Drinking water disinfection byproducts and risk of orofacial clefts in the National Birth Defects Prevention Study
Funding information: Centers for Disease Control and Prevention; Grant number U01DD001035
Abstract
Background
Maternal exposure to drinking water disinfection byproducts (DBP)s may contribute to orofacial cleft (OFC) development, but studies are sparse and beset with limitations.
Methods
Population-based, maternal interview reports of drinking water filtration and consumption for 680 OFC cases (535 isolated) and 1826 controls were linked with DBP concentration data using maternal residential addresses and public water system monitoring data. Maternal individual-level exposures to trihalomethanes (THM)s and haloacetic acids (HAA)s (µg/L of water consumed) were estimated from reported consumption at home, work, and school. Compared to no exposure, associations with multisource maternal exposure <1/2 or ≥1/2 the Maximum Contaminant Levels (MCL)s for total THMs (TTHM)s and HAAs (HAA5) or Maximum Contaminant Level Goals (MCLG)s for individual THMs and HAAs (if non-zero) were estimated for all OFCs and isolated OFCs, cleft palate (CP), and cleft lip ± cleft palate (CL/P) using logistic regression analyses.
Results
Compared to controls, associations were near or below unity for maternal TTHM, HAA5, and individual THM exposures with all OFCs and isolated OFCs, CP, and CL/P. Associations also were near or below unity for individual HAAs with statistically significant, inverse associations observed with each OFC outcome group except CL/P.
Conclusions
This study examined associations for maternal reports of drinking water filtration and consumption and maternal DBP exposure from drinking water with OFCs in offspring. Associations observed were near or below unity and mostly nonsignificant. Continued, improved research using maternal individual-level exposure data will be useful in better characterizing these associations.
1 INTRODUCTION
Orofacial clefts (OFC)s are among the most common human birth defects and are estimated to affect 1 per 700 live births worldwide (Mossey & Castillia, 2003). Disruption of the development of the lip or palate may result in distinct OFC subtypes, including cleft lip only (CL), cleft lip with cleft palate (CLP), or cleft palate only (CP) (Burdi, 2006). Numerous candidate gene studies and more recent genome-wide association studies have identified several susceptibility loci for OFCs. Similarly, numerous epidemiologic studies have examined maternal and paternal exposures for OFCs (reviewed in Leslie & Marazita, 2013; reviewed in Mehrotra, 2015). With the exception of maternal cigarette smoking (Little, Cardy, & Munger, 2004; Sabbagh et al., 2015), findings for most exposures investigated are mixed (Mossey, Little, Munger, Dixon, & Shaw, 2009), and studies of some exposures, such as drinking water contaminants, are sparse (Brender et al., 2013; reviewed in Nieuwenhuijsen et al., 2009).
Water disinfection byproducts (DBP)s are common drinking water contaminants and are formed when disinfectants (e.g., chlorine) react with bromide and natural organic matter in raw (untreated) water during the drinking water treatment process (Singer, 1994). Under the Safe Drinking Water Act, the United States (U.S.) Environmental Protection Agency (EPA) has set National Primary Drinking Water Regulations for several DBPs, including the more commonly measured trihalomethanes (THM)s and haloacetic acids (HAA)s. The U.S. EPA currently regulates THMs as total THMs (TTHM)s—the sum of bromoform, chloroform, bromodichloromethane, and dibromochloromethane; the Maximum Contaminant Level (MCL) for TTHM is currently set at 80 μg/L (U.S. EPA, 2010). HAAs are regulated as HAA5—the sum of monochloroacetic acid, dichloroacetic acid, trichloroacetic acid, bromoacetic acid, and dibromoacetic acid; the MCL for HAA5 is currently set at 60 μg/L (U.S. EPA, 2010). With more than 300 million people in the U.S. receiving their drinking water from public water systems (U.S. EPA, 2015), exposure to TTHMs and HAA5 is quite common among U.S. residents.
The role of THMs and HAAs in abnormal fetal development is unclear. In some animal studies, THMs and HAAs have been reported to decrease birth weight, increase pregnancy loss, and increase the risk of various birth defects (reviewed in Graves, Matanoski, & Tardiff, 2001; reviewed in Tardiff, Carson, & Ginevan, 2006). One study reported a significant increase in CP in the offspring of mice exposed to inhaled chloroform during days 8 through 15 of gestation (Murray, Schwetz, McBride, & Staples, 1979). Few epidemiologic studies, however, have explored associations between drinking water DBPs and OFCs. A record linkage study conducted in New Jersey (Bove et al., 1995) reported a statistically significant, positive association between mothers with public water system concentrations of TTHMs during pregnancy ≥100 μg/L compared to concentrations of TTHMs ≤20 μg/L and OFCs in their offspring. A recent study conducted in Massachusetts also reported a statistically significant, positive association for maternal exposure to a combination of nine DBPs (TTHM + HAA5) with CP, as well as positive associations for HAA5 and several individual THMs and HAAs with CP; associations for CL/P were largely near or below unity (Kaufman et al., 2018). Additional studies of TTHM exposures have reported either nonsignificant, positive associations (Hwang, Jaakkola, & Guo, 2008; Righi et al., 2012; Shaw et al., 2003) or null associations (Dodds, King, Woolcott, & Pole, 1999; Nieuwenhuijsen et al., 2008) between varying levels of maternal TTHM exposures measured in public water systems and OFCs in offspring. A study that examined maternal exposure to individual THMs reported positive associations between OFCs and mothers exposed to concentrations of chloroform between 50 and 74 μg/L and ≥100 μg/L (Dodds & King, 2001). A recent meta-analysis reported no associations between OFCs and any water chlorination or TTHM exposure (Nieuwenhuijsen et al., 2009).
To date, epidemiologic studies of DBPs and OFCs have relied on ecological measures of DBP exposures only, rather than measures generated from individual-level reports of estimated water consumption, which may have introduced exposure misclassification. Also, no study considered individual-level estimates of water consumption outside of the home (e.g., at work or school) or of alternative routes of DBP exposure (e.g., through bathing or showering), also potentially introducing exposure misclassification. To improve upon these limitations, we linked interview reports of water filtration and consumption from a large, U.S. population-based case-control study with public water system monitoring data, accounting for temporal and spatial fluctuations in DBP concentrations, to examine associations of maternal individual-level estimates of exposure to THMs and to HAAs with OFCs in their offspring.
2 METHODS
2.1 Study sample
The National Birth Defects Prevention Study (NBDPS) was a multisite, population-based case-control study funded by the Centers for Disease Control and Prevention. As detailed elsewhere (Reefhuis et al., 2015), interview reports were collected from mothers of cases and controls with estimated dates of delivery (EDD)s from October 1, 1997–December 31, 2011. Case deliveries were live births, stillbirths, or elective terminations diagnosed with one or more of over 30 major structural birth defects identified from the population-based birth defect surveillance program at each NBDPS site; cases with monogenic or chromosomal etiologies or whose OFCs were secondary to another defect were excluded. Eligible cases for the current project were those diagnosed with CL (modified British Paediatric Association [BPA] codes 749.101–749.103, 749.110, 749.120, 749.195); CP (BPA codes 749.001–749.003, 749.010, 749.020, 749.030, 749.041–749.043, 749.050, 749.060, 749.070, 749.090); or CLP (BPA codes 749.201–749.203, 749.210, 749.220, 749.290). OFC cases were further classified as isolated (no other major defects) or multiple (at least one additional major, unrelated defect) (Rasmussen et al., 2003). Eligible controls were live births without major birth defect diagnoses randomly selected from birth certificates or birth hospitals in the corresponding surveillance catchment areas for each NBDPS site. The NBDPS sites were Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. North Carolina and Utah joined the NBDPS in 2003. For this analysis, cases and controls from California and New Jersey were excluded because access to water quality data from individual public water systems at these sites was not available, and controls from Utah delivered in 2003 were excluded because Utah did not contribute OFC cases during that year.
2.2 Data collection
Mothers of eligible OFC cases and controls were asked to complete the NBDPS interview by telephone in either English- or Spanish-language (a copy of the interview is available from the corresponding author upon request). Briefly, the interview included items regarding parental sociodemographics, family history of birth defects, and occupation as well as maternal medical history, prenatal care, diet, lifestyle, and residence history beginning three months prior to conception (B3) through the EDD or end of pregnancy. Interviews conducted with mothers of cases and controls delivered between January 1, 2000 and December 31, 2005 included a detailed drinking water module that asked about maternal water sources, residential water treatment, drinking water consumption, and additional water use activities. Participation rates for the interview for all study years (1997–2011) were 71% for mothers of OFC cases and 64% for mothers of all controls. Our analyses included 1582 mothers of OFC cases and 3962 mothers of controls who completed an interview with the detailed drinking water module.
2.3 DBP concentration estimates
The critical period for lip and palate development is the first three months of gestation (Burdi, 2006). The estimated date of conception (EDC) for each mother was calculated by subtracting 280 days (40 weeks) from the EDD to obtain the last menstrual period date, then adding 14 days. Using the EDC and assigning 30 days per month, we estimated maternal exposure to DBPs from tap water for the preconception month (B1) immediately before the EDC through the first three months of pregnancy (P1, P2, P3) by applying a structured algorithm that combined interview data and DBP water sample results. The preconception month was included as part of the critical exposure period due to the potential for maternal prepregnancy behaviors to extend into early pregnancy.
In the interview, mothers were asked to report the full address, along with the residency start date (month, year) and end date (month, year), for each residence reported. Addresses were geocoded using Centrus (Group 1 Software; Lanham, MD); 97% of addresses were matched at any level, and 89% were matched at the address level. Public water systems were identified by linking the geocoded maternal residence to digitized public water system service area maps; 2010 census place shapes were used to approximate service boundaries if the public water system service area map was not available. For a city served by multiple public water systems that lacked information on the public water system boundaries within the district, the public water system that served the largest number of residences in the city was linked to the maternal geocoded residence.
An attempt was made to obtain DBP sampling results (contaminant levels, sampling date, location) from linked public water systems. DBP data are available on monitoring schedules for all public water systems in accordance with Safe Drinking Water Act regulations and guidelines (U.S. EPA, 2010). Nationally, public water systems are required to monitor THM and HAA levels; however, the frequency of mandatory reporting varies depending on the size of the public water system and the population served. Larger systems are required to monitor quarterly—compliance is based on a running annual average of quarterly samples. Small surface water and small groundwater systems are required to monitor annually. As such, for some cases and controls, TTHM or HAA5 levels were not available from the linked public water systems that served the respective maternal geocoded residences from B1 through P3.
Exposure measurement error can occur when sampling results from a public water system are assigned to a single residence due to temporal (e.g., monthly or seasonal) and spatial fluctuations in DBP concentrations throughout the system. To account for these fluctuations, we estimated an inverse-time weighted mean using all sample measurement days (up to a maximum of 10) for each available THM and HAA during the critical exposure period for each case and control mother, giving a higher weight to those measurements that occurred closest to the EDC. For mothers served by systems with multiple DBP sample measurements taken in a single day at different locations throughout the system during the critical exposure period, we used the mean concentration for each THM and HAA to estimate exposure for that day. Additionally, we explored a weighting factor to reduce the statistical contribution of mothers whose public water systems had high spatial variability in DBP concentrations throughout the system within individual measurement days (Waller, Swan, Windham, & Fenster, 2001); this factor was omitted from our final analyses due to a large proportion of mothers in our analytic sample whose public water systems provided only a single measurement for each DBP per measurement day.
2.4 Maternal water consumption estimates
During the interview, mothers were asked whether: the drinking water at the residence closest to the EDC was from a private well or public water system; the water was chemically disinfected (private well only); and the water used for drinking or cooking was filtered (none, whole house filter, faucet filter, etc.), and if filtered, the type of filter (membrane, charcoal, etc.) and frequency of filter changes per year. For each reported residence, mothers were also asked about the water source(s) (unfiltered tap, filtered tap, bottled, other) used for drinking, number of 8 oz. glasses of water consumed per day from each source, sources used to make hot drinks and for cooking, and details about changes in drinking water consumption from B3 through the end of the pregnancy (the month of a change in amount, number of 8 oz. glasses of water consumed per day after a change in amount, water sources used after a change in source). Additionally, mothers were asked about the water source(s) (unfiltered tap, filtered tap, drinking fountain [coded as unfiltered tap], bottled/cooler, brought from home, other) used for drinking at school (if enrolled) or at each job (if employed), and the average number of 8 oz. glasses of water consumed per day from each source at school and work. We reviewed the “other” responses for water source at home, work, and school and recoded them into one of the predefined sources, where possible. Additional interview items asked about water use activities, including washing dishes and clothes, showering and bathing, and swimming.
Total water consumption during the critical exposure period from each water source was estimated using the number of 8 oz. glasses of water consumed per day at home and while at each job and school, and the estimated number of days spent at each job and school during the critical exposure period (for an example, see Box 1). We did not use the responses about water sources used to make hot drinks and for cooking, as associated consumption amounts were not reported. A mother's estimated average daily consumption from a water source was calculated by dividing her total estimated consumption by 120 days.

Water consumption example without amount or source changes
For mothers who reported changing their daily amount of water consumption or starting or stopping living at a residence, working at a job, or attending school, the date of each event was collected in the interview at the level of calendar month and year. If the year was reported but not the month, the change was assumed to occur in July. To determine if and when any of these events occurred during the critical exposure period, we converted the calendar month and year to the 30-day period (B1, P1, P2, P3) that contained the largest number of days within the identified calendar month. For example, January would be assigned P1 for a mother with an EDC of January 10th but assigned B1 for a mother with an EDC of January 20th. If two 30-day periods contained the same number of days for a given calendar month, the earlier 30-day period was assigned. Changes to consumption amount were assumed to apply to one-half of the 30-day period during which they were reported. Mothers were assumed to be at a residence, job, or school during one-half of the 30-day period during which they started or stopped being at that location.
Although the interview asked about the timing of any change in the amount of drinking water consumed at home, it did not ask about the distribution of the change by water source. If more than one water source was reported, we estimated the source distribution of total home drinking water consumption after the change using unweighted and weighted approaches (Figure 1). Our unweighted approach distributed total consumption across reported water sources according to the proportions before the change in consumption amount. Our weighted approach used a ranking (lowest to highest) of water sources by DBP concentration (bottled water, filtered tap water, unfiltered tap water). Arbitrary weights of 3, 2, and 1 were used if all three water sources were reported, and arbitrary weights of 3 and 1 were used if only two water sources were reported. Our low-DBP-weighted approach assigned the highest weight value to the water source with the assumed lowest DBP concentration. Conversely, our high-DBP-weighted approach assigned the highest weight value to the water source with the assumed highest DBP concentration (for an example, see Box 2).
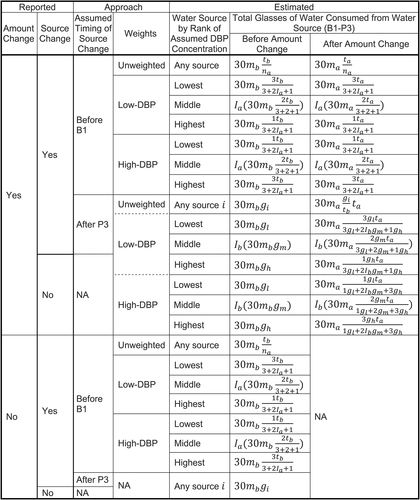
Algorithm developed to estimate distribution of total water consumption when multiple sources were reported, National Birth Defects Prevention Study (2000–2005 deliveries).B1 = preconception month; EDC = estimated date of conception; NA = not applicable; P3 = third month of pregnancy.
gl = glasses of water consumed per day from source with lowest DBP concentration before any reported source or amount change.
gm = glasses of water consumed per day from source with middle DBP concentration before any reported source or amount change.
gh = glasses of water consumed per day from source with highest DBP concentration before any reported source or amount change.
Ib=1(nb=3) = indicator that three water sources are reported before any reported water source change.
Ia=1(na=3) = indicator that three water sources are reported after any reported water source change.
mb = number of pregnancy months in critical exposure period living at residence closest to EDC before any reported amount change.
ma = number of pregnancy months in critical exposure period living at residence closest to EDC after any reported amount change.
nb = number of water sources reported before any reported water source change.
na = number of water sources reported after any reported water source change.
tb = gl+Ibgm+gh = total number of glasses of water consumed per day before any reported amount change.
ta = total number of glasses of water consumed per day after any reported amount change.

Water consumption example with amount change
Although a mother could report a change in water source, the interview did not ask about the date corresponding to the change. As such, we estimated consumption from each water source using two different assumptions for mothers who reported changing sources. The first assumption was that the change in water source occurred before the critical exposure period; the second assumption was that the change in water source occurred after the critical exposure period. For the first assumption, because the interview did not ask about the distribution of total water consumption by water source after a reported change in source, we estimated the distribution to be proportional to the weights of the respective consumption estimation approach (for an example, see Box 3). For the second assumption, the water source change did not affect consumption estimates because it did not apply to the critical exposure period. In calculating total consumption that included amounts from work or school, we assumed the distribution of water sources that a mother reported bringing from home to work or school was proportional to the estimated distribution of home water consumption.

Water consumption example with source change
2.5 Maternal DBP ingestion estimates from public water systems
In our analyses, a mother's exposure to DBPs in tap water depends on the levels of DBPs in the public water system(s) serving her residence, work, and school; her average daily water consumption from each water source; and the use of any water filters at her residence, work, and school that affected DBP levels. We assumed that private well water (due to minimal reported disinfection treatments) and bottled water had 0 µg/L of DBP exposure. We also assumed a mother's work and school were in the same water district as her residence. If a mother reported use of a filtration system at home, the reported brand names for filtration systems and filters provided were queried in the list of NSF International certified drinking water treatment units (http://info.nsf.org/Certified/DWTU/) to determine whether the system or filter could remove DBPs. If no brand name was reported, the effectiveness in DBP removal of the filter or system was determined by the description of the filtration method. Types of filters that are known to remove DBPs were estimated to reduce the DBP concentration to 10% of that measured in the public water system. Reported types of filters unable to remove DBPs or those with undetermined capacity to remove DBPs were assumed to reduce the DBP concentration to 90% of that measured in the public water system. The interview did not ask about information regarding the types of filters used at work or school, so these filters also were assumed to reduce the DBP concentration to 90% of that measured in the public water system.
2.6 Maternal dermal and inhalation DBP exposures
A positive association between longer showers taken by mothers (duration ≥15 min) and cleft lip ± cleft palate (CL/P) has been previously reported using NBDPS data (Agopian, Waller, Lupo, Canfield, & Mitchell, 2013); therefore, we included average shower duration in our analysis as a covariate. Because our focus was on DBP exposures from drinking water consumption, we did not consider additional reported dermal or inhalation exposures from washing dishes, washing clothes, bathing, and swimming in our analyses.
2.7 Statistical analysis
Case and control mothers were excluded from analysis if they reported a diagnosis of pregestational diabetes due to the strong association between pregestational diabetes and birth defects, particularly OFCs (Aberg, Westbom, & Kallen, 2001; Becerra, Khoury, Cordero, & Erickson, 1990; Correa et al., 2008; Spilson, Kim, & Chung, 2001). Mothers were eligible to be included in the analyses if they resided at the same residence throughout the critical exposure period, and they either did not drink tap water provided by a public water system during the critical exposure period, or they drank tap water provided by a public water system during the critical exposure period and their DBP ingestion could be estimated. Mothers’ DBP ingestion could be estimated if: (a) they reported the year(s) and number of days per week they attended every reported job and school outside the home; (b) they reported water sources and consumption amounts at their residence and at all jobs and schools outside the home during the critical exposure period; (c) all reported water sources could be categorized as “unfiltered tap,” “filtered tap,” or “bottled;” (d) their residence was successfully geocoded; (e) the public water system that served their residence was identified; and (f) the DBP measurements of that water system were known while they resided at that address during the critical exposure period.
Selected child and maternal characteristics and maternal exposures during the critical exposure period were assessed as covariates. To evaluate the representativeness of OFC cases and controls available for analysis, we compared their covariates to those of all OFC cases and all controls delivered during 2000–2005, respectively, using chi-square goodness-of-fit tests (calculating exact p-values if expected cell counts <5). We also compared covariates of all OFC cases and controls eligible for analysis using chi-square tests of independence or Fisher's exact tests (if expected cell counts <5) to determine statistically significant differences (p < .05).
Child characteristics examined were sex (male, female), gestational age (<37, 37–45 weeks), family history of OFC (first-degree relative, other relative, none), and plurality (single birth, twin, other multiple birth). Maternal characteristics examined were age at delivery (<20, 20–24, 25–29, 30–34, ≥35 years), education at delivery (<12, 12, 13–15, ≥16 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), gravidity (first pregnancy, second pregnancy, third or higher pregnancy), prepregnancy body mass index (BMI) (<18.5, 18.5–25.0, 25.0–30.0, ≥30.0 kg/m2), and study site (Arkansas, Georgia, Iowa, Massachusetts, New York, North Carolina, Texas, Utah). Maternal exposures during the critical exposure period examined were cigarette smoking (no active or passive smoking, active smoking only, passive smoking only [exposed to cigarette smoking at home or workplace], active and passive smoking), alcohol consumption (no drinking, binge drinking [≥4 drinks on one occasion], drinking but no binge drinking), use of a folic acid-containing supplement (yes, no), and average shower duration (<15, ≥15 min).
Crude odds ratios (cORs), adjusted odds ratios (aORs), and 95% confidence intervals (CI)s were estimated using logistic regression analysis. Associations between OFCs and DBPs were analyzed for all OFCs, isolated OFCs, and two subtypes of isolated OFCs: CP and CL/P. We examined potential associations between each outcome group and maternal exposure to TTHMs and HAA5 in µg per liter of water consumed. Categories of exposure were based on one-half the MCLs (40 µg/L for TTHMs and 30 µg/L for HAA5), and mothers unexposed to TTHMs or HAA5 were used as the referent groups for the respective analyses. Crude analyses were conducted only for categories of TTHM and HAA5 exposures that included at least three exposed and three referent case mothers.
Adjusted analyses were conducted only for categories of TTHM and HAA5 exposures that included at least five exposed and five referent case mothers. Covariates that were found to be statistically associated (p < .05) with the outcome group and exposure using chi-square tests of independence or Fisher's exact tests (if expected cell counts <5) were added separately to the exposure-only model; covariates that changed the cOR estimate by more than 10% were included in the adjusted model. Because a previous NBDPS study reported that increases in maternal water consumption were inversely associated with CL (Alman, Coffman, Siega-Riz, Luben, & National Birth Defects Prevention Study, 2017), we considered total maternal water consumption during the critical exposure period as a potential covariate in adjusted analyses. We observed that total maternal water consumption was not associated with any OFC outcome group using logistic regression analysis, and thus, it was not included in any adjusted models.
We also examined potential associations between the outcome groups and individual THM and HAA contaminants in µg per liter of water consumed. Categories of exposure were based on one-half the Maximum Contaminant Level Goals (MCLG)s, if available and non-zero (35 µg/L for chloroform, 30 µg/L for dibromochloromethane, 35 µg/L for monochloroacetic acid, and 10 µg/L for trichloroacetic acid) (U.S. EPA, 2010). Contaminants with MCLGs of 0 µg/L (bromoform, bromodichloromethane, and dichloroacetic acid) or no MCLGs (bromoacetic acid and dibromoacetic acid) (U.S. EPA, 2010) were analyzed as dichotomous indicators of any maternal exposure from drinking water. Mothers unexposed to a specific contaminant were used as the referent group for the analyses of that contaminant. Mothers from Massachusetts and Utah were excluded from our analysis of individual contaminants because these sites did not report individual THM and HAA contaminant concentrations.
As a subanalysis, we examined potential associations between the same outcome groups and concentrations of TTHMs, HAA5, and individual THM and HAA contaminants in the public water systems linked to maternal residences without accounting for filtration and consumption. These results are more directly comparable to previous studies that lacked this information (Dodds et al., 1999; Hwang et al., 2008; Kaufman et al., 2018; Nieuwenhuijsen et al., 2008; Righi et al., 2012; Shaw et al., 2003). Concentrations less than one-half the MCLs or MCLGs were used as the referent groups, except for contaminants with MCLGs of 0 µg/L or no MCLGs for which concentrations of 0 µg/L were used.
To assess the magnitude of improvement of exposure classification in our study compared to ecological studies, we compared mothers’ classifications of TTHM and HAA5 exposures per liter of water consumed with their classifications of TTHM and HAA5 concentrations in public water systems. All analyses were conducted using the Statistical Analysis System (SAS) version 9.4 statistical software (SAS institute, Cary, NC).
3 RESULTS
Overall, 5544 (cases = 1582, controls = 3962) mothers completed an interview. Of these, 66 (cases = 29, controls = 37) mothers were excluded due to a reported diagnosis of pregestational diabetes or an incomplete response for diagnosis or type of diabetes. Using the assumption that any reported source changes occurred after the critical exposure period, among the remaining 5478 (cases = 1553, controls = 3925) mothers, 1346 (cases = 409, controls = 937) had insufficient interview data or relocated during the critical exposure period, 1367 (cases = 377, controls = 990) reported that they did not drink tap water provided by a public water system during the critical exposure period, and 2765 (cases = 767, controls = 1998) reported that they drank tap water provided by a public water system during the critical exposure period. Of the last group, 1139 (cases = 303, controls = 836) mothers had their addresses successfully geocoded and were linked to a public water system for which DBP measurements were available; these 1139 mothers along with the 1367 mothers who reported no tap water consumption were eligible for analysis (cases = 680, controls = 1826). The 680 eligible OFC cases included 535 isolated cases, 146 with CP and 389 with CL/P; the remaining 145 OFC cases had multiple defects.
No statistical differences (p < .05) were observed for child characteristics between controls eligible for analysis and all controls whose mothers completed the interview and reported no pregestational diabetes; likewise, no statistical differences were observed between eligible OFC cases and all OFC cases. For maternal characteristics and exposures, statistical differences were observed between mothers of eligible controls and all controls for age at delivery, gravidity, prepregnancy BMI, study site, and smoking. Furthermore, statistical differences were observed between mothers of eligible OFC cases and all OFC cases for age at delivery, education at delivery, and study site (Table 1).
| All Controls | Eligiblea Controls | All OFCs | Eligiblea OFCs | Eligible vs All Controls | Eligible vs All OFCs | Eligible Controls vs Eligible OFCs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariateb | Nc | % | Nc | % | Nc | % | Nc | % | p | p | p |
| Total | 3925 | 1826 | 1553 | 680 | |||||||
| Child characteristics | |||||||||||
| Sex | .971 | .865 | .001 | ||||||||
| Male | 2017 | 51.4 | 937 | 51.4 | 919 | 59.3 | 401 | 9.0 | |||
| Female | 1904 | 48.6 | 886 | 48.6 | 631 | 40.7 | 279 | 41.0 | |||
| Gestational age (weeks) | .711 | .511 | <.001 | ||||||||
| Preterm (<37) | 395 | 10.1 | 179 | 9.8 | 255 | 16.4 | 118 | 17.4 | |||
| Term (≥37) | 3530 | 89.9 | 1647 | 90.2 | 1298 | 83.6 | 562 | 82.7 | |||
| Family history of OFC | .346 | .918 | <.001 | ||||||||
| First-degree relative | 10 | .3 | 3 | .2 | 92 | 5.9 | 39 | 5.7 | |||
| Other relative | 42 | 1.1 | 25 | 1.4 | 217 | 14.0 | 92 | 13.5 | |||
| None | 3873 | 98.7 | 1798 | 98.5 | 1244 | 80.1 | 549 | 80.7 | |||
| Plurality | .822d | .892d | .317 | ||||||||
| Single birth | 3794 | 97.5 | 1763 | 97.2 | 1481 | 96.3 | 648 | 96.1 | |||
| Twin | 91 | 2.3 | 46 | 2.5 | 52 | 3.4 | 23 | 3.4 | |||
| Other multiple birth | 7 | .2 | 4 | .2 | 5 | .3 | 3 | .5 | |||
| Maternal characteristics | |||||||||||
| Age at delivery (years) | .001 | .004 | .491 | ||||||||
| <20 | 388 | 9.9 | 147 | 8.1 | 150 | 9.7 | 53 | 7.8 | |||
| 20–24 | 893 | 22.8 | 367 | 20.1 | 398 | 25.6 | 154 | 22.7 | |||
| 25–29 | 1065 | 27.1 | 502 | 27.5 | 431 | 27.8 | 174 | 25.6 | |||
| 30–34 | 1024 | 26.1 | 516 | 28.3 | 353 | 22.7 | 180 | 26.5 | |||
| ≥35 | 555 | 14.1 | 294 | 16.1 | 221 | 14.2 | 119 | 17.5 | |||
| Education at delivery (years) | .494 | .159 | .032 | ||||||||
| <12 | 610 | 15.8 | 282 | 15.4 | 252 | 16.4 | 91 | 13.4 | |||
| 12 | 926 | 24.0 | 442 | 24.2 | 432 | 28.1 | 188 | 27.7 | |||
| 13–15 | 1008 | 26.1 | 454 | 24.9 | 407 | 26.5 | 191 | 28.1 | |||
| ≥16 | 1314 | 34.1 | 648 | 35.5 | 448 | 29.1 | 210 | 30.9 | |||
| Race/Ethnicity | .142 | .824 | .001 | ||||||||
| Non-Hispanic white | 2414 | 61.9 | 1095 | 60.3 | 1044 | 67.5 | 454 | 67.0 | |||
| Non-Hispanic black | 487 | 12.5 | 237 | 13.1 | 111 | 7.2 | 51 | 7.5 | |||
| Hispanic | 711 | 18.2 | 362 | 19.9 | 273 | 17.7 | 126 | 18.6 | |||
| Other | 291 | 7.5 | 122 | 6.7 | 118 | 7.6 | 47 | 6.9 | |||
| Gravidity | .033 | .052 | .436 | ||||||||
| First pregnancy | 1148 | 29.3 | 484 | 26.5 | 481 | 31.0 | 182 | 26.8 | |||
| Second pregnancy | 1169 | 29.8 | 567 | 31.1 | 479 | 30.9 | 227 | 33.4 | |||
| Third or higher pregnancy | 1602 | 40.9 | 775 | 42.4 | 590 | 38.1 | 271 | 39.9 | |||
| Pre-pregnancy BMI (kg/m2) | .008 | .622 | .006 | ||||||||
| Underweight (<18.5) | 206 | 5.5 | 68 | 3.9 | 107 | 7.2 | 42 | 6.4 | |||
| Normal (18.5–25.0) | 2055 | 54.5 | 953 | 54.6 | 792 | 52.9 | 342 | 52.4 | |||
| Overweight (25.0–30.0) | 877 | 23.3 | 444 | 25.4 | 335 | 22.4 | 143 | 21.9 | |||
| Obese (≥30.0) | 632 | 16.8 | 281 | 16.1 | 262 | 17.5 | 126 | 19.3 | |||
| Study Site | <.001 | .018 | .002 | ||||||||
| Arkansas | 651 | 16.6 | 273 | 15.0 | 216 | 13.9 | 80 | 11.8 | |||
| Georgia | 536 | 13.7 | 235 | 12.9 | 246 | 15.8 | 108 | 15.9 | |||
| Iowa | 540 | 13.8 | 257 | 14.1 | 229 | 14.8 | 94 | 13.8 | |||
| Massachusetts | 573 | 14.6 | 327 | 17.9 | 252 | 16.2 | 141 | 20.7 | |||
| New York | 410 | 10.5 | 156 | 8.5 | 174 | 11.2 | 64 | 9.4 | |||
| North Carolinae | 406 | 10.3 | 223 | 12.2 | 101 | 6.5 | 53 | 7.8 | |||
| Texas | 582 | 14.8 | 295 | 16.2 | 236 | 15.2 | 106 | 15.6 | |||
| Utahf | 227 | 5.8 | 60 | 3.3 | 99 | 6.4 | 34 | 5.0 | |||
| Maternal exposures | |||||||||||
| Cigarette smoking | <.001 | .081 | <.001 | ||||||||
| No active or passive smoking | 2603 | 67.5 | 1295 | 71.2 | 917 | 59.6 | 427 | 62.9 | |||
| Active smoking only | 293 | 7.6 | 135 | 7.4 | 149 | 9.7 | 69 | 10.2 | |||
| Passive smoking only | 507 | 13.1 | 232 | 12.8 | 212 | 13.8 | 93 | 13.7 | |||
| Active and passive smoking | 454 | 11.8 | 158 | 8.7 | 260 | 16.9 | 90 | 13.3 | |||
| Alcohol consumption | .174 | .129 | .169 | ||||||||
| No drinking | 2466 | 64.3 | 1200 | 66.2 | 930 | 60.8 | 418 | 62.3 | |||
| Binge drinking (≥4 drinks/occasion) | 430 | 11.2 | 183 | 10.1 | 202 | 13.2 | 71 | 10.6 | |||
| Drinking but no binge drinking | 940 | 24.5 | 430 | 23.7 | 397 | 26.0 | 182 | 27.1 | |||
| Folic-acid containing supplement | .084 | .850 | .073 | ||||||||
| Yes | 3406 | 88.1 | 1623 | 89.4 | 1332 | 87.1 | 586 | 86.8 | |||
| No | 462 | 11.9 | 193 | 10.6 | 198 | 12.9 | 89 | 13.2 | |||
| Average shower duration | .191 | .901 | .003 | ||||||||
| <15 min | 1935 | 51.9 | 947 | 53.4 | 694 | 46.8 | 307 | 46.6 | |||
| ≥15 min | 1794 | 48.1 | 825 | 46.6 | 788 | 53.2 | 352 | 53.4 | |||
- BMI = body mass index; OFC = orofacial cleft.
- Because of rounding, percentages might not total 100. Bold p-value indicates p-value < .05.
- a Unweighted exposure estimation assuming any source change occurred after the critical exposure period.
- b Daily maternal water consumption was analyzed as a continuous variable (data not shown).
- c Missing values not included in chi-square tests.
- d Exact p-value.
- e Includes deliveries during 2003–2005.
- f Includes deliveries during 2004–2005.
Comparing child characteristics between eligible OFC cases and controls, cases were statistically more likely to be male, preterm, and have a first-degree relative or other relative with an OFC. We also observed statistical differences between mothers of eligible cases and controls for education, race/ethnicity, BMI, and study site. Comparing exposures between mothers of eligible cases and controls, we also observed an excess of case mothers who were active or passive smokers and case mothers who took ≥15 min showers (Table 1).
Table 2 presents results for associations for all OFCs and isolated OFCs, CP, and CL/P with maternal THM and HAA exposure per liter of water consumed, estimated using the unweighted algorithm and assuming any reported source changes occurred after the critical exposure period. If no covariates met the criteria for inclusion in an adjusted model, the cOR and 95% CI was reported for the given association. No statistically significant, positive associations were observed for any OFC outcome group with maternal exposure to TTHMs, HAA5, or individual contaminants. We observed significant, inverse associations for all OFCs with any maternal exposure to bromoacetic acid, for isolated OFCs with any maternal exposure to dibromoacetic acid, and for CP with maternal exposure to monochloroacetic acid less than one-half the MCLG of 35 µg/L (no mothers had an average ingestion concentration of 35 µg/L or greater) and any maternal exposure to bromoacetic acid and dibromoacetic acid. No substantive differences in the pattern of results were found using the weighted exposure estimation approaches or the assumption that any reported source changes occurred before the critical exposure period (data not shown).
| Controls | All OFCs | Isolated OFCs | Isolated CP | Isolated CL/P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposurea | N | % | N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) |
| TTHMsb | ||||||||||||||
| No exposure | 1005 | 55.3 | 388 | 57.1 | Reference | 303 | 56.7 | Reference | 81 | 57.0 | Reference | 222 | 56.6 | Reference |
| <½ MCL | 640 | 35.2 | 236 | 34.8 | 1.0 (.8–1.2) i | 191 | 35.8 | 1.0 (.8–1.2)i | 52 | 36.6 | 1.0 (.7–1.4) | 139 | 35.5 | 1.0 (.8–1.2) |
| ≥½ MCL | 174 | 9.6 | 55 | 8.1 | .9 (.7–1.3) i | 40 | 7.5 | .9 (.6–1.3)i | 9 | 6.3 | .6 (.3–1.3) | 31 | 7.9 | .8 (.5–1.2) |
| Individual THMs | ||||||||||||||
| Bromoform c | ||||||||||||||
| No exposure | 1018 | 74.5 | 373 | 76.9 | Reference | 304 | 77.8 | Reference | 87 | 81.3 | Reference | 217 | 76.4 | Reference |
| Exposed | 348 | 25.5 | 112 | 23.1 | .9 (.7–1.1) | 87 | 22.3 | .8 (.6–1.1) | 20 | 18.7 | .7 (.4–1.1) | 67 | 23.6 | .9 (.7–1.2) |
| Chloroformd | ||||||||||||||
| No exposure | 760 | 55.6 | 280 | 57.7 | Reference | 223 | 57.0 | Reference | 62 | 57.9 | Reference | 161 | 56.7 | Reference |
| <½ MCLG | 523 | 38.3 | 181 | 37.3 | .9 (.8–1.2) | 150 | 38.4 | 1.0 (.8–1.2) | 41 | 38.3 | 1.0 (.6–1.4) | 109 | 38.4 | 1.0 (.8–1.3) |
| ≥½ MCLG | 83 | 6.1 | 24 | 5.0 | .8 (.5–1.3) | 18 | 4.6 | .7 (.4–1.3) | 4 | 3.7 | .6 (.2–1.7) | 14 | 4.9 | .8 (.4–1.4) |
| Bromodichloromethanec | ||||||||||||||
| No exposure | 760 | 55.7 | 276 | 56.9 | Reference | 220 | 56.3 | Reference | 61 | 57.0 | Reference | 159 | 56.0 | Reference |
| Exposed | 605 | 44.3 | 209 | 43.1 | 1.0 (.8–1.2) | 171 | 43.7 | 1.0 (.8–1.2) | 46 | 43.0 | .9 (.6–1.4) | 125 | 44.0 | 1.0 (.8–1.3) |
| Dibromochloromethanee | ||||||||||||||
| No exposure | 792 | 58.0 | 284 | 58.6 | Reference | 228 | 58.3 | Reference | 62 | 57.9 | Reference | 166 | 58.5 | Reference |
| <½ MCLG | 570 | 41.8 | 200 | 41.2 | 1.0 (.8–1.2) | 162 | 41.4 | 1.0 (.8–1.2) | 45 | 42.1 | 1.0 (.7–1.5) | 117 | 41.2 | 1.0 (.8–1.3) |
| ≥½ MCLG | 3 | .2 | 1 | .2 | NC | 1 | .3 | NC | 0 | 0.0 | NC | 1 | .4 | NC |
| HAA5f | ||||||||||||||
| No exposure | 1018 | 63.7 | 396 | 65.8 | Reference | 308 | 65.5 | Reference | 81 | 66.4 | Reference | 227 | 65.2 | Reference |
| <½ MCL | 475 | 29.7 | 173 | 28.7 | 1.0 (.8–1.2) i | 139 | 29.6 | 1.0 (.8–1.2)i | 37 | 30.3 | 1.0 (.7–1.5) | 102 | 29.3 | 1.0 (.8–1.3)j |
| ≥½ MCL | 105 | 6.6 | 33 | 5.5 | .9 (.6–1.4) i | 23 | 4.9 | .8 (.5–1.3)i | 4 | 3.3 | .5 (.2–1.3) | 19 | 5.5 | .9 (.5–1.5)j |
| Indivdual HAAs | ||||||||||||||
| Monochloroacetic acidd | ||||||||||||||
| No exposure | 944 | 75.3 | 347 | 79.8 | Reference | 277 | 79.6 | Reference | 77 | 85.6 | Reference | 200 | 77.5 | Reference |
| <½ MCLG | 309 | 24.7 | 88 | 20.2 | .9 (.6–1.2) i | 71 | 20.4 | .9 (.6–1.2)i | 13 | 14.4 | .5 (.3–.9) | 58 | 22.5 | .9 (.6–1.2) |
| ≥½ MCLG | 0 | 0.0 | 0 | 0.0 | NC | 0 | 0.0 | NC | 0 | 0.0 | NC | 0 | 0.0 | NC |
| Dichloroacetic acidc | ||||||||||||||
| No exposure | 763 | 60.9 | 275 | 63.2 | Reference | 218 | 62.6 | Reference | 59 | 65.6 | Reference | 159 | 61.6 | Reference |
| Exposed | 490 | 39.1 | 160 | 36.8 | .9 (.7–1.1) | 130 | 37.4 | .9 (.7–1.2) | 31 | 34.4 | .8 (.5–1.3) | 99 | 38.4 | 1.0 (.7–1.3) |
| Trichloroacetic acidg | ||||||||||||||
| No exposure | 772 | 61.6 | 281 | 64.6 | Reference | 228 | 63.8 | Reference | 59 | 65.6 | Reference | 163 | 63.2 | Reference |
| <½ MCLG | 349 | 27.9 | 113 | 26.0 | .9 (.7–1.1) | 98 | 28.2 | 1.0 (.7–1.3) | 25 | 27.8 | .9 (.6–1.5) | 73 | 28.3 | 1.0 (.7–1.3) |
| ≥½ MCLG | 132 | 10.5 | 41 | 9.4 | .9 (.6–1.2) | 28 | 8.1 | .7 (.5–1.1) | 6 | 6.7 | .6 (.3–1.4) | 22 | 8.5 | .8 (.5–1.3) |
| Bromoacetic acidh | ||||||||||||||
| No exposure | 1019 | 81.3 | 378 | 86.9 | Reference | 302 | 86.8 | Reference | 85 | 94.4 | Reference | 217 | 84.1 | Reference |
| Exposed | 234 | 18.7 | 57 | 13.1 | .7 (.5–.9) | 46 | 13.2 | .7 (.5–1.0)k | 5 | 5.6 | .3 (.1–.6) | 41 | 15.9 | 1.0 (.7–1.4)k |
| Dibromoacetic acidh | ||||||||||||||
| No exposure | 969 | 77.3 | 358 | 82.3 | Reference | 291 | 83.6 | Reference | 79 | 87.8 | Reference | 212 | 82.2 | Reference |
| Exposed | 284 | 22.7 | 77 | 17.7 | .7 (.6 – 1.0) | 57 | 16.4 | .7 (.5–.9) | 11 | 12.2 | .5 (.2–.9) | 46 | 17.8 | .7 (.5–1.0) |
- CI = confidence interval; HAAs = haloacetic acids; HAA5 = group of five most common haloacetic acids; MCL = maximum contaminant level; MCLG = maximum contaminant level goal; NC = not calculated; OFC = orofacial cleft; OR = odds ratio; THMs = trihalomethanes; TTHMs = total trihalomethanes.
- Because of rounding, percentages might not total 100.
- a Unweighted exposure estimation assuming any source change occurred after the critical exposure period.
- b ½ MCL for TTHMs is 40 µg/L.
- c MCLG for bromoform, bromodichloromethane, and dichloroacetic acid is 0 µg/L.
- d ½ MCLG for chloroform and monochloroacetic acid is 35 µg/L.
- e ½ MCLG for dibromochloromethane is 30 µg/L.
- f ½ MCL for HAA5 is 30 µg/L.
- g ½ MCLG for trichloroacetic acid is 10 µg/L.
- h No MCLG for bromoacetic acid or dibromoacetic acid.
- i Adjusted for study site.
- j Adjusted for maternal race/ethnicity.
- k Adjusted for family history of OFC.
Results of the subanalysis of associations of the OFC outcome groups with THM and HAA concentrations in public water systems are presented in Table 3. Similar to the analysis of THM and HAA exposures per liter of water consumed, there were no statistically significant, positive associations for any OFC outcome group with concentration of TTHMs, HAA5, or individual contaminants. We observed significant, inverse associations for all OFCs, isolated OFCs, and isolated CL/P with non-zero concentration of dibromoacetic acid and for CP with concentration of TTHMs at or above one-half the MCL of 40 µg/L, concentration of chloroform at or above one-half the MCLG of 35 µg/L, concentration of trichloroacetic acid at or above one-half the MCLG of 10 µg/L, and non-zero concentration of bromoacetic acid.
| Controls | All OFCs | Isolated OFCs | Isolated CP | Isolated CL/P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | N | % | N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) |
| TTHMsa | ||||||||||||||
| <½ MCL | 705 | 55.2 | 278 | 57.1 | Reference | 224 | 58.0 | Reference | 68 | 68.0 | Reference | 156 | 54.6 | Reference |
| ≥½ MCL | 573 | 44.8 | 209 | 42.9 | 1.0 (.8–1.3) h | 162 | 42.0 | 1.0 (.8–1.3)h | 32 | 32.0 | .6 (.4–.9) | 130 | 45.5 | 1.2 (.9–1.5)h |
| Individual THMs | ||||||||||||||
| Bromoform b | ||||||||||||||
| No exposure | 401 | 42.3 | 164 | 47.3 | Reference | 134 | 48.2 | Reference | 42 | 53.2 | Reference | 92 | 46.2 | Reference |
| Exposed | 548 | 57.7 | 183 | 52.7 | .6 (.4–1.0) h | 144 | 51.8 | .6 (.4–1.0)h,i | 37 | 46.8 | .6 (.4–1.0) | 107 | 53.8 | 0.7 (0.5–1.0)i |
| Chloroformc | ||||||||||||||
| <½ MCLG | 697 | 73.5 | 256 | 73.8 | Reference | 211 | 75.9 | Reference | 67 | 84.8 | Reference | 144 | 72.4 | Reference |
| ≥½ MCLG | 252 | 26.6 | 91 | 26.2 | 1.0 (0.7 – 1.3) | 67 | 24.1 | 0.9 (0.6 – 1.2) | 12 | 15.2 | 0.5 (0.3 – 0.9) | 55 | 27.6 | 1.1 (0.8 – 1.5) |
| Bromodichloromethaneb | ||||||||||||||
| No exposure | 25 | 2.6 | 14 | 4.0 | Reference | 10 | 3.6 | Reference | 4 | 5.1 | Reference | 6 | 3.0 | Reference |
| Exposed | 923 | 97.4 | 333 | 96.0 | 0.6 (0.3–1.3) | 268 | 96.4 | 0.8 (0.4–1.8)h | 75 | 94.9 | 0.5 (0.2–1.5) | 193 | 97.0 | 1.0 (0.4–2.4)i |
| Dibromochloromethaned | ||||||||||||||
| <½ MCLG | 938 | 99.0 | 343 | 98.9 | Reference | 276 | 99.3 | Reference | 79 | 100.0 | Reference | 197 | 99.0 | Reference |
| ≥½ MCLG | 10 | 1.1 | 4 | 1.2 | 1.1 (0.3–3.5) | 2 | 0.7 | NC | 0 | 0.0 | NC | 2 | 1.0 | NC |
| HAA5e | ||||||||||||||
| <½ MCL | 587 | 61.7 | 239 | 65.1 | Reference | 186 | 65.3 | Reference | 48 | 69.6 | Reference | 138 | 63.9 | Reference |
| ≥½ MCL | 365 | 38.3 | 128 | 34.9 | 1.0 (.8–1.3) h | 99 | 34.7 | 1.0 (.7–1.3)h | 21 | 30.4 | .7 (.4–1.2) | 78 | 36.1 | 1.1 (.8–1.5)h |
| Individual HAAs | ||||||||||||||
| Monochloroacetic acid c | ||||||||||||||
| <½ MCLG | 773 | 99.6 | 273 | 99.6 | Reference | 215 | 99.5 | Reference | 55 | 100.0 | Reference | 160 | 99.4 | Reference |
| ≥½ MCLG | 3 | .4 | 1 | .4 | NC | 1 | .5 | NC | 0 | 0.0 | NC | 1 | .6 | NC |
| Dichloroacetic acidb | ||||||||||||||
| No exposure | 34 | 4.4 | 13 | 4.7 | Reference | 7 | 3.2 | Reference | 2 | 3.6 | Reference | 5 | 3.1 | Reference |
| Exposed | 742 | 95.6 | 261 | 95.3 | .9 (.5–1.8) | 209 | 96.8 | 1.4 (.6–3.1) | 53 | 96.4 | NC | 156 | 96.9 | 1.7 (.6–4.4)i |
| Trichloroacetic acidf | ||||||||||||||
| <½ MCLG | 374 | 48.2 | 146 | 53.3 | Reference | 120 | 55.6 | Reference | 35 | 63.6 | Reference | 85 | 52.8 | Reference |
| ≥½ MCLG | 402 | 51.8 | 128 | 46.7 | .8 (.6–1.1) | 96 | 44.4 | .7 (.6–1.0) | 20 | 36.4 | .5 (.3–0.9) | 76 | 47.2 | 1.0 (.7–1.3)i |
| Bromoacetic acidg | ||||||||||||||
| No exposure | 436 | 56.2 | 170 | 62.0 | Reference | 131 | 60.7 | Reference | 41 | 74.6 | Reference | 90 | 55.9 | Reference |
| Exposed | 340 | 43.8 | 104 | 38.0 | .8 (.6–1.0) | 85 | 39.4 | .8 (.6–1.1) | 14 | 25.5 | .4 (.2–.8) | 71 | 44.1 | 1.3 (.8–2.0)h |
| Dibromoacetic acidg | ||||||||||||||
| No exposure | 329 | 42.4 | 136 | 49.6 | Reference | 110 | 50.9 | Reference | 31 | 56.4 | Reference | 79 | 49.1 | Reference |
| Exposed | 447 | 57.6 | 138 | 50.4 | .7 (.5–.9) i | 106 | 49.1 | .6 (.4–.8)i | 24 | 43.6 | .6 (.3–1.0) | 82 | 50.9 | .6 (.4–0.9)i |
- CI = confidence interval; HAAs = haloacetic acids; HAA5 = group of five most common haloacetic acids; MCL = maximum contaminant level; MCLG = maximum contaminant level goal; NC = not calculated; OFC = orofacial cleft; OR = odds ratio; THMs = trihalomethanes; TTHMs = total trihalomethanes.
- Because of rounding, percentages might not total 100.
- a ½ MCL for TTHMs is 40 µg/L.
- b MCLG for bromoform, bromodichloromethane, and dichloroacetic acid is 0 µg/L.
- c ½ MCLG for chloroform and monochloroacetic acid is 35 µg/L.
- d ½ MCLG for dibromochloromethane is 30 µg/L.
- e ½ MCL for HAA5 is 30 µg/L.
- f ½ MCLG for trichloroacetic acid is 10 µg/L.
- g No MCLG for bromoacetic acid or dibromoacetic acid.
- h Adjusted for study site.
- i Adjusted for maternal race/ethnicity.
To examine the potential for misclassification of maternal DBP exposure, Table 4 shows our results classifying maternal DBP exposure applying the conventional ecologic exposure assessment versus our more detailed, individual level exposure assessment. Overall, 1629 of 5478 (29.7%) mothers were eligible for both analyses of TTHM exposure, and 1216 (22.2%) mothers were eligible for both analyses of HAA5 exposure. Classification of TTHM exposure was the same in both analyses for 832 (51.1%) mothers, and classification of HAA5 exposure was the same in both analyses for 609 (50.1%) mothers.
| Concentration in Tap Water | ||||
|---|---|---|---|---|
| <½ MCL | ≥½ MCL | |||
| Exposurea per liter of water consumed | N | % | N | % |
| TTHMsb | ||||
| No exposure | 299 | 18.4 | 225 | 13.8 |
| <½ MCL | 603 | 37.0 | 273 | 16.8 |
| ≥½ MCL | 0 | 0.0 | 229 | 14.1 |
| HAA5c | ||||
| No exposure | 288 | 23.7 | 142 | 11.7 |
| <½ MCL | 471 | 38.7 | 177 | 14.6 |
| ≥½ MCL | 0 | 0.0 | 138 | 11.3 |
- HAA5 = group of five most common haloacetic acids; MCL = maximum contaminant level; TTHMs = total trihalomethanes.
- Because of rounding, percentages might not total 100.
- a Unweighted exposure estimation assuming any source change occurred after the critical exposure period.
- b ½ MCL for TTHMs is 40 µg/L.
- c ½ MCL for HAA5 is 30 µg/L.
4 DISCUSSION
To examine the relation between individual-level DBP exposure and OFCs, we linked maternal interview reports of water filtration and consumption during pregnancy from the NBDPS with public water system monitoring data. Compared to controls, no statistically significant associations were observed for all OFCs or isolated OFCs, CP, or CL/P with maternal exposure to TTHMs, HAA5, or individual THMs from drinking water during the critical exposure period, with most estimates near unity. No significant, positive associations were observed for any OFC outcome group with exposure to individual HAAs; significant, inverse associations were observed for all OFCs with bromoacetic acid, for isolated OFCs with dibromoacetic acid, and for CP with monochloroacetic acid, bromoacetic acid, and dibromoacetic acid.
Our study incorporated maternal individual-level water filtration and consumption information to estimate the relation between DBP exposure and OFCs; as such, the results of our analysis of DBP exposure per liter of water consumed could not be compared directly to previous studies. Conversely, the results of our subanalysis of DBP concentrations in public water systems did not rely upon maternal individual-level water filtration and consumption information, and therefore are more comparable to previous studies. Our findings of no statistically significant, positive associations between DBPs and OFCs were similar to those of most studies using ecological measures of DBP exposure (Dodds et al., 1999; Hwang et al., 2008; Nieuwenhuijsen et al., 2008; Righi et al., 2012; Shaw et al., 2003), as well as a meta-analysis examining chlorination and TTHM exposure (Nieuwenhuijsen et al., 2009). Similarly, our findings of no significant, positive associations between OFCs and chloroform were similar to previous studies that examined individual THMs (Dodds & King, 2001; Kaufman et al., 2018). Our findings of no significant, positive associations between HAAs and CL/P and a significant, inverse association between dibromoacetic acid and CP parallel associations reported from a previous Massachusetts study, although the inverse associations we observed between HAAs and CP generally contrast the Massachusetts study's positive or near-unity associations for monochloroacetic acid, dichloroacetic acid, and trichloroacetic acid with CP (Kaufman et al., 2018). Some previous animal studies have reported no teratogenic effects following maternal administration of several DBPs (reviewed in Graves et al., 2001); one animal study reported a significant increase in CP in offspring of mice exposed to ≥100 ppm inhaled chloroform, although the effect was observed at doses that likely exceed those seen in humans (Murray et al., 1979).
A strength of our study was the use of data from the NBDPS, a large, population-based case control study. Review of medical record data by clinical geneticists reduced the potential for case misclassification and allowed for the examination of individual OFC subtypes. The NBDPS interview collected information on use of water from a private well or public water system, which allowed improved specificity in assigning DBP concentrations to public water users only. Furthermore, the NBDPS interview also collected detailed information on individual water consumption at home, work, and school, as well as water filtration at home for the critical exposure period. This information helped to reduce potential exposure misclassification (Whitaker, Nieuwenhuijsen, & Best, 2003) that may occur from use of residence location as a proxy for DBP exposure, as used in previous studies (Bove et al., 1995; Dodds et al., 1999; Dodds & King, 2001; Hwang et al., 2008; Nieuwenhuijsen et al., 2008; Righi et al., 2012; Shaw et al., 2003). Mothers also reported information on shower and bathing habits, which may influence OFC risk (Agopian et al., 2013); heating of water may volatilize DBPs, leading to inhalation and dermal exposures. Our study was also able to examine individual THMs and HAAs; the metabolism and toxicity of individual DBPs may vary and use of TTHMs or HAA5 as a proxy measure for individual DBPs may mask the effects of individual DBPs. Furthermore, potential temporal and spatial fluctuations of DBPs were addressed in the estimation of DBP exposure for each mother.
Although our study had numerous strengths and improved upon the methods used in previous studies, several limitations remained. The primary limitation of our study was the proportion of mothers ineligible for any exposure analyses due to our inability to link these mothers to their corresponding DBP values from their water systems. Even with successful linkage, the use of maternal retrospective self-reports created the potential for imprecision in recall of water consumption during pregnancy. Related to this, some estimates observed were based on small numbers of exposed mothers and were imprecise. Also, although mothers could report changes in water consumption amount at home during pregnancy, the distribution of the change by water source could not be reported. To attempt to address this limitation, we estimated changes to the source distribution of home drinking water consumption when multiple sources were reported using unweighted and weighted approaches; the results of analyses using these approaches were not substantively different. Another limitation was that mothers could report changes in water sources at home during pregnancy, but the timing of a change in source was not requested. We attempted to address this limitation by estimating DBP exposure using two assumptions. One assumption was that changes in source occurred before the critical exposure period, and the other was that such changes occurred after the critical exposure period; results did not differ substantively between these assumptions. Also, mothers reported the source of water for hot drinks, but corresponding consumption estimates were not requested, potentially leading to exposure misclassification. Additionally, we only were able to estimate associations for the individual THMs and HAAs regulated by the U.S. EPA; unmeasured DBPs may present different risks than those measured. Although participant mothers of controls in the NBDPS were previously found to be statistically similar for several characteristics to mothers of all live births in the same geographic areas (Cogswell et al., 2009), we observed that the eligible mothers in our analyses were not representative of NBDPS mothers of OFC cases and controls for some characteristics and exposures; however, only one characteristic (study site) was retained in any of our adjusted models. Lastly, we did not control for multiple comparisons; thus, findings observed may have been due to chance.
Using maternal reports of water filtration and consumption data from the NBDPS, we observed associations near or below unity for all OFCs, isolated OFCs, CL/P, and CP with TTHMs, HAA5, and individual THMs and HAAs. No statistically significant, positive associations were observed for any outcome groups with any exposure groups; however, statistically significant, inverse associations were observed for all OFCs with bromoacetic acid, isolated OFCs with dibromoacetic acid, and CP with monochloroacetic acid, bromoacetic acid, and dibromoacetic acid. Compared to our subanalysis that did not account for filtration and consumption, the exposure classifications using information on filtration and consumption for TTHMs and HAA5 were discrepant for nearly one-half of mothers, reducing potential overestimation of these exposures. Continued, improved research using maternal individual-level exposure data could be impactful in better characterizing the relation between DBPs and OFCs.
ACKNOWLEDGMENTS
We thank the study participants and study staff at each site who contributed to the NBDPS. This work was supported by funding from the Centers for Disease Control and Prevention (U01DD001035). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The views expressed in this manuscript are those of the the authors and do not necessarily represent views or policies of the U.S. Environmental Protection Agency.
CLINICAL TRIAL REGISTRATION
N/A
CONFLICT OF INTEREST
None.




