Atypical structural connectivity of language networks in autism spectrum disorder: A meta-analysis of diffusion tensor imaging studies
Abstract
Patients with autism spectrum disorder (ASD) often show pervasive and complex language impairments that are closely associated with aberrant structural connectivity of language networks. However, the characteristics of white matter connectivity in ASD have remained inconclusive in previous diffusion tensor imaging (DTI) studies. The current meta-analysis aimed to comprehensively elucidate the abnormality in language-related white matter connectivity in individuals with ASD. We searched PubMed, Web of Science, Scopus, and Medline databases to identify relevant studies. The standardized mean difference was calculated to measure the pooled difference in DTI metrics in each tract between the ASD and typically developing (TD) groups. The moderating effects of age, sex, language ability, and symptom severity were investigated using subgroup and meta-regression analysis. Thirty-three DTI studies involving 831 individuals with ASD and 836 TD controls were included in the meta-analysis. ASD subjects showed significantly lower fractional anisotropy or higher mean diffusivity across language-associated tracts than TD controls. These abnormalities tended to be more prominent in the left language networks than in the right. In addition, children with ASD exhibit more pronounced and pervasive disturbances in white matter connectivity than adults. These results support the under-connectivity hypothesis and demonstrate the widespread abnormal microstructure of language-related tracts in patients with ASD. Otherwise, white matter abnormalities in the autistic brain could vary depending on the developmental stage and hemisphere.
Lay Summary
This meta-analysis explored abnormalities in white matter connectivity in language networks of individuals with ASD. Significantly reduced white matter integrity was found in all language-associated tracts in subjects with ASD compared with TD controls. In addition, structural disturbances of language networks in the autistic brain exhibit a leftward tendency, and more prominent abnormalities are observed in younger people with ASD than in adults.
INTRODUCTION
Autism spectrum disorder (ASD) is a heterogeneous developmental disorder characterized by impairments in social interactions, communicative ability, and restricted interest/repetitive behaviors (American Psychiatric Association, 2013). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5, language impairments are not essential for the diagnosis of ASD. Nevertheless, language impairments are still considered a common symptom in individuals with ASD. Indeed, approximately 63% of children with ASD present with co-occurring language dysfunctions (Levy et al., 2010). Additionally, ~30% of children with ASD remain minimally verbal or nonverbal until school age (Norrelgen et al., 2015; Tager-Flusberg & Kasari, 2013). Indeed, delay in language expression is often the parent's earliest concern (Gladfelter & Goffman, 2018; Pierce et al., 2019). Many clinical studies have also indicated that language impairments as standard and apparent features of ASD play a critical role in the detection, diagnosis, assessment, and intervention of ASD (Hudry et al., 2010; Mody & Belliveau, 2013). The manifestations of language impairment in ASD are complex and vary considerably. Language deficits in ASD exist at various language processing levels, such as phonological, semantic, syntactic, and pragmatic processing (Ehlen et al., 2020; Groen et al., 2008). The abnormal language profiles in ASD also present remarkable individual variety and distinct subgroups (Rapin et al., 2009; Tager-Flusberg, 2014). Therefore, elucidating the neurocognitive mechanisms underlying these symptoms is essential for understanding language deficits in ASD.
Accumulating evidence suggests that abnormal connectivity of brain structures plays a vital role in the clinical symptoms of ASD (Libero & Kana, 2013; Mohammad-Rezazadeh et al., 2016). Studies have also indicated that under-connectivity in language networks is critically related to linguistic dysfunction in ASD (Groen et al., 2008; Mody et al., 2013). The structural connectivity of language networks can be divided into dorsal and ventral pathways according to the orientation of language-related white matter tracts (Friederici, 2009; Hickok & Poeppel, 2007). The dorsal route, including arcuate fasciculus and superior longitudinal fasciculus (AF/SLF), is associated with the temporal and frontal cortex and supports the production processes from sound to speech (Giampiccolo & Duffau, 2022). The ventral pathway mainly consists of the uncinate fasciculus (UF), inferior longitudinal fasciculus (ILF), and inferior frontal-occipital fasciculus (IFOF), which connects the occipital, temporal, and frontal areas and is considered to be involved in language comprehension and meaning processing (Friederici, 2020; Friederici & Gierhan, 2013).
Diffusion tensor imaging (DTI) has been widely used to investigate the abnormal connectivity of language-related tracts in ASD. It can verify neuroanatomical characteristics by measuring the diffusion of water molecules in the brain tissue and detecting the orientation and integrity of white matter tracts based on quantitative metrics, including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD).
Although extensive DTI studies have been conducted, the findings of the white matter characteristics in ASD across these studies remain inconclusive and even contradictory. Several studies have indicated that individuals with ASD present a significant FA reduction in the AF, SLF, UF, IFOF, or ILF compared with typically developing (TD) controls (Naigles et al., 2017; Shukla et al., 2011; Zhang et al., 2018). Furthermore, many studies have reported increased MD in language-related tracts of subjects with ASD (Billeci et al., 2012; Fletcher et al., 2010). Some studies demonstrated no significant difference in FA or MD in these tracts (such as AF, SLF, and UF) between the ASD and TD groups (Karahanoğlu et al., 2018; Liu et al., 2019). Several studies have found increased FA or reduced MD in the AF, IFOF, and SLF in the ASD group (Fitzgerald et al., 2018; Wolff et al., 2012). These inconsistent findings are likely related to variations in the characteristics of participants (e.g., sex, age, cognitive ability, symptom severity) and methodological approaches (e.g., data acquisition, analytic pipeline), as well as anatomical factors (e.g., hemisphere) among individual studies. A recent scoping review (Cermak et al., 2022) indicated that inconsistent findings on the association between white matter microstructure and language profiles in individuals still need to be further elucidated.
Given the inconsistency and heterogeneity across previous studies, it is necessary to conduct a meta-analysis of DTI studies specific to the atypical structural connectivity of language networks in ASD. The primary aim of this meta-analysis was to elucidate whether the structural connectivity of language networks in individuals with ASD was altered compared with TD controls. The secondary objective was to examine how demographic features (e.g., age, sex, language ability, and symptom severity) contribute to the heterogeneity of the anatomical characteristics using subgroup analysis and meta-regression. We expect that our meta-analysis will provide more comprehensive and robust information for further language development studies and speech therapy in ASD.
METHODS
This systematic review and meta-analysis was conducted based on a predefined protocol registered in the PROSPERO database (registration number: CRD42021282765; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=282765) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021).
Literature search
We performed a comprehensive literature search of four electronic databases (PubMed, Web of Science, Scopus, and Medline) to identify relevant studies. We developed search strategies, consisting of a combination of the words in titles/abstracts and MeSH terms based on the P (Participants)—E (Exposure)—S (Study design) pattern. The MeSH term for “P” was “autism,” “autism spectrum disorder,” or “asperger syndrome”; The MeSH term for “E” was “diffusion tensor imaging” or “diffusion MRI”; The MeSH term for “S” was “cohort studies” or “case–control studies.” The time restrictions of publications were limited to between January 2001 and November 2021. However, we did not apply language restrictions. Details of the search strategy are provided in Data S1. In addition, we manually conducted citation searches of recent review articles and reference lists of the included studies.
Study selection
The first and second stages of study screening, based on the titles and abstracts, were conducted using Rayyan (https://www.rayyan.ai). Subsequently, we reviewed the full text of all potential articles against the inclusion and exclusion criteria based on the PECOS strategy. Eligible studies met the following conditions: (a) Participants: enrolled individuals with ASD or AS, who were diagnosed by DSM, International Code of Diseases (ICD), Autism Diagnostic Observation Schedule (ADOS), or Autism Diagnostic Interview-Revised (ADI-R); (b) Exposure: DTI was used to investigate the features of language-related white matter tracts (AF, SLF, UF, IFOF, or ILF), which utilized tract-specific or atlas-based analyses. This is because tract-specific and atlas-based methods are more precise for localizing the structural connections of the white matter tracts; (c) Comparison: compared with the age-matched TD control group without developmental disorders or mental illness; (d) Outcome: sufficient data on FA or MD values in language-related tracts were reported. (e) Study design: observational studies were eligible for this review.
Studies were excluded for the following reasons: (a) Participants were below 3 years old as neural system development is still unstable before 3 years old; (b) did not refer to language-related tracts; (c) studies using voxel-based or TBSS methods; (d) the comparison group did not include a TD population; (e) did not provide available raw data; (f) not an observational or original study. Two assessors (LM and WY) independently conducted the study selection, and any disagreements were resolved through a consensus process.
Data extraction and quality assessment
We extracted basic and participants' information as follows: first author, publication year, country, participants' features (e.g., sample size, mean age, sex ratio, IQ scores), and diagnostic/assessment tools. In addition, we coded the methodological characteristics, including the parameters of imaging acquisition (e.g., Tesla, TE/TR, FOV, number of directions, b value), data processing methods, measures of head motion, involved tracts, and DTI metrics. For the meta-analysis, we extracted the mean FA/MD values and their standard deviations (SD) in relevant language tracts for the ASD and TD subjects, respectively. When studies reported multiple sub-region outcomes of a single white matter tract (e.g., SLF including three sub-regions: SLF1, SLF2, and SLF3), we computed the mean of outcomes for each sub-region, and used these integrated results as the data of pooled analysis.
The included studies were evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS). NOS is a quality tool that consists of nine evaluation items divided into three domains (selection, comparability, and exposure). Besides, considering the significant impact of head motion on the quality of DTI data, we added “Measures of Head Motion” as an additional evaluation item in the quality assessment. Studies can be defined as of adequate quality when at least six items are satisfied. Data extraction and quality assessment were independently performed by two reviewers (LM and WY).
Meta-analysis
The standardized mean difference (SMD) with 95% confidence intervals (95% CI) was calculated based on Cohen's d and used as the effect size. A positive SMD indicates that the ASD group had higher outcomes than the TD controls and vice versa. The effect size benchmarks were 0.2, 0.5, and 0.8, which represent small, medium, and large effects, respectively (Cohen, 1992). In addition, the statistical significance of the effect size was set at a p-value of <0.05. We used a meta-analysis to estimate the group differences in FA/MD metrics in each tract between the ASD and TD groups. Due to the diversity of methodology among the included studies, we used the recommended random-effects model (DerSimonian-Laird method) for the estimation of pooled effect size, which is more flexible to address heterogeneity between studies (Borenstein et al., 2009; DerSimonian & Laird, 1986). The random-effects model assumes that the true effect sizes of included studies vary from study to study and follow a distribution. In addition, study weights are more balanced under the random-effects model than under the fixed-effect model (Borenstein et al., 2009). The fixed-effect model, on the other hand, does not address between-study heterogeneity. Considering the discrepancies in DTI studies, we excluded the conspicuous outlier studies that were beyond two SD from the mean effect size. In addition, we used the Galbraith plot to investigate the contribution of each study to the overall heterogeneity. Moreover, we combined the AF and SLF into the analysis previous studies (Friederici & Gierhan, 2013). Furthermore, we independently performed meta-analyses for the right and left hemispheres in each tract.
We estimated I2 statistics to examine between-study heterogeneity, representing the percentage of variance in the pooled effect size that contributes to heterogeneity (Higgins & Thompson, 2002). The I2 can be classified as low (25%–50%), moderate (50%–75%), and high (75%–100%) (Higgins et al., 2003).
Publication bias was evaluated using funnel plots and Egger's regression test (Egger et al., 1997). The asymmetry of the funnel plots and the significance level of Egger's test (p < 0.05) indicate the presence of publication bias. The trim-and-fill method (Duval & Tweedie, 2000) was applied if a publication bias was found.
To assess the robustness of the meta-analysis results, we performed a sensitivity analysis by sequentially excluding each study and subsequently re-conducting the meta-analysis for the remaining studies. If a significant alteration appears in the leave-one-out sensitivity analysis, then the pooled estimate lacks stability.
In addition, we conducted subgroup and meta-regression analyses to investigate the moderating effects of subjects' variables. In the subgroup analysis, the included studies were assigned to the child group (age range: 3–18 years) and the adult group (age range: >18 years), according to the mean age of participants. We investigated the moderating effects of age range according to the pooled effect size and p-value in each subgroup. The minimum number of studies in the subgroup analysis was three. In addition, we performed a meta-regression using the residual maximum likelihood model to examine the moderating effects of verbal IQ, sex ratio, and symptom severity in ASD. A minimum of five studies were included in each meta-regression analysis. Statistical efficacy (p-value <0.01) was used to identify the moderating effect, given the limited number of studies included in the meta-regression. Statistical analysis was performed using Stata version 16.0 (StataCrop, LLC).
RESULTS
Study selection
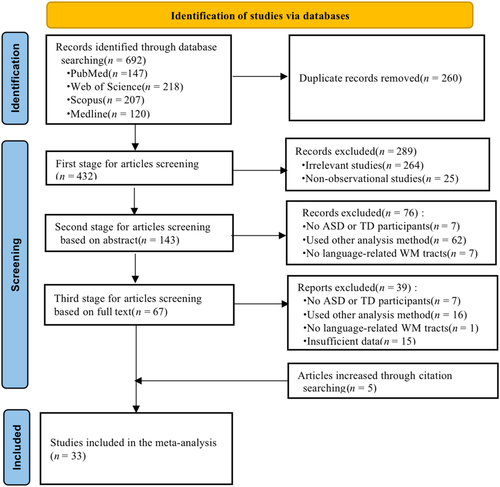
Figure 1 shows the procedure for study selection. The database search yielded 692 studies, of which 432 remained after removing duplicate studies. After screening titles and abstracts, 67 articles remained and underwent full-text review. Of these, seven studies were excluded because they did not involve both ASD and TD groups, 16 studies did not meet the inclusion criteria of the analytic method, and one study did not involve language-related tracts. Of the remaining 43 candidates, 15 studies were excluded because of insufficient data, as they did not report detailed FA or MD values. Additionally, five studies were identified through a citation search. Finally, 33 studies involving 1667 participants (N_ASD = 831, N_TD = 836) were included in our meta-analysis.
Study characteristics
The characteristics of the included studies are summarized in Table 1. More than 80% of the included studies were from the United States or Europe. The mean age of the ASD subjects ranged from 5.0 to 32.9 years old. Twenty-four studies investigated the child group, and nine focused on the adult group. Regarding the sex ratio, 13 studies examined only male subjects, and the percentage of females was also small in the remaining studies. Among the 33 included studies, 31 examined high-functioning autism (IQ > 70), and 21 studies precisely matched the IQ of the ASD and TD groups.
| Study | Country | Number of subjects | Demographic characters of ASD | Diagnosis and assessment | |||||
|---|---|---|---|---|---|---|---|---|---|
| Study design | Sex ratio (% male) | Mean age (SD) | FSIQ/VIQ (SD) | Diagnosis type | Diagnostic criteria | IQ/VIQ assessment | |||
| Andica et al. (2021) | Japan | Cross-sectional | 26 ASD 25 TD | 73.08 | 32.93 (9.24) | >80 | HFA | DSM-IV; AQ | NR |
| Kato et al. (2019) | Japan | Cross-sectional | 17 ASD 18 TD | 100 | 12.0 (1.5) | 105.12(13.24) | HFA | DSM-5, ADOS-G | WISC-IV; DN-CAS |
| Lei et al. (2019) | UK | Cross-sectional | 81 ASD 39 TD | 69.14 | M:9.63 (4.02) F:9.30 (4.25) | M:97.34 (21.00) F: 97.20 (21.34) | HFA | DSM-5, ADI-R, ADOS; SRS-2 | WAIS |
| Boets et al. (2018) | Belgium | Cross-sectional | 17 ASD 17 TD | 100 | 13.8 (1.3) | 105 (14) | HFA | DSM-IV; SRS | NR |
| Fitzgerald et al. (2018) | Ireland | Cross-sectional | 45 ASD 45 TD | 100 | 16.55 (3.04) | 109.5 (15.9) | HFA | ADI-R, ADOS-G | NR |
| Zeestraten et al. (2017) | UK | Cross-sectional | 98 ASD 115 TD | 62.24 | M:26.0 (7.0) F:25.4 (6.1) | M:115.3 (12.6) F:113.7 (15.0) | AS | ICD-10, ADI-R, ADOS-Module 4 | WAIS |
| Catani et al. (2016) | UK | Cross-sectional | 61 ASD 61 TD | 100 | 26 (6.9) | 111 (13) | HFA, AS | ICD-10, DSM-IV, ADI-R, ADOS; AQ | WAIS |
| Libero et al. (2016) | USA | Cross-sectional | 42 ASD 44 TD | 85.71 | 19.9 (1.27) | 112.9 (1.99) | HFA | ADI-R, ADOS-G; AQ; RAADS-R | WAIS |
| Moseley et al. (2016) | UK | Cross-sectional | 18 ASD 14 TD | 83.3 | 30.39 (9.99) | 112.72 (22.56) | HFA, AS | DSM-IV; AQ | NR |
| Samson et al. (2016) | Switzerland | Cross-sectional | 18 ASD 18 TD | 88.9 | 13.06 (3.57) | 104 (18.25) | HFA | DSM-IV, ADI-R, ADOS; SRS | Stanford Binet-5 |
| Lu et al. (2016) | USA | Cross-sectional | 25 ASD 20 TD | 68 | 11.3 (3.48) | 108.9 (15.28) | HFA | ADOS; | CELF-4; KBIT-2 |
| Roine et al. (2015) | Finland | Cross-sectional | 14 ASD 19 TD | 100 | 28.6 (5.7) | 125.1 (14.5) | AS | ICD-10; AQ | WASI-III |
| Joseph et al. (2014) | USA | Cross-sectional | 20 ASD 20 TD | 90 | 5.91 (1.25) | 96 (23) | HFA | ADI-R, ADOS | CELF-III; KBIT-2; DAS; OWLS |
| Roberts et al. (2014) | USA | Cross-sectional | 18 ASD 25 TD | 88.9 | 11.47 (3.25) | >75 | HFA | ADOS; SRS | WISC-IV, CELF-4 |
| Sharda et al. (2015) | India | Cross-sectional | 22 ASD 22 TD | 72.7 | 11.0 (3.4) | 83.14 (17.8) | ASD | DSM-IV, ADOS-G | WASI |
| Verly et al. (2014) | Belgium | Cross-sectional | 17 ASD 25 TD | 82.3 | 13.95 (1.34) | 88.24 (19.20) | HFA | DSM-IV; SRS | WISC-III, CELF-4, PPVT |
| McGrath et al. (2013) | Ireland | Cross-sectional | 25 ASD 25 TD | 100 | 17.28 (2.87) | 106.84 (14.54) | HFA | ADOS-G, ADI-R | WISC–IV, WAIS-III |
| Mills et al. (2013) | USA | Cross-sectional | 10 ASD 17 TD | 80 | 9.2 (1.8) | 94.2 (16) | HFA | DSM-IV, ADI-R, ADOS | WISC–IV, CELF-4 |
| Peeva et al. (2013) | USA | Cross-sectional | 18 ASD 18 TD | 83.3 | 25.6 (9.2) | 112.4 (9.7) | HFA | DSM-IV, ADI-R, ADOS-Module 4 | American National Adult Reading Test |
| Billeci et al. (2012) | Italy | Cross-sectional | 22 ASD 10 TD | NR | 5.54 (2.03) | 70.5 (23.31) | ASD | DSM-IV, ADOS-G; CARS | WPPSI, WISC-R |
| Lai et al. (2012) | USA | Cross-sectional | 16 ASD 18 TD | 87.5 | 11.02 (3.72) | <80 | ASD | DSM-IV, ADI-R | Clinical observation of words uttered |
| Nagae et al. (2012) | USA | Cross-sectional | 18 ASD 25 TD | NR | 11.3 (6.7–17.5) | 108 (10) | HFA | ADOS; SRS | WISC-IV, CELF-4 |
| Poustka et al. (2012) | Germany | Cross-sectional | 18 ASD 18 TD | 88.9 | 9.7 (2.1) | 111.0 (14.4) | HFA | ICD-10, ADI-R, ADOS | Raven's Colored Progressive Matrices Test |
| Verhoeven et al. (2012) | Belgium | Cross-sectional | 19 ASD 21 TD | 84.2 | 13.8 (1.6) | 90.5 (18.7) | HFA | DSM-IV; SRS | WISC-III, CELF-4 |
| Ameis et al. (2011) | Canada | Cross-sectional | 19 ASD 16 TD | 84.21 | 12.4 (3.1) | 98.5 (20.4) | HFA | DSM-IV, ADI-R, ADOS | WISC-IV |
| Cheon et al. (2011) | Korea | Cross-sectional | 17 ASD 17 TD | 100 | 11.0 (2.1) | 112.1 (12.0) | HFA | DSM-IV, ADI-R-K, ADOS-K; SRS | WISC-R-III |
| Jou et al. (2011) | USA | Cross-sectional | 10 ASD 10 TD | 100 | 13.06 (3.85) | 91.0 (24.79) | HFA | DSM-IV, ADI-R, ADOS | WISC-III |
| Thomas et al. (2011) | USA | Cross-sectional | 12 ASD 18 TD | 100 | 28.5 (9.7) | 106.92 (10.47) | HFA | DSM-IV, ADI-R, ADOS-G | WAIS-III |
| Fletcher et al. (2010) | USA | Cross-sectional | 10 ASD 10 TD | 100 | 14.25 (1.92) | 103.7 (18.55) | HFA | ICD-10, ADI-R, ADOS-G | WISC, CELF-III |
| Knaus et al. (2010) | USA | Cross-sectional | 14 ASD 20 TD | 100 | 16.09 (2.3) | 103.29 (NR) | HFA | DSM-IV, ADI-R, ADOS | CELF-III; KBIT-2 |
| Kumar et al. (2010) | USA | Cross-sectional | 32 ASD 16 TD | 90.6 | 5.0 (2.5–8.9) | >80 | HFA | DSM-IV; CARS-2 | WPPSI-III, WISC-IV |
| Brito et al. (2009) | Brazil | Cross-sectional | 8 ASD 8 TD | 100 | 9.53 (1.83) | NR | ASD | DSM-IV | NR |
| Pugliese et al. (2009) | UK | Cross-sectional | 24 ASD 42 TD | 100 | 23.3 (12.4) | 104.7 (12.05) | AS | ICD-10, ADI-R, ADOS | WAIS |
- Abbreviations: ADI-R, autism diagnostic interview-revised; ADOS, autism diagnostic observation schedule; ADOS-G, autism diagnostic observation schedule-generic; AQ, autism-spectrum quotient; AS, Asperger syndrome; ASD, autism spectrum disorder; CARS, Childhood Autism Rating Scale; CELF, clinical evaluation of language fundamentals; DAS, Differential Ability Scales; DN-CAS, Das-Naglieri cognitive assessment system; DSM, diagnostic and statistical manual of mental disorder; F, female; FSIQ, full-scale IQ; HFA, high-functioning autism; ICD-10, international statistical classification of diseases; KBIT, Kaufman brief intelligence tests; M, male; NR, not reported; OWLS, Oral and Written Language Scales; PPVT, Peabody Picture Vocabulary Test; RAADS-R, Ritvo Autism-Asperger Diagnostic Scale-Revised; SD, standard deviation; SRS, Social Responsiveness Scale; TD, typically developing individuals; VIQ, verbal IQ; WASI, Wechsler Abbreviated Scale of Intelligence; WISC, Wechsler Intelligence Scale for Children; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
The methodological characteristics of the included studies were significantly heterogeneous (Table 2). For data acquisition, a 3 T scanner (25 studies) was utilized more widely than a 1.5 T scanner; the number of directions ranged from six to 72, and the b value varied between 600 and 2000. Regarding data processing, six studies applied atlas-based analysis, 16 adopted deterministic tractography, and eight employed probabilistic tractography. The remaining studies used more advanced tractography, such as automated fiber quantification (AFQ) and constrained spherical deconvolution (CSD)-based tractography. All studies have accounted for head motion in the procedure of image acquisition or data processing. Most studies corrected eddy currents and head motion with a software package (such as FSL or Explore-DTI) in the data pre-processing. Besides, a few studies disposed of the artifacts and head motion in the image acquisition (Kumar et al., 2010; Samson et al., 2016; Thomas et al., 2011). For the ROI tracts, 29 articles investigated the dorsal pathway (AF/SLF) (such as Andica et al., 2021; Kato et al., 2019; Lei et al., 2019), and 17, 13, and 17 studies involved UF, IFOF, and ILF, respectively. For DTI metrics, FA and MD were reported more generally than other parameters (e.g., AD or RD) in the included studies. The results of the quality assessment are presented in Table S1.
| Study | Image acquisition | Analysis method | ROI tract | DTI metrics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tesla | TE (ms) | TR (ms) | FOV (mm2) | No. of directions | b value (s/mm) | Software | Type of analysis | Measures of head motions | AF | SLF | ILF | IFOF | UF | FA | MD | AD | RD | Vol | Nb | LI | |
| Andica et al. (2021) | 3 | 100 | 9810 | 256*256 | 32 | 2000 | FSL | Atlas-based | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | × | ○ | × | × | × |
| Kato et al. (2019) | 3 | 74.3 | 12,000 | 260*260 | 25 | 1000 | Explore-DTI | Deter | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | × | × | × |
| Lei et al. (2019) | 3 | 85 | 6200 | 240*240 | 30 | NR | FSL | Atlas-based | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | × | × | × |
| Boets et al. (2018) | 3 | 65 | 7600 | 240*200 | 60 | 1300 | TRACULA | Prob | ○ | × | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | × | × | × |
| Fitzgerald et al. (2018) | 3 | 79 | 20,122 | 248*248 | 61 | 1500 | Explore-DTI | Deter | ○ | × | ○ | × | × | × | ○ | × | × | × | × | × | ○ |
| Zeestraten et al. (2017) | 3 | 104.5 | 20R-R intervals | 307*307 | 32 | 1300 | Explore-DTI/FSL | Deter | ○ | ○ | × | ○ | ○ | ○ | ○ | × | × | × | × | × | × |
| Catani et al. (2016) | 3 | 104.5 | 20R-R intervals | 307*307 | 32 | 1300 | Explore-DTI | Deter | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | × | ○ | × | ○ | × |
| Libero et al. (2016) | 3 | 90 | 7000 | 220*220 | 46 | 1000 | mrDiffusion Package | Deter (AFQ) | ○ | × | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | × | × | × |
| Moseley et al. (2016) | 3 | 90 | 7800 | 192*192 | 64 | 1000 | FSL | Prob | ○ | ○ | × | × | × | × | ○ | ○ | × | × | ○ | × | × |
| Samson et al. (2016) | 3 | NR | NR | NR | 30 | 1200 | AFQ | Deter | ○ | × | × | × | × | ○ | ○ | × | × | × | × | × | × |
| Lu et al. (2016) | NR | 84 | 9300 | 256*256 | 30 | 700 | TRACULA | Automatic Prob | ○ | ○ | × | ○ | × | × | ○ | ○ | ○ | ○ | × | × | × |
| Roine et al. (2015) | 3 | 98 | 10,000 | 240*240 | 60 | 1000 | Explore-DTI | CSD-based | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | × | × | × | × | ○ |
| Joseph et al. (2014) | 3 | 91 | 10,646 | 230*230 | 15 | 1000 | FSL | Prob | ○ | ○ | × | × | × | × | ○ | ○ | ○ | ○ | × | × | ○ |
| Roberts et al. (2014) | 3 | 70 | 14,000 | 256*256 | 30 | 1000 | DTIStudio | Deter | ○ | ○ | × | × | × | × | ○ | ○ | ○ | ○ | × | × | × |
| Sharda et al. (2015) | 3 | 45 | 8000 | 240*240 | 16 | 1000 | FSL | Atlas-based | ○ | ○ | ○ | ○ | × | ○ | ○ | × | × | × | × | × | ○ |
| Verly et al. (2014) | 3 | 55 | 11,043 | 220*220 | 45 | 800 | Explore-DTI | Deter | ○ | × | ○ | × | × | × | ○ | × | × | × | × | ○ | × |
| McGrath et al. (2013) | 3 | 79 | 20,122 | 128*128 | 61 | 1500 | Explore-DTI | CSD-based | ○ | ○ | × | × | ○ | × | ○ | × | × | × | × | × | × |
| Mills et al. (2013) | 1.5 | NR | NR | 240*240 | 51 | 600/800/1000 | DTIStudio | Deter | ○ | ○ | ○ | ○ | ○ | ○ | ○ | × | × | × | × | × | × |
| Billeci et al. (2012) | 1.5 | 107 | 11,000 | 190*190 | 25 | 1000 | FSL | Deter | ○ | ○ | × | × | × | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Lai et al. (2012) | 1.5 | 81.9 | 8500 | NR | 25 | 1000 | FSL | Prob | ○ | ○ | × | × | × | × | ○ | × | × | × | × | × | × |
| Peeva et al. (2013) | 3 | 82 | 8400 | NR | 72 | 700 | FSL | Prob | ○ | ○ | × | × | × | × | ○ | × | × | × | ○ | ○ | × |
| Negae et al. (2012) | 3 | 70 | 14,000 | 256*256 | 30 | 1000 | DTIStudio | Deter | ○ | × | ○ | × | × | × | ○ | ○ | × | × | × | × | × |
| Poustka et al. (2012) | 1.5 | 78 | 4700 | 192*192 | 6 | 1000 | NeuroQLab | Deter | ○ | × | ○ | × | × | ○ | ○ | × | × | × | × | × | × |
| Verhoeven et al. (2012) | 3 | 55 | 11,043 | 220*220 | 45 | 800 | Explore-DTI | Deter | ○ | × | ○ | × | × | × | ○ | × | × | × | × | × | × |
| Ameis et al. (2011) | 3 | 80 | 4100 | 210*210 | 12 | 1250 | FSL | Atlas-based | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | × | × | × |
| Cheon et al. (2011) | 1.5 | 86 | 6500 | 230*230 | 30 | 900 | FSL | Atlas-based | ○ | × | × | ○ | × | ○ | ○ | ○ | ○ | ○ | × | × | × |
| Jou et al. (2011) | 1.5 | 92 | 11,200 | 256*256 | 6 | 1000 | BioImage | Deter | ○ | × | ○ | ○ | ○ | × | ○ | × | × | × | ○ | × | × |
| Thomas et al. (2011) | 3 | 82 | 4900 | 210*210 | 12 | 850 | DTIStudio | Deter | ○ | × | × | ○ | ○ | ○ | ○ | × | × | × | ○ | ○ | ○ |
| Fletcher et al. (2010) | 3 | 84 | 7000 | 256*256 | 12 | 1000 | ITK-SNAP | Prob | ○ | ○ | × | × | × | × | ○ | ○ | ○ | ○ | ○ | × | ○ |
| Knaus et al. (2010) | 3 | 73 | NR | 230*230 | 15 | 1000 | FSL | Prob | ○ | ○ | × | × | × | × | ○ | × | × | × | × | × | × |
| Kumar et al. (2010) | 3 | 79 | NR | 240*240 | 6 | 1000 | DTIStudio | Deter | ○ | ○ | × | × | ○ | ○ | ○ | × | × | × | ○ | × | × |
| Brito et al. (2009) | 1.5 | 90 | 3100 | 250*250 | 12 | 1000 | DTI Task Card | Atlas-based | ○ | × | ○ | ○ | × | × | ○ | ○ | × | × | × | × | × |
| Pugliese et al. (2009) | 3 | 107 | 15R-R intervals | 240*240 | NR | 1300 | NR | Deter | ○ | × | × | ○ | ○ | ○ | ○ | ○ | × | × | × | ○ | × |
- Abbreviations: ×, no referred in individual studies; ○, referred in individual studies; AD, axial diffusivity; AF, arcuate fasciculus; AFQ, automated fiber quantification; CSD-based, constrained spherical deconvolution-based tractography; Deter, deterministic tractography; FA, fractional anisotropy; FOV, field of view; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; L-, left hemisphere; LI, laterality index; MD, mean diffusivity; Nb, number of streamlines; Prob, probabilistic tractography; R-, right hemisphere; RD, radial diffusivity; ROI, region of interest; SLF, superior longitudinal fasciculus; TE, echo time; TR, repetition time; UF, uncinate fasciculus; Vol, volume.
Pooled effect size
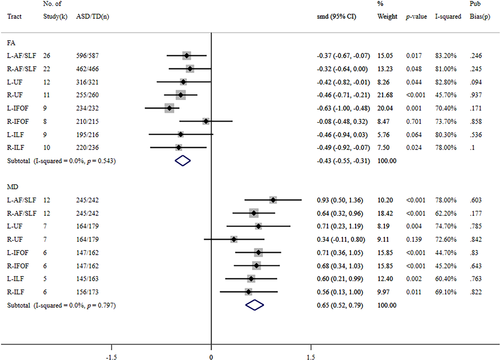
The meta-analysis results are shown in Figure 2. In the pooled analyses, lower FA or higher MD was found in all tracts in individuals with ASD than in TD controls. Meanwhile, the effect size for FA value showed statistical significance in bilateral AF/SLF, bilateral UF, left IFOF, and right ILF. Regarding the MD, results reached the significance level in bilateral AF/SLF, left UF, bilateral IFOF, and left ILF.
Dorsal pathway (AF/SLF)
After removing two outlier data sets (Libero et al., 2016; Zeestraten et al., 2017 [Male group]) (Figure S17), 26 studies involving 596 ASD and 587 TD individuals were integrated into the pooled analysis of the FA values in the left dorsal pathway. Significantly reduced FA in L-AF/SLF appeared in ASD subjects compared to TD controls (SMD = −0.37, 95% CI −0.67 ~ −0.07, p = 0.017, I2 = 83.2%). After two data sets were removed as outliers (Zeestraten et al., 2017 [Male and female groups]) (Figure S17), 22 studies (N_ASD = 462, N_TD = 466) were pooled into the meta-analysis for the right dorsal pathway. A significant FA reduction was observed in subjects with ASD (SMD = −0.32, 95% CI −0.64 ~ 0.00, p = 0.048, I2 = 81.0%). MD data sets for the dorsal pathway were obtained from 12 studies that examined 245 individuals with ASD and 242 TD controls. MD values in the bilateral dorsal pathway were significantly increased in the ASD group, and the effect size in the left hemisphere was larger compared with that in the right hemisphere (left: SMD = 0.93, 95% CI 0.50 ~ 1.36, p < 0.001, I2 = 78.00%; right: SMD = 0.64, 95% CI 0.32 ~ 0.96, p < 0.001, I2 = 62.20%).
Uncinate fasciculus
In addition to one outlier data set (Zeestraten et al., 2017 [Male group]) (Figure S18), 12 studies (N_ASD = 316, N_TD = 321) remained in the pooled analysis of the FA value in the left UF. Significantly reduced FA was found in the ASD group compared to in the TD controls (SMD = −0.42, 95% CI −0.82 ~ −0.01, p = 0.044, I2 = 82.80%). Excluding two outlier data sets (Zeestraten et al., 2017 [Male and female groups]) (Figure S18), 11 studies were included in the meta-analysis for the right UF. The FA value in ASD subjects was significantly lower than that in TD controls (SMD = −0.46, 95% CI −0.71 ~ −0.21, p < 0.001, I2 = 45.70%). Pooled analysis of seven studies with MD data sets (N_ASD = 164, N_TD = 179) revealed that, individuals with ASD appeared to have significantly higher MD than TD controls in the left UF (SMD = 0.71, 95% CI 0.23 ~ 1.19, p = 0.004, I2 = 74.70%), however no significant group difference in the right UF (SMD = 0.34, 95% CI −0.11 ~ 0.80, p = 0.139, I2 = 72.60%).
Inferior frontal-occipital fasciculus
After removing one outlier data set (Thomas et al., 2011) (Figure S19), nine studies remained in the pooled analysis of the FA values in the left IFOF. Significantly decreased FA was found in the ASD group in the left IFOF (SMD = −0.63, 95% CI −1.00 ~ −0.48, p = 0.001, I2 = 70.40%), whereas the group difference in the right IFOF did not reach significance (k = 8, SMD = −0.08, 95% CI −0.48 ~ 0.32, p = 0.701, I2 = 73.70%). For the MD data, six studies that investigated 147 ASD and 162 TD subjects were included in the meta-analysis. Pooled effects of MD data sets revealed that the ASD group showed a considerably significant MD increase in bilateral IFOF (left: SMD = 0.71, 95% CI 0.36 ~ 1.05, p < 0.001; right: SMD = 0.68, 95% CI 0.34 ~ 1.03, p < 0.001). In addition, heterogeneity was relatively low in both hemispheres (left: I2 = 44.70%; right: I2 = 45.20%).
Inferior longitudinal fasciculus
After removal of one outlier study (Jou et al., 2011) (Figure S20), 9 and 10 data sets were preserved in the meta-analysis of FA values in the left and right ILF, respectively. The pooled effects of FA values showed no statistical difference between ASD individuals and TD controls in the left ILF (k = 9, SMD = −0.46, 95% CI −0.94 ~ 0.03, p = 0.064), whereas ASD subjects showed significantly lower FA in the right ILF (k = 10, SMD = −0.49, 95% CI −0.92 ~ −0.07, p = 0.024). Between-study heterogeneity was considerably large in either hemisphere (left: I2 = 80.30%; right: I2 = 78.00%). The MD data sets for the meta-analysis of the left and right ILF were from five and six studies (excluding one outlier data set, Ameis et al., 2011 [Adolescent group]) (Figure S20), respectively. Pooled analysis of the bilateral ILF showed significantly increased MD in the ASD group and moderate between-study heterogeneity (left: SMD = 0.60, 95% CI 0.21 ~ 0.99, p = 0.002, I2 = 60.40%; right: SMD = 0.56, 95% CI 0.13 ~ 1.00, p = 0.011, I2 = 69.10%).
Publication bias and sensitivity analysis
No significant evidence of publication bias was observed in our meta-analysis, as confirmed through visual inspection of the funnel plot (Figures S21–S24). In addition, Egger's regression test of each pooled analysis did not generate any significant small-study effect (p-value ranged from 0.094 to 0.937) (Figure 2). In addition, the leave-one-out sensitivity analysis showed that the direction of the effect size and statistical significance remained stable during the sequential removal of individual studies in all pooled analyses (Figures S25–S28).
Subgroup analysis
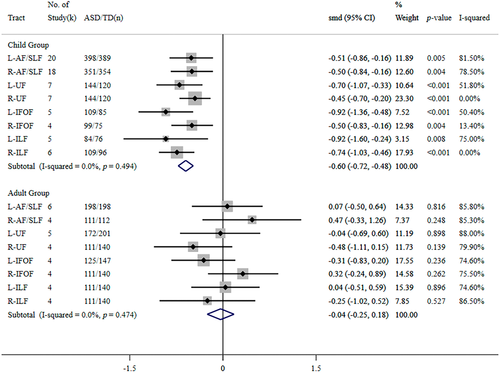
Relatively higher levels of between-study heterogeneity were observed for the majority of the outcomes, except for the FA value in the right UF and MD values in the bilateral IFOF, as presented in Figure 2. We performed a subgroup analysis based on the participants' age (child group versus adult group) to examine this heterogeneity in the pooled analyses. Subgroup analysis of FA data sets (Figure 3) revealed significantly lower FA in children with ASD than in TD controls in all white matter tracts but no significant differences in adult groups. Meanwhile, in the pooled analyses for the younger group, the left hemisphere showed a relatively larger effect size than the right hemisphere.
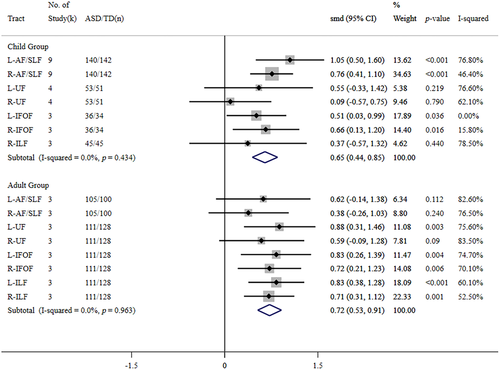
In contrast, subgroup analysis of the MD data sets (Figure 4) indicated that the pooled effects of MD values significantly increased in children with ASD in the bilateral dorsal pathway (L/R-AF/SLF) and bilateral IFOF. In contrast, adult subjects with ASD showed significantly increased MD in the left UF, bilateral IFOF, and bilateral ILF. Due to the insufficient MD data sets in this study, we could not perform a subgroup analysis for the left ILF in the younger group.
Meta-regression analysis
To examine the moderating effects of the sex ratio and verbal IQ, we performed meta-regression analyses of the FA values in all tracts and MD values in the dorsal pathway, including more than five studies. However, due to the limited number of studies, we only performed a meta-regression of symptom severity using FA data sets in the dorsal pathway. The results of the meta-regression analysis are presented in Table 3. We did not find any statistically significant results in the meta-regression mentioned above. In other words, variances in the sex ratio, verbal IQ, and symptom severity do not seem to be the factors that bring about between-study heterogeneity in our pooled results.
| Tract | Dependent variable | Sex ratio | Verbal IQ | SRS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | β | SE | p | k | β | SE | p | k | β | SE | p | |||
| Dorsal Pathway | AF/SLF | L_FA | 23 | 0.001 | 0.009 | 0.293 | 12 | 0.046 | 0.023 | 0.073 | 6 | −0.002 | 0.016 | 0.897 |
| R_FA | 20 | 0.0005 | 0.009 | 0.962 | 11 | 0.034 | 0.031 | 0.307 | 6 | −0.011 | 0.016 | 0.537 | ||
| L_MD | 10 | 0.013 | 0.02 | 0.532 | 3 | — | — | — | — | — | — | — | ||
| R_MD | 10 | −0.001 | 0.014 | 0.923 | 3 | — | — | — | — | — | — | — | ||
| Ventral Pathway | UF | L_FA | 11 | 0.008 | 0.006 | 0.211 | 8 | 0.067 | 0.031 | 0.068 | — | — | — | — |
| R_FA | 11 | 0.002 | 0.005 | 0.76 | 7 | 0.042 | 0.022 | 0.16 | — | — | — | — | ||
| ILF | L_FA | 10 | 0.008 | 0.009 | 0.388 | 7 | 0.053 | 0.045 | 0.286 | — | — | — | — | |
| R_FA | 10 | 0.004 | 0.008 | 0.604 | 8 | 0.049 | 0.026 | 0.111 | — | — | — | — | ||
| IFOF | L_FA | 10 | 0.011 | 0.008 | 0.22 | 5 | — | — | — | — | — | — | — | |
| R_FA | 8 | 0.001 | 0.007 | 0.191 | 5 | — | — | — | — | — | — | — | ||
- Note: k: Number of studies; —: No sufficient studies; Factor significance was set at p < 0.01, and there were no statistically significant results in any meta-regression.
DISCUSSION
Our meta-analysis included 33 DTI studies involving 831 individuals with ASD and 836 TD controls. According to the pooled results, we found significantly lower FA and higher MD in the bilateral dorsal pathway, left UF, left IFOF, and right ILF in ASD subjects compared with TD controls, significantly decreased FA in the right UF, and increased MD in the right IFOF and left ILF. In particular, the sub-analyses by age group indicated that children, but not adults, with ASD showed remarkable FA reduction among all tracts. On the other hand, MD increased in bilateral language-associated fibers, except for UF and ILF, in ASD children, and in most ventral fibers, except R-UF, in ASD adults. Moreover, abnormalities in language-associated fibers appeared more prominent in the left hemisphere than in the opposite hemisphere, especially in the child group. These results demonstrate that individuals with ASD tend to exhibit widespread abnormal white matter connectivity in their language networks. Our study is the first report of a sub-analysis by age group and indicates that these abnormalities could vary depending on the developmental stage and hemisphere.
Abnormal connectivity of language networks in ASD
The abnormal connectivity of language-related tracts in patients with ASD is concordant with the previous hypoconnectivity hypothesis (Just et al., 2012). Hoppenbrouwers et al. (2014) combined qualitative and quantitative reviews and demonstrated that ASD is a disconnection syndrome with widespread disruption of white matter integrity. In addition, hypoconnectivity of association tracts in ASD has been frequently reported in previous systematic reviews (Ameis & Catani, 2015; Rane et al., 2015; Vissers et al., 2012). Aoki et al.'s (2013) meta-analysis synthesized 14 DTI tractography studies reporting decreased FA and increased MD in the SLF and UF in subjects with ASD. The hypoconnectivity of language-related tracts in ASD may result from the aberrant microstructure in white matter tracts, such as disturbed fiber myelination, decreased axonal density, or impaired cellular membranes (Travers et al., 2012). In line with these findings, previous structural MRI studies have suggested unusual morphological alterations in the autistic brain. For example, several meta-analyses of VBM studies have indicated that individuals with ASD have significant volume reduction in the SLF and ILF (Duerden et al., 2012) and reduced density in the IFOF and ILF (Nickl-Jockschat et al., 2012). Furthermore, our findings are concordant with the results of previous fMRI studies. Several experimental studies with a multimodal approach (Jung et al., 2019; Lai et al., 2012) have reported a concurrent reduction of structural connectivity and functional activity in the language-related areas of ASD patients. In addition, Lai et al. (2012) indicated that the ASD group showed significant correlations between the tract integrity of language pathways and functional activity in the left inferior frontal gyrus during the auditory task with speech stimuli. Moreover, Olesen et al. (2003) combined analysis of DTI and fMRI data, which revealed positive correlations between the maturation of white matter and increased gray matter activity, especially in frontoparietal regions. The development of gray matter activity seems to be mediated by the maturation of white matter (Olesen et al., 2003). However, it should be noted that the white matter–gray matter correlations are not yet well-understood, and vary by diagnostic group, DTI parameter, tasks, and seed points (Richards et al., 2015). In addition, the target of DTI, structural MRI, and fMRI is distinctive, and comprehensive imaging studies with the multi-modal approach are necessary to uncover the relationship between white matter connectivity and brain functional efficiency in the future.
The altered structural connectivity mentioned above plays a critical role in language impairment in ASD (Cauda et al., 2014). First, the dorsal pathway encompasses the AF and SLF, which are widely distributed across many important language-related regions, such as the middle temporal gyrus, superior temporal gyrus, inferior frontal gyrus, and angular gyrus (Dick & Tremblay, 2012; Friederici, 2015; Kamali et al., 2014). Its disruption contributes to speech arrest, dysarthria, and syntactical impairments (Chang et al., 2015; Kljajevic, 2014). The hypoconnectivity of the dorsal pathway may be closely associated with various speech dysfunctions pervasively observed in patients with ASD, such as delayed phonological development, articulation distortions (Chenausky et al., 2019; Schoen et al., 2011), and grammatical disorders (more errors in grammatical morphemes and clitics) (Roberts et al., 2004; Terzi et al., 2014). The ventral pathway mainly consists of the UF, IFOF, and ILF (Hagoort, 2019). The UF is a temporal-frontal association tract that projects from the temporal pole to the orbitofrontal cortex (Catani et al., 2013). It is relevant for sentence-level comprehension and pragmatic function (Friederici, 2017), which are the most commonly affected linguistic abilities in ASD individuals (Hage et al., 2021). The IFOF is a long-range intrahemispheric tract that originates from the occipital cortex, runs through the posteroinferior temporal area, terminates in the inferior frontal gyrus and dorsolateral prefrontal cortex, correlates with semantic and phrase-level syntactical processes, and assists in reading or listening comprehension tasks (Friederici, 2015). The ILF is considered the indirect ventral pathway, which interconnects the visual areas in the occipital lobe, MTG, and temporal lobe, and mainly involves visual information processing such as object recognition and visual–linguistic information mapping (Duffau et al., 2013). Lesions in the ILF and IFOF are associated with alexia, nominal aphasia, and semantic paraphasia (Chang et al., 2015; Ivanova et al., 2016). Hence, the under-connectivity of the IFOF and ILF may be associated with idiosyncratic word usage and atypical semantic processing that frequently appears in patients with ASD (Boucher, 2012).
Accordingly, our results support the under-connectivity hypothesis and demonstrate the widespread abnormal microstructure of language-related tracts in patients with ASD. Furthermore, taken together with neurocognitive insights, we speculate that in ASD, the under-connectivity of the dorsal pathway may contribute to phonological paraphasia and syntactical errors, and disruption of the ventral pathway is associated with semantic paraphasia and comprehension problems. Nevertheless, the elaborate relationships between the microstructural properties of white matter tracts and language processing remain under-researched. The causality of these relationships and the neurological underpinnings of language impairments in ASD require clarification using more specific experimental studies.
Moderating effects of developmental stage
The subgroup analysis revealed that the atypical structural connectivity in ASD showed considerable differences according to the developmental stage. Specifically, children with ASD exhibit more pronounced and pervasive white matter connectivity disturbances than the adult group. This indicates that structural abnormalities in language-related tracts tend to be modest with age. In line with our findings, Bakhtiari et al. (2012) found that adolescents with ASD showed decreased FA in the IFOF, ILF, and SLF, whereas no difference in FA was observed between ASD and TD adults. A similar tendency was observed in structural MRI studies that examined morphological abnormalities in ASD. A prior meta-analysis of VBM studies (Duerden et al., 2012) investigated the difference in brain morphology between children and adults with ASD and reported that significantly reduced volume in the SLF and ILF only existed in children with ASD and adolescents but not in adults.
On the other hand, MD increases were observed in language-related tracts in children and adults with ASD. The distinction between FA and MD results in adults was also reported in previous experimental DTI studies (Andica et al., 2021; Catani et al., 2016). These deviations may result from the distinctive trajectories of diffusion parameters with age. Several lifespan studies have identified white matter developmental trajectories in healthy individuals, suggesting rapid increases in FA and gentle decreases in MD during childhood and puberty (Lebel et al., 2008; Peters et al., 2012; Tamnes et al., 2018). The FA peaks in adolescence to young adulthood, while the minimum MD generally occurs 3 to 6 years later than the FA peaks (Lebel et al., 2012; Lebel et al., 2019; Lebel & Deoni, 2018). In addition, several studies focused on the developmental trajectory of the autistic brain, demonstrating that aberrant white matter microstructure was caused by delayed development in later childhood and adolescence (Girault & Piven, 2020), whereas the atypical development tends to normalize in adulthood (Khundrakpam et al., 2017; Zheng et al., 2021). Considering the different developmental rates of FA and MD values, abnormal FA reductions in ASD are likely to be relieved before early adulthood, whereas disturbed MD will continue from childhood to adulthood. Changes in FA and MD are driven by numerous cellular factors, such as myelination, axonal packing, membrane permeability, axon diameter, the proliferation of oligodendrocytes, and tissue water content (Beaulieu, 2002; Beaulieu, 2014). Unfortunately, the differences in physiological processes between FA and MD values cannot be distinguished, which should be expounded upon by more neurobiological studies (Lebel et al., 2019).
The relative remission of structural disturbance may be due to the moderating effects of environmental factors (such as education, intervention, and cognitive experience) in the development of ASD. Several experimental studies have examined the association between therapy and structural/functional brain development in ASD, suggesting that behavioral interventions in early life contribute to normalizing atypical patterns of white matter integrity (Saaybi et al., 2019) and brain activity (Dawson et al., 2012). Moreover, previous DTI studies have explored the association between white matter maturation and the environment, showing that unfavorable environmental factors (such as socioemotional deprivation) in early life will lead to the maldevelopment of white matter (Makinodan et al., 2017). However, in this study, we could not obtain sufficient information about interventions for participants with ASD. Therefore, the specific white matter changes according to the environmental moderating effects remain to be elaborated on in the future using long-term longitudinal studies.
Left-dominant abnormality in ASD
In our study, more significant and larger effect sizes of FA and MD values were observed in the left tracts than in the right, particularly in the younger subgroup. This indicates that individuals with ASD tend to exhibit more prominent abnormalities in their left language networks. The trend of left-dominant abnormalities in ASD has been reported in several previous studies. For instance, more pronounced FA reductions (Lange et al., 2010; Perkins et al., 2014) or MD elevation (Peterson et al., 2015) in the left hemisphere. Aoki et al.'s (2013) meta-analysis also reported that individuals with ASD have significantly decreased FA in the left SLF and UF, but not on the reverse side. In addition, previous structural MRI studies investigated volume alteration in the autistic brain, finding significant volume reduction in the left language-related areas but no significance in the right (Rojas et al., 2002). These findings indicated that left-dominant tendencies are generally recognized in both morphological and white matter abnormalities in individuals with ASD.
Furthermore, numerous lines of evidence have shown atypical asymmetry of structural and functional brain organization in patients with ASD. For example, ASD individuals showed significantly less left-lateralization than TD controls in DTI metrics of the language-related tracts, such as AF (Fitzgerald et al., 2018; Joseph et al., 2014), SLF, ILF, and IFOF (Carper et al., 2016). Several DTI studies have reported right-lateralization in widespread white matter regions (Fu et al., 2020), including the AF (Wan et al., 2012). This aberrant asymmetry pattern was also observed in the morphological structures of patients with ASD. de Fossé et al. (2004) reported that individuals with ASD and language impairment showed atypical rightward asymmetry of volume in the language cortex. Postema et al. (2019) also suggested decreased asymmetry in the brain volume and cortical thickness in widespread language-related regions in patients with ASD. Several fMRI studies have demonstrated altered functional asymmetry in the autistic brain. For instance, individuals with ASD showed less left-lateralized responses to phonemic cues (Minagawa-Kawai et al., 2009) and speech sounds (Eyler et al., 2012) in the temporal cortex. Jouravlev et al. (2020) reported that individuals with ASD exhibit reduced left-lateralization in comprehension tasks. Moreover, individuals with ASD also show atypical rightward asymmetry in widespread functional networks (such as visual, auditory, motor, exclusive, language, and attentional networks) (Cardinale et al., 2013) and white matter networks (Wei et al., 2018). Therefore, the unusual pattern of hemispheric asymmetries in patients with ASD seems to be a pervasive feature observed in various brain measures. Enhanced maturation and activity of the right hemisphere are thought to underlie the reduced left-lateralized pattern of ASD (Jouravlev et al., 2020), but the exact mechanism remains to be determined. Otherwise, the atypical lateralizations mentioned above are closely associated with language dysfunction symptoms in ASD (Knaus et al., 2010; Peterson et al., 2015). The atypical lateralization of language pathways in infants may be an early predictor of future language development and ASD risk (Liu et al., 2019). However, the specific correlations between aberrant lateralization and particular language impairments in patients with ASD should be elucidated in future studies.
Limitations
Our meta-analysis had several limitations. First, there was significant between-study heterogeneity in most of the pooled results. We performed subgroup analysis and meta-regression to identify the potential study-level factors that contributed to this heterogeneity. However, due to the insufficient number of relevant studies (especially studies focused on the ventral pathway and adult group), we could not explore the causes of heterogeneity adequately. Second, the included studies varied in methodological factors, such as data acquisition (e.g., field strength, imaging sequences, and image acquisition parameters) and analysis details (e.g., analysis software, fitting model, and tractography parameters). These methodological variabilities could have led to the significant heterogeneity in our findings. We tried to perform the sensitivity analysis to explore the contribution of these methodological variables to the heterogeneity, but we could not because of the imbalanced number of raw studies. The impact of various methodologies on between-study heterogeneity must be further illuminated with more extensive and sufficient data sets. Third, considering the unstable neural system development in the infant period, we excluded studies that examined participants below 3 years old. However, white matter development in early life is a crucial topic that should be independently investigated in future ASD studies. Fourth, our meta-analysis did not include longitudinal studies due to the limited original literature. However, longitudinal studies are critical to uncovering age-related changes in white matter in autistic brains. Fifth, our meta-analysis could not directly analyze the correlation between DTI metrics, language performance, and symptom severity in ASD. We extracted and summarized the correlation results of the included studies, which were difficult to synthesize due to the distinct variables, assessment scales, and statistical indexes in the original correlation analysis. Therefore, the specific relationships between cognitive characteristics and white matter connectivity in the language processes of autism remain to be determined in the future. Finally, it should be noted that some limitations remain in the DTI technique. For instance, the tensor model used in DTI is insufficient for dealing with the crossing white matter fibers. Moreover, the sensitivity of DTI metrics to the specific tissue properties and biological processes needs to be improved (Tournier, 2019). Recently, various advanced acquisition techniques (e.g., high angular resolution diffusion imaging, diffusion spectrum imaging) and computation models (e.g., neurite orientation dispersion and density imaging, diffusion kurtosis imaging) have been developed to overcome these limitations of DTI. Future research will be needed to focus on newer imaging techniques to provide more detailed information about altered white matter microstructure of the autistic brain.
CONCLUSIONS
Our meta-analysis investigated the abnormal white matter microstructure of language networks in individuals with ASD. The autistic brain showed pervasive hypoconnectivity in language-related tracts. In addition, structural disturbances of language networks in patients with ASD exhibit a leftward tendency and atypical brain lateralization. We suggest that atypical structural connectivity is closely associated with various language dysfunctions in patients with ASD. Otherwise, the deviations of white matter disturbance were observed in different age groups; younger people with ASD showed more significant and apparent abnormalities than adults. Accordingly, aberrant white matter connectivity may alleviate with age due to heightened developmental moderating effects in the autistic brain. The current literature provides an understanding of neurocognitive characteristics in the language networks of patients with ASD. We hope that this meta-analysis will contribute to future language development studies and speech therapy for patients with ASD.
ACKNOWLEDGMENT
The authors thank all of the researchers who participated in the studies involved in the current article.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
ETHICS STATEMENT
The current study was exempted from institutional review board review because it was based on a reanalysis of published data.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PROSPERO at https://www.crd.york.ac.uk/prospero/, reference number CRD42021282765.




