 1.2) | 4 | 0.5 (0.2, 1.4) d | 30 | 1.0 (0.6, 1.7) | 48 | 0.9 (0.6, 1.3) | 98 | 1.1 (0.8, 1.6) |
| >75th | 220 | 19 | 0.8 (0.4, 1.6) | 11 | 1.6 (0.5, 4.4) | 11 | 1.5 (0.5, 4.2) | 19 | 0.6 (0.3, 1.2) | 8 | 1.1 (0.4, 3.1) | 26 | 1.1 (0.6, 1.9) | 40 | 0.7 (0.4, 1.2) | 127 | 0.9 (0.6, 1.3) |
| HAA5j | |||||||||||||||||
| ≤50th | 427 | 43 | REF | 29 | REF | 26 | REF | 58 | REF | 22 | REF | 72 | REF | 124 | REF | 159 | REF |
| 50th–75th | 213 | 24 | 1.2 (0.6, 2.0) | 14 | 1.2 (0.6, 2.3) | 10 | 0.9 (0.4, 2.0) | 19 | 0.7 (0.4, 1.3) | 5 | 0.6 (0.2, 1.5) | 41 | 1.3 (0.8, 2.0) | 63 | 1.0 (0.7, 1.5) | 114 | 1.5 (1.1, 2.1) |
| >75th | 214 | 29 | 1.4 (0.8, 2.4) | 11 | 0.9 (0.4, 2.0) | 15 | 1.4 (0.6, 2.9) | 28 | 1.0 (0.6, 1.8) | 6 | 0.8 (0.3, 2.0) | 33 | 0.9 (0.5, 1.6) | 56 | 0.8 (0.6, 1.2) | 107 | 1.5 (1.1, 2.2) |
| MBAAk | |||||||||||||||||
| ≤75th | 557 | 55 | REF | 30 | REF | 33 | REF | 63 | REF | 18 | REF | 95 | REF | 146 | REF | 232 | REF |
| >75th | 138 | 19 | 1.3 (0.7, 2.4) | 5 | 0.6 (0.2, 1.8) | 7 | 0.5 (0.2, 1.4) | 10 | 0.6 (0.2, 1.2) | 6 | 1.8 (0.6, 5.2) | 20 | 0.6 (0.3, 1.1) | 40 | 1.0 (0.7, 1.6) | 85 | 1.4 (1.0, 2.1) |
| MCAAl | |||||||||||||||||
| ≤75th | 521 | 56 | REF | 26 | REF | 24 | REF | 54 | REF | 14 | REF | 91 | REF | 143 | REF | 193 | REF |
| >75th | 174 | 19 | 1.0 (0.5, 1.8) | 9 | 0.9 (0.3, 2.4) | 16 | 2.0 (0.8, 4.9) | 20 | 1.2 (0.6, 2.4) | 10 | 3.6 (1.2, 10.5) | 24 | 0.5 (0.3, 0.9) | 44 | 0.8 (0.5, 1.3) | 125 | 1.4 (1.0, 2.0) |
| DBAAm | |||||||||||||||||
| ≤75th | 534 | 60 | REF | 25 | REF | 30 | REF | 60 | REF | 16 | REF | 87 | REF | 149 | REF | 162 | REF |
| >75th | 161 | 15 | 0.8 (0.4, 1.7) | 10 | 1.2 (0.4, 3.1) | 9 | 0.8 (0.3, 2.1) | 14 | 0.6 (0.2, 1.3) | 8 | 1.8 (0.5, 5.1) | 28 | 1.3 (0.7, 2.3) | 38 | 0.9 (0.5, 1.5) | 156 | 1.2 (0.7, 1.8) |
| DCAAn | |||||||||||||||||
| ≤50th | 354 | 37 | REF | 16 | REF | 19 | REF | 34 | REF | 14 | REF | 56 | REF | 91 | REF | 150 | REF |
| 50th–75th | 167 | 18 | 1.0 (0.5, 1.9) | 12 | 1.9 (0.8, 4.4) | 8 | 0.8 (0.3, 2.1) | 20 | 1.3 (0.7, 2.5) | 6 | 1.1 (0.4, 3.1) | 34 | 1.3 (0.8, 2.2) | 58 | 1.3 (0.9, 2.0) | 88 | 1.6 (1.1, 2.4) |
| >75th | 174 | 20 | 1.1 (0.6, 2.0) | 7 | 1.0 (0.3, 2.5) | 12 | 1.3 (0.5, 3.0) | 20 | 0.9 (0.5, 1.9) | 4 | 0.6 (0.2, 1.7)d | 25 | 0.9 (0.5, 1.5) | 37 | 0.8 (0.5, 1.2) | 80 | 1.5 (1.0, 2.2) |
| TCAAo | |||||||||||||||||
| ≤50th | 347 | 36 | REF | 17 | REF | 19 | REF | 35 | REF | 12 | REF | 56 | REF | 98 | REF | 194 | REF |
| 50th—75th | 174 | 13 | 0.6 (0.3, 1.2) | 8 | 1.0 (0.4, 2.4) | 8 | 1.2 (0.5, 3.2) | 17 | 1.0 (0.5, 1.9) | 6 | 1.3 (0.4, 3.9) | 27 | 1.0 (0.6, 1.7) | 43 | 0.9 (0.5, 1.3) | 56 | 1.0 (0.6, 1.5) |
| >75th | 174 | 26 | 1.4 (0.8, 2.6) | 10 | 1.2 (0.5, 3.0) | 13 | 1.6 (0.7, 3.9) | 22 | 1.1 (0.6, 2.1) | 6 | 1.5 (0.5, 4.5) | 32 | 1.0 (0.6, 1.8) | 46 | 0.8 (0.5, 1.2) | 68 | 1.3 (0.8, 2.0) |
- Note: Bold values indicates estimate ≥2.0 or ≤0.5.
- Abbreviations: AS = aortic stenosis, ASD = atrial septal defect, BDCM = bromodichloromethane, BRF = bromoform, C = controls, CHLF = chloroform, CI = confidence interval, CoA = coarctation of the aorta, DBAA = dibromoacetic acid, DBCM = dibromochloromethane, DCAA = dichloroacetic acid, d-TGA = d-transposition of the great arteries, Exp = exposure, HAA5 = group of five haloacetic acids most commonly found in drinking water, HLHS = hypoplastic left heart syndrome, MBAA = monobromoacetic acid, MCAA = monochloroacetic acid, NC = not calculated, PVS = pulmonary valve stenosis, REF = referent group, TCAA = trichloroacetic acid, THM4 = total trihalomethanes, TOF = tetralogy of Fallot, VSD = ventricular septal defect.
- a Exposures based on measurements found in public water supply linked to maternal residence.
- b All models adjusted for maternal age, race, body mass index, education, and study site.
- c Study sites MA and UT, only contributed measurement values for TTH4 and HAA5.
- d Unadjusted estimate.
- e Quantile cut points for THM4: 50th 36.6 μg/L, 75th 52.6 μg/L.
- f Quantile cut points for CHLF: 50th 20.0 μg/L, 75th 35.2 μg/L.
- g Quantile cut points for BRF: 75th 2.3 μg/L.
- h Quantile cut points for BDCM: 50th 7.5 μg/L, 75th 11.2 μg/L.
- i Quantile cut points for DBCM: 50th 2.5 μg/L, 75th 7.0 μg/L.
- j Quantile cut points for HAA5: 50th 22.9 μg/L, 75th 37.1 μg/L.
- k Quantile cut points for MBAA: 75th 1.0 μg/L.
- l Quantile cut points for MCAA: 75th 3.2 μg/L.
- m Quantile cut points for DBAA: 75th 2.0 μg/L.
- n Quantile cut points for DCAA: 50th 12.5 μg/L, 75th 20.3 μg/L.
- o Quantile cut points for TCAA: 50th 9.8 μg/L, 75th 15.8 μg/L.
In Table 4, we show the aOR estimates for the association between maternal ingestion of DBPs and eight CHDs. Across all CHD categories and DBP exposures, we did not find any moderately elevated aORs. However, compared with other CHD categories, VSDs had the highest proportion of aORs greater than 1.0 but not greater than 2.0 across all DBP exposure categories compared with the other CHD groupings. Yet, most were only minimally elevated and imprecise. We observed several moderately reduced estimates between various THM and HAA species and specific conotruncal and left obstructive CHDs. Among those with moderate reductions, most were observed for an upper quantile for which counts were generally smaller and estimates imprecise.
| C | TOF | d-TGA | HLHS | CoA | AS | PVS | VSD | ASD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBP exposurec | N | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) | N | OR (95%CI) |
| THM4e | |||||||||||||||||
| ≤50th | 565 | 58 | REF | 34 | REF | 28 | REF | 69 | REF | 25 | REF | 72 | REF | 118 | REF | 234 | REF |
| 50th–75th | 283 | 33 | 1.1 (0.7, 1.7) | 13 | 0.9 (0.5, 1.7) | 14 | 1.1 (0.5, 2.1) | 32 | 1.0 (0.6, 1.6) | 10 | 0.8 (0.4, 1.7) | 52 | 1.4 (0.9, 2.0) | 74 | 1.2 (0.9, 1.7) | 107 | 1.0 (0.7, 1.3) |
| >75th | 282 | 21 | 0.7 (0.4, 1.2) | 17 | 1.3 (0.7, 2.4) | 9 | 0.7 (0.3, 1.5) | 16 | 0.5 (0.3, 0.9) | 4 | 0.4 (0.1, 0.9) d | 33 | 0.9 (0.6, 1.4) | 68 | 1.1 (0.8, 1.6) | 80 | 0.7 (0.5, 1.0) |
| CHLFf | |||||||||||||||||
| ≤50th | 458 | 37 | REF | 14 | REF | 16 | REF | 40 | REF | 13 | REF | 36 | REF | 72 | REF | 164 | REF |
| 50th–75th | 229 | 18 | 0.8 (0.4, 1.4) | 6 | 0.8 (0.3, 2.2) | 8 | 0.9 (0.4, 2.1) | 18 | 0.7 (0.4, 1.4) | 9 | 0.9 (0.3, 2.1) | 33 | 1.4 (0.8, 2.4) | 58 | 1.2 (0.8, 1.8) | 87 | 1.2 (0.8, 1.7) |
| >75th | 229 | 22 | 0.9 (0.5, 1.7) | 12 | 1.7 (0.7, 4.2) | 6 | 0.6 (0.2, 1.8) | 18 | 0.7 (0.4, 1.4) | 2 | NC | 34 | 1.3 (0.7, 2.3) | 53 | 1.1 (0.7, 1.7) | 68 | 1.0 (0.7, 1.6) |
| BRFg | |||||||||||||||||
| ≤75th | 589 | 54 | REF | 27 | REF | 21 | REF | 62 | REF | 17 | REF | 80 | REF | 135 | REF | 222 | REF |
| >75th | 228 | 23 | 0.9 (0.5, 1.7) | 5 | 0.6 (0.2, 1.6) | 10 | 1.7 (0.6, 4.4) | 14 | 0.6 (0.3, 1.1) | 7 | 0.8 (0.3, 2.3) | 23 | 0.6 (0.3, 1.0) | 48 | 0.9 (0.6, 1.4) | 98 | 0.7 (0.5, 0.9) |
| BDCMh | |||||||||||||||||
| ≤50th | 359 | 41 | REF | 14 | REF | 16 | REF | 41 | REF | 12 | REF | 42 | REF | 65 | REF | 152 | REF |
| 50th–75th | 229 | 22 | 0.8 (0.4, 1.4) | 9 | 1.2 (0.5, 2.8) | 8 | 0.7 (0.3, 1.8) | 22 | 0.9 (0.5, 1.6) | 9 | 1.1 (0.4, 2.6) | 36 | 1.2 (0.7, 2.0) | 76 | 1.8 (1.2, 2.7) | 94 | 1.2 (0.8, 1.7) |
| >75th | 229 | 14 | 0.5 (0.3, 1.0) | 9 | 1.2 (0.5, 2.9) | 6 | 0.8 (0.3, 2.0) | 13 | 0.5 (0.3, 1.1) | 3 | 0.4 (0.1, 1.3) d | 25 | 0.9 (0.5, 1.6) | 42 | 1.0 (0.7, 1.6) | 73 | 0.9 (0.6, 1.3) |
| DBCMi | |||||||||||||||||
| ≤50th | 359 | 32 | REF | 14 | REF | 13 | REF | 43 | REF | 12 | REF | 42 | REF | 68 | REF | 144 | REF |
| 50th–75th | 229 | 29 | 1.4 (0.8, 2.5) | 10 | 1.4 (0.6, 3.5) | 9 | 1.3 (0.5, 3.1) | 22 | 0.9 (0.5, 1.7) | 8 | 0.8 (0.3, 2.0) | 32 | 1.0 (0.6, 1.8) | 66 | 1.5 (1.0, 2.2) | 82 | 1.2 (0.8, 1.7) |
| >75th | 229 | 16 | 0.7 (0.3, 1.3) | 8 | 1.1 (0.4, 2.7) | 8 | 1.2 (0.4, 3.0) | 11 | 0.4 (0.2, 0.8) | 4 | 0.6 (0.2, 1.6)d | 29 | 1.1 (0.6, 1.8) | 48 | 1.2 (0.8, 1.8) | 92 | 0.9 (0.6, 1.3) |
| HAA5j | |||||||||||||||||
| ≤50th | 354 | 36 | REF | 20 | REF | 24 | REF | 49 | REF | 19 | REF | 49 | REF | 87 | REF | 169 | REF |
| 50th–75th | 222 | 27 | 1.3 (0.7, 2.2) | 12 | 1.1 (0.5, 2.4) | 12 | 0.8 (0.3, 1.6) | 27 | 0.9 (0.5, 1.6) | 9 | 0.7 (0.3, 1.6) | 39 | 1.2 (0.7, 1.9) | 67 | 1.2 (0.8, 1.7) | 83 | 1.1 (0.7, 1.5) |
| >75th | 222 | 19 | 0.9 (0.5, 1.7) | 15 | 1.6 (0.8, 3.5) | 7 | 0.5 (0.2, 1.2) | 19 | 0.7 (0.4, 1.3) | 2 | NC | 36 | 1.2 (0.7, 2.0) | 63 | 1.1 (0.8, 1.7) | 93 | 1.0 (0.7, 1.4) |
| MBAAk | |||||||||||||||||
| ≤75th | 447 | 39 | REF | 26 | REF | 23 | REF | 50 | REF | 17 | REF | 63 | REF | 112 | REF | 205 | REF |
| >75th | 196 | 23 | 1.5 (0.8, 2.9) | 4 | 0.4 (0.1, 1.0) d | 9 | 0.8 (0.3, 2.2) | 14 | 0.6 (0.3, 1.2) | 4 | 0.6 (0.2, 1.5)d | 33 | 1.0 (0.5, 1.7) | 55 | 1.1 (0.7, 1.7) | 86 | 1.0 (0.7, 1.6) |
| MCAAl | |||||||||||||||||
| ≤75th | 445 | 41 | REF | 21 | REF | 21 | REF | 47 | REF | 16 | REF | 61 | REF | 115 | REF | 199 | REF |
| >75th | 198 | 20 | 1.1 (0.5, 2.0) | 9 | 1.1 (0.4, 2.7) | 10 | 1.2 (0.4, 3.2) | 17 | 0.9 (0.4, 1.7) | 5 | 0.6 (0.2, 1.8) | 35 | 1.1 (0.6, 1.8) | 52 | 1.0 (0.6, 1.5) | 91 | 1.1 (0.7, 1.6) |
| DBAAm | |||||||||||||||||
| ≤75th | 448 | 39 | REF | 24 | REF | 21 | REF | 50 | REF | 17 | REF | 69 | REF | 114 | REF | 193 | REF |
| >75th | 195 | 23 | 1.4 (0.8, 2.6) | 6 | 0.6 (0.2, 1.5) | 10 | 1.2 (0.5, 3.0) | 14 | 0.7 (0.3, 1.4) | 4 | 0.6 (0.2, 1.6)d | 27 | 0.8 (0.4, 1.3) | 53 | 1.1 (0.7, 1.7) | 97 | 0.8 (0.6, 1.2) |
| DCAAn | |||||||||||||||||
| ≤50th | 248 | 24 | REF | 10 | REF | 14 | REF | 31 | REF | 12 | REF | 28 | REF | 54 | REF | 130 | REF |
| 50th–75th | 198 | 22 | 1.2 (0.6, 2.2) | 10 | 1.4 (0.5, 3.6) | 10 | 0.8 (0.3, 1.9) | 17 | 0.7 (0.3, 1.3) | 7 | 0.6 (0.2, 1.6) | 35 | 1.3 (0.7, 2.4) | 61 | 1.4 (0.9, 2.1) | 86 | 1.4 (0.9, 2.1) |
| >75th | 197 | 16 | 0.8 (0.4, 1.7) | 10 | 1.3 (0.5, 3.6) | 7 | 0.7 (0.3, 1.9) | 16 | 0.6 (0.3, 1.3) | 2 | NC | 33 | 1.4 (0.8, 2.6) | 53 | 1.2 (0.8, 1.9) | 76 | 1.0 (0.7, 1.5) |
| TCAAo | |||||||||||||||||
| ≤50th | 248 | 26 | REF | 13 | REF | 16 | REF | 30 | REF | 12 | REF | 32 | REF | 58 | REF | 151 | REF |
| 50th–75th | 198 | 14 | 0.7 (0.3, 1.4) | 5 | 0.5 (0.2, 1.4) | 10 | 0.7 (0.3, 1.6) | 16 | 0.7 (0.3, 1.3) | 7 | 0.6 (0.2, 1.5) | 28 | 0.9 (0.5, 1.7) | 49 | 1.0 (0.6, 1.5) | 68 | 0.9 (0.6, 1.3) |
| >75th | 197 | 22 | 1.1 (0.5, 2.0) | 12 | 1.1 (0.5, 2.8) | 6 | 0.5 (0.2, 1.3) | 18 | 0.8 (0.4, 1.5) | 2 | NC | 36 | 1.2 (0.7, 2.2) | 60 | 1.2 (0.8, 1.9) | 73 | 0.9 (0.6, 1.4) |
- Note: Bold values indicate estimate ≥2.0 or ≤0.5.
- Abbreviations: AS = aortic stenosis, ASD = atrial septal defect, BDCM = bromodichloromethane, BRF = bromoform, C = controls, CHLF = chloroform, CI = confidence interval, CoA = coarctation of the aorta, DBAA = dibromoacetic acid, DBCM = dibromochloromethane, DCAA = dichloroacetic acid, d-TGA = d-transposition of the great arteries, HAA5 = group of five haloacetic acids most commonly found in drinking water, HLHS = hypoplastic left heart syndrome, MBAA = monobromoacetic acid, MCAA = monochloroacetic acid, NC = not calculated, PVS = pulmonary valve stenosis, REF = referent group, TCAA = trichloroacetic acid, THM4 = total trihalomethanes, TOF = tetralogy of Fallot, VSD = ventricular septal defect.
- a Unweighted exposure estimation assuming any source change occurred after the critical exposure period and excludes well water drinkers.
- b All models adjusted for maternal age, race/ethnicity, body mass index, education, and study site.
- c Study Sites MA and UT, only contributed measurement values for THM4 and HAA5.
- d Unadjusted estimate.
- e Quantile cut points for THM4: 50th 8.52 μg/day, 75th 40.2 μg/day.
- f Quantile cut points for CHLF: 50th 2.08 μg/day, 75th 17.7 μg/day.
- g Quantile cut points for BRF: 75th 0.64 μg/day.
- h Quantile cut points for BDCM: 50th 1.36 μg/day, 75th 7.71 μg/day.
- i Quantile cut points for DBCM: 50th 0.33 μg/day, 75th 2.81 μg/day.
- j Quantile cut points for HAA5: 50th 3.00 μg/day, 75th 20.8 μg/day.
- k Quantile cut points for MBAA: 75th 0.076 μg/day.
- l Quantile cut points for MCAA: 75th 0.95 μg/day.
- m Quantile cut points for DBAA: 75th 0.47 μg/day.
- n Quantile cut points for DCAA: 50th 1.11 μg/day, 75th 10.9 μg/day.
- o Quantile cut points for TCAA: 50th 0.77 μg/day, 75th 6.89 μg/day.
4 Discussion
Our study of household DBP exposure included a broad range of positive, null, and negative estimates across eight specific CHDs and two summary exposures (THM4 and HAA5) as well as nine individual species of DBPs. In addition to examining household exposure to DBPs, we also assessed the association between DBP exposure and CHDs accounting for ingestion patterns. The results taking ingestion patterns into account also included a range of positive, null, and negative associations with only a small number of moderately reduced estimates and no convincing evidence of meaningful patterns or elevated risks.
Comparing the results of the household exposure to those from the ingestion exposure reveal mostly similar findings. All the moderately elevated estimates in the household exposure results were attenuated in the maternal ingestion analyses. Differences between our “household” DBP concentration metric and our ingestion metric could possibly be attributed to the incorporation of water use behavior information in the exposure assessment. However, it is worth noting that the sample sizes differ between the two analyses, so it is possible that both samples do not include the same cases and controls due to various exclusions made when defining each sample (see Figure 1). We have most confidence in the HAA ingestion metric, given that THM ingestion is expected to play a very minor role in multiroute exposures. The THM ingestion metric has less relevance because of the importance of dermal and inhalation routes of THM exposure. Thus, THM exposure may be better reflected by the household exposure metric.
Epidemiologic studies provide some evidence of an association between exposure to DBPs and the risk of CHDs, but findings are inconsistent. In a review of the literature up to 2008, Nieuwenhuijsen et al. (2009) summarized the results of eight epidemiologic studies on the topic of drinking water DBPs and risk of CHDs. Several of the eight studies reported results for one to three CHD subtypes and observed increased ORs for associations between high versus low DBPs and VSDs (Hwang, Jaakkola, and Guo 2008; Nieuwenhuijsen et al. 2008), but did not find increased risks for other CHD subtypes (Hwang, Jaakkola, and Guo 2008; Nieuwenhuijsen et al. 2008). The meta-analysis summary estimate for VSDs was 1.59 (95%CI = 1.21, 2.07) (Nieuwenhuijsen et al. 2009). A more recent study, not included in the meta-analysis, utilized data from the MA birth defects registry and reported very similar results for elevated THM4 exposure and also found elevated ORs for some CHD subtypes and individual species of DBPs (Wright et al. 2017). While we also observed similar results, there are differences in methods related to both the outcomes and exposures analyzed.
We took advantage of the detailed NBDPS case classification by trained clinical geneticists and examined specific CHDs, with the understanding that CHDs have heterogeneous etiologies. We were able to include a total of eight CHD subtypes in our analysis, many of which have not been previously examined except in one other study (Wright et al. 2017). Wright et al. (2017) also included individual DBPs in their analyses and with the exception of TOF, observed significantly elevated ORs for the remaining CHD subtypes included (d-TGA, ASD, VSD, and PVS) and bromoform exposure, while we did not. For the association between HAA5 and both TOF and d-TGA, we observed evidence of an association, although comparatively weak and statistically nonsignificant. Additionally, our results differed in that we did not observe strong evidence of any association between TOF and TCAA and DCAA. Other differences included our ability to incorporate personal water use habits to assess the amount of water consumed (by source and whether filtered or not), which was not included in the exposure assessment by Wright et al. (2017). Finally, another difference between our study and Wright et al. (2017) was their use of birth certificate data as the source for individual-level covariates to adjust for confounding in their models, whereas our study used self-reported information collected from maternal interviews.
Our analysis has several limitations, particularly nondifferential exposure assessment. First, for the ingestion metric, we relied on self-reported questionnaire data, which is subject to error in recalling behaviors over 1 year in the past or bias if mothers of cases were more or less likely to report drinking unfiltered tap water. Second, our exposure assessment did not account for exposure to THMs via dermal absorption or inhalation while showering, bathing, or cooking and is therefore an unmeasured source of potential exposure. This is another limitation of the ingestion metric. Finally, we used the DBP measurement data linked to the maternal residence to estimate DBP exposure from tap water ingestion at the mother's job location. Our study did not collect addresses for work locations outside the home and were therefore unable to obtain DBP measurement values from PWSs serving mother's workplaces. Mothers may have spent a considerable amount of time at work and thus drank tap water there that could have a different concentration of DBPs than their drinking water at home. While this could have also contributed to nondifferential exposure misclassification, recent analyses attempting to characterize the impact of such bias suggest that the impact would be small (Zaganjor et al. 2022). It should be noted that these same limitations do not apply to the household exposure metric. The household exposure measurements do not rely on recall and although no attempt is made to account for individual differences in ingestion, showering, bathing, or cooking, household levels have been shown to successfully distinguish high exposure and low exposure to volatile THMs in particular (Rivera-Núñez et al. 2012).
Our analysis has several strengths. The DBP ingestion exposure assessment developed for this study incorporated detailed information collected in the interview on water use behaviors from multiple sources and maternal home address information throughout pregnancy. This was then used in combination with summary and individual DBP species measurement data, accounting for spatial and temporal variability, to estimate exposure during the critical period of heart development. Additionally, the NBDPS used a population-based case–control design, with study centers across the US, active case ascertainment, and included cases that were liveborn, stillborn, and elective terminations. Finally, the NBDPS employed systematic clinician review and classification of all eligible case medical record abstracts, ensuring categories and groupings consisted of well-characterized phenotypes. Additional strengths included our ability to identify or exclude participants based on certain criteria obtained through the interview data to limit the influence of certain biases. This included factors such as excluding those with indication of pregestational or gestational diabetes and excluding private well water drinkers.
5 Conclusion
Our analysis did not find strong evidence of an association between specific CHDs and maternal exposure to DBPs. This analysis presents an examination of the association between CHDs and DBP species utilizing a detailed individual-level exposure assessment, which incorporated information on participant behavior as it relates to water use. Additionally, we employed a careful case classification of CHDs, including some specific categories that have not been included in other studies. Despite the overall large study size, we observed small numbers in many exposure categories and for certain CHDs, limiting our ability to estimate precise effect measure estimates.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The study questionnaires and process for accessing the data used in this study are described at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html. The codebook may be made available upon request.




