To covet what we see: Autistic traits modulate the relationship between looking and choosing
Abstract
Behavioral studies indicate that autistic traits predict reduced gaze toward social stimuli. Moreover, experiments that require participants to make an explicit choice between stimuli indicate reduced preferences for social stimuli in individuals with high autistic traits. These observations, in combination, fit with the idea that gaze is actively involved in the formation of choices—gaze toward a stimulus increases the likelihood of its subsequent selection. Although these aspects of gaze and choice behavior have been well characterized separately, it remains unclear how autistic traits affect the relationship between gaze and socially relevant choices. In a choice-based eye-tracking paradigm, we observed that autistic traits predict less frequent and delayed selection of social stimuli. Critically, eye tracking revealed novel phenomena underlying these choice behaviors: first, the relationship between gaze and choice behavior was weaker in individuals with high autistic traits—an increase in gaze to a stimulus was associated with a smaller increase in choice probability. Second, time-series analyses revealed that gaze became predictive of choice behaviors at longer latencies in observers with high autistic traits. This dissociation between gaze and choice in individuals with high autistic traits may reflect wider atypicalities in value coding. Such atypicalities may predict the development of atypical social behaviors associated with the autism phenotype.
Lay Summary
When presented with multiple stimuli to choose from, we tend to look more toward the stimuli we later choose. Here, we found that this relationship between looking and choosing was reduced in individuals with high autistic traits. These data indicate that autistic traits may be associated with atypical processing of value, which may contribute to the reduced preferences for social stimuli exhibited by individuals with autism.
Introduction
A large body of evidence indicates that individuals with autism exhibit reduced tendencies to seek and exchange information about other people. This constitutes part of the diagnostic criteria for autism spectrum disorders (ASDs) (American Psychiatric Association, 2013). Several dominant models propose that early motivational and attentional social deficits could disrupt the developmental framework for complex social and cognitive processes to develop later in life (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Toth, Munson, Meltzoff, & Dawson, 2006). As a spectrum disorder, traits associated with ASD are observed to varying degrees throughout the population (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001). As such, one approach to studying autism is to investigate how autistic traits predict behaviors. This approach is motivated by the observation that autistic traits are associated with similar aetiologies at extreme ends (Robinson et al., 2011). Dimensional approaches are more methodologically tractable than case–control designs and have good explanatory power in generalizing findings to clinical samples (Bölte, Westerwald, Holtmann, Freitag, & Poustka, 2011; Chandler et al., 2007; Stevenson et al., 2018) .
Historically, eye tracking has provided a valuable tool for studying the autism phenotype (Howard, Zhang, & Benson, 2019). Gaze provides an online, behavioral correlate of cognitive processes. However, most research examining the relationship between gaze behavior and autistic traits has focused on atypical visual behavior during free-viewing tasks (Chita-Tegmark, 2016; Frazier et al., 2017; Klin, Lin, Gorrindo, Ramsay, & Jones, 2009), or tasks with “correct” answers, such as identifying emotional expressions (Pelphrey et al., 2002; Spezio, Adolphs, Hurley, & Piven, 2007). These studies generally indicate that autistic traits are associated with reduced attention to or abnormal sampling of social stimuli.
By contrast, little is known about whether these atypical gaze behaviors extend to subjective decisions that are highly relevant to social behavior. Humans frequently encounter situations that require explicit, subjective choices to be made between social and nonsocial rewards. For example, an individual may be faced with a choice between meeting friends, or staying at home to play with toys. Indeed, when presented with analogous situations in experimental contexts, individuals with high autistic traits invest less effort to obtain social rewards (Dubey, Ropar, & de C Hamilton, 2017; Dubey, Ropar, & Hamilton, 2015; Traynor, Gough, Duku, Shore, & Hall, 2019). Understanding the mechanisms underlying such choices is highly relevant to the autism phenotype.
Such an investigation is also timely because it is now established that there are strong reciprocal links between gaze and choice behavior. The most basic demonstration of this is that in choice situations, individuals tend to look for longer at images they later chose (Cavanagh, Wiecki, Kochar, & Frank, 2014; Fiedler & Glöckner, 2012; Folke, Jacobsen, Fleming, & De Martino, 2016). This relationship is also supported by the demonstration that external manipulation of gaze can induce changes in choice probabilities (Armel, Beaumel, & Rangel, 2008; Shimojo, Simion, Shimojo, & Scheier, 2003). Motivated by these findings, recent proposals hold that gaze does not merely sample information during choice, but is actively involved in constructing preferences (Cavanagh et al., 2014; Thomas, Molter, Krajbich, Heekeren, & Mohr, 2019). This reciprocal link between gaze and preference may reflect a wider, fundamental principle of value coding—that increased exposure to a stimulus tends to increase positive affective responses to it. One classic exemplar of this “exposure-affect coding” comes from social psychology—the “mere exposure effect” demonstrates that individuals form an increased preference for a stimulus as a consequence of mere repeated exposure. This effect is robust and generalizes across cultures and stimulus conditions (Zajonc, 2001).
Increasingly, it is also becoming appreciated that there are individual differences in the strength of the association between gaze and choice (Smith & Krajbich, 2018; Thomas et al., 2019). For instance, analyzing four-choice data sets, Thomas et al. (2019) observed that some individuals exhibit choice behaviors that are strongly influenced by gaze, whereas others exhibit choice behavior that is almost independent of gaze. A subset of individuals exhibited strong biases towards choosing items that were more fixated, to the extent that their choices actually contradicted their previous valuations of the competing stimuli. Consequently, modeling these individual differences in the strength of the gaze–choice relationship led to superior prediction of choices compared to a model that assumed the same relationship for each observer. Moreover, these individual differences generalize across diverse contexts (Smith & Krajbich, 2018)—observers with a strong gaze–choice relationship in one context exhibited a strong relationship in another context (e.g., with and without risk, across food and monetary rewards). Such observations indicate that the gaze–choice relationship is some robust trait, rather than reflecting the peculiarities of specific experimental conditions.
These studies demonstrate the significant individual variability in this gaze–choice relationship and its importance for understanding choice behavior. However, it is unknown how such a relationship may be associated with other individual-level traits of the observer. For instance, differences in the gaze–choice relationship may contribute to some of the social behavioral atypicalities associated with the autism phenotype. One simple prediction of the gaze–choice relationship is that stimuli that capture attention are more likely to be preferred. We would therefore predict an individual with a strong gaze–choice relationship to develop preferences for salient stimuli that have evolved to capture attention exogenously early in development, such as faces (Shah, Gaule, Bird, & Cook, 2013), or biological motion patterns (Simion et al. 2008). This bias would then potentiate a mutual feedback loop that leads to increased preferences for, and gaze toward social stimuli. This trajectory is consistent with behaviors observed in individuals with low autistic traits (Dubey et al., 2015; Williams & Cross, 2018). By contrast, we would predict an individual with a weak gaze–choice relationship to not be as likely to form preferences for these attention-grabbing stimuli, predisposing the individual toward social difficulties later in development. This latter trajectory is consistent with behaviors observed in individuals with high autistic traits (Chevallier et al., 2012; Johnson, 2005). Taken together, we may therefore predict individuals with higher autistic traits to exhibit a weaker relationship between gaze and choice.
The current study examines how autistic traits modulate gaze behavior, choice behavior and their interaction during socially relevant decisions. We test if autistic traits are predictive of choices between social and nonsocial stimuli. We then investigate the underlying mechanisms of these choice behaviors by examining sampling strategies and temporal trajectories of gaze behavior during choice.
Method
Participants
Our full sample consisted of 53 participants (32 Female, 21 Male, M age = 35.05, SE = 1.79). Thirty of these were students and staff recruited from the University of Reading campus. In addition to these 30 participants, to expand the range of autistic traits, we also recruited 23 adults with a Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision (DSM-IV TR)-based diagnosis of an ASD from a recognized clinic, who were recruited through the Centre for Autism database of research volunteers. To provide a measure of cognitive ability of these ASD participants, each completed the Wechsler Abbreviated Scale of Intelligence (WASI), which estimates the participant's cognitive ability as a percentile of the general population (Wechsler, 2008). The mean WASI was 115 (SE = 2.20), indicating a high level of intellectual functioning and cognitive ability comparable to the University population (Lassiter, Bell, Hutchinson & Matthews, 2001; Wilson et al., 2014). To provide a measure of autistic traits, after the experiment, participants completed the autism quotient (AQ). The AQ scores captured a wide range of values (range: 8–45, M = 25.9, SE = 1.76). This range extends to the extreme percentiles of the distribution of scores typically found in large Neurotypical (NT) and ASD samples (Baron-Cohen et al., 2001). Moreover, 53 participants provide good statistical power to detect autism-related differences in social choice behaviors (see Supporting Information Section S1 for power calculation). Ethical clearance was obtained from the University of Reading Ethics Committee, and all participants gave fully informed consent.
Stimuli
We employed 30 pairs of static social and nonsocial reward images, which were the same as those used in Chakrabarti, Haffey, Canzano, Taylor, and McSorley (2017). Images were recovered from the international Affective Picture System (Lang, Bradley & Cuthbert, 1997) and publicly available creative common licensed databases. Social images were scenes involving happy groups of people, whereas nonsocial images involved food, natural scenery, and money (see Supporting Information Section S2 for further details of selection criteria). To minimize the influence of extraneous sensory and affective differences between image pairs, all pairs were matched as closely as possible in terms of low-level properties (luminance, contrast, Koch saliency) as well as valence and arousal based on data from a pilot experiment (see Supporting Information S2 and Figure S1 for further details on matching). Participants were seated 60 cm from a 1280 × 1024 pixel resolution monitor with an inbuilt Tobii T60 eye tracker. Stimuli subtended 5.59° × 4.19° of visual angle. In addition, to further characterize the influence of low-level confounds, we presented two stimulus types. One set of images were intact, and another were phase scrambled. This manipulation maintains the mean luminance, contrast, spatial frequency and colour profile of the intact images, but renders them unrecognizable (Figure 1(A)). The logic is that if simple low-level variability between image pairs drives a bias toward social images, we would expect to find a social bias of the same magnitude in the intact and scrambled condition. Stimuli were presented using MATLAB with Psychtoolbox extensions (Brainard, 1997).

Procedure
Following a 9-point calibration, participants completed the choice task: Observers were informed that they would be presented with pairs of images and that they were required to indicate their preferred image via button press. Figure 1(B) depicts the trial sequence: In the fixation phase, observers were presented with a fixation cross for 500 ms. Subsequently, in the free-inspection phase, observers were presented with a pair of social and nonsocial stimuli until their response was made. Finally, in the choice phase, a green frame was presented around the chosen image to indicate the preferred choice. To illustrate, Figure 1(C) depicts representative data obtained from one trial. An observer looks towards each alternative, implementing a comparison before responding. Observers completed 240 trials in total (30 image pairs, 2 stimulus types × 4 repetitions). The repeated presentation of image pairs allowed us to monitor the consistency of choice responses. This provided us with an index of how stable each observer's choice behavior was. In addition, it allowed us to counterbalance the side of the display that each image was presented on, thereby protecting against lateral biases influencing choice. This measure is important given that the gaze–choice relationship predicts choices biased towards the initially fixated image (Krajbich & Rangel, 2011)—and that attention is often initially biased towards the left.
Results
Data reduction and analytic approach
The raw data supporting this article are publicly available via the “Figshare” repository (Hedger, 2020). Using Grafix Fixations Coder software (Saez de Urabain, Johnson, & Smith, 2015), we combined raw gaze coordinates from the left and right eyes into a single set of X and Y coordinates and smoothed this time series via a bilateral filtering algorithm (Durand & Dorsey, 2002). We interpolated missing data portions that were briefer than 150 ms. This 150-ms cutoff was based on previous literature that indicates saccadic programming takes around 130 ms and so interpolations of less than 150 ms should prevent interpolating an entire saccade-fixation-saccade sequence (Wass, Smith, & Johnson, 2013). We removed trials for which gaze failed to record for more than 60% of the trial (5.02% of the data). Trials corresponding to response time outliers were defined as being more than 3 SD from the log-transformed mean RT of each observer (separately for intact and scrambled trials). Rejecting these trials removed a further 0.67% of the data.
Unless specified otherwise, to evaluate hypotheses, we fit generalized linear mixed effects models. Each fixed effect that was evaluated was entered into the model with a corresponding by-subject random slope (Barr, 2013). Reported p values were obtained by likelihood ratio tests that compare models with the coefficients to those without them (recommended by Barr, Levy, Scheepers, & Tily, 2013). Note that since our study was not planned as, or optimized for a case control design, we employ a dimensional approach to the analyses (with AQ as the primary explanatory variable). Nonetheless, for completeness, we also report equivalent group-based analyses (see Supporting Information Section S3 and Figure S2). These results are qualitatively and quantitatively very similar to those reported in the present manuscript.
Choice behavior
Choice proportions
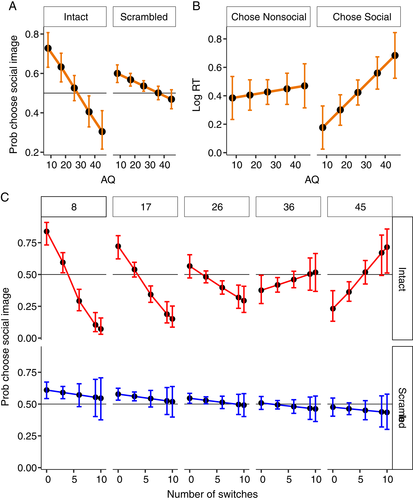
Across all stimulus types and observers, the grand mean indicated that social stimuli were chosen on 52.70% of trials (SE = 2.02). Choice data (proportion social images chosen) were analyzed in a logistic regression model with stimulus type and AQ as fixed effects. No main effect of stimulus type was detected χ2 (1) = 0.490, p = 0.751—social stimuli were selected with equal frequency for both intact and scrambled stimulus types (intact = 52%, scrambled = 54%). Critically, though, a robust interaction between AQ and stimulus type was detected χ2 (1) = 10.99, p = 0.009 (Figure 2(A)). Higher AQ predicted reduced selection of social stimuli, and this effect was substantially larger in the intact condition (β = −0.04) than the scrambled condition (β = −0.01).
Choice consistency
The repetition of each stimulus pair allowed us to measure the “consistency” of choices. Low consistency may indicate inattention, or impulsive responses. For each stimulus pair, we determined the proportions of “chose social” and “chose nonsocial” responses and recorded the larger of these values (implying a minimum consistency of 0.5, and a maximum of 1). Observers were very consistent in their responses for intact stimuli (M = 0.96, SE = 0.008) and this consistency was lowered for scrambled images (M = 0.86, SE = 0.005), presumably because of the lack of recognizable structure. No relationship was detected between consistency and AQ for either stimulus type (intact: r(51) = 0.162, p = 0.246, scrambled: r(51) = 0.156, p = 0.264).
Choice latencies
The mean choice latency was 1815.75 ms (SD = 674 ms). Latency data (log response times - RTs) were analyzed in a general linear model with choice (chose social, chose nonsocial) stimulus type (intact, scrambled) and AQ as fixed effects. A three-way interaction was detected between AQ, stimulus type and stimulus choice χ2 (1) = 12.60, p = <0.001. There was no interaction between AQ and choice in the scrambled condition χ2 (1) = 403, p = 0.525. However, critically, in the intact condition, AQ predicted increased choice latency in trials where social images were chosen (β = 0.0137, p < 0.001), but not when nonsocial images were chosen (β = 0.002, p = 0.507). This interaction is depicted in Figure 2(B). Critically, this is inconsistent with a generalized increase in response latency in high AQ observers: the increase in latency was restricted to trials wherein intact social stimuli were chosen.
The above analyses indicate that autistic traits are associated with a reduced preference for, and delayed selection of social rewards. Importantly, the specificity of these effects is consistent with reduced social reward responsivity, not with inattention, generalized slowing of responses, or basic sensory properties of the stimuli. First, AQ did not predict consistency of responses. Second, delayed responses were not observed when nonsocial images were selected. Third, the substantially reduced influence of AQ in the scrambled condition indicate that these effects are explained by some semantic evaluation of the images and not by simple preferences based on low-level properties. With these effects established, we proceeded to investigate the oculomotor correlates of these behaviors.
Relationship between gaze and choice behavior
Switching behavior
The latency of choice responses (as indexed by RT) is proportional to the uncertainty regarding the choice. However, a more sensitive measure may be obtained from eye movements. As an oculomotor index of choice uncertainty, we calculated the number of switches in gaze between the competing stimuli. We reasoned that a larger number of switches was proportional to uncertainty regarding the preferred stimulus. Choice data (proportion social images chosen) were analyzed in a logistic regression model with number of switches, stimulus type, and AQ as fixed effects. This revealed robust three-way interaction between AQ, switches, and stimulus type on choice behavior χ2 (1) = 16.80, p < 0.001. This interaction is depicted in Figure 2(C). The upper panel shows data for intact images. For low AQ observers, increased switches predicted a decreased likelihood that the social image was chosen. By contrast, for higher AQ observers, increased switches predicted increased likelihood that a social image would be chosen. Expressed differently, high AQ predicted high uncertainty in choosing social images and low uncertainty in choosing nonsocial images (with the opposite pattern for low AQ). By contrast, in trials involving scrambled images (lower panel) the relationship between switches and choice behavior is roughly invariant across AQ. Again, this speaks against a low-level explanation for this effect.
Gaze proportions
Gaze data were collapsed into the proportion of trial time directed toward the social image. Choice data (proportion social images chosen) were analyzed in a logistic regression model with proportion gaze to social image and AQ as fixed effects. This revealed a main effect of gaze proportion χ2 (1) = 82.76, p = 0.001, such that increased gaze to the social image predicted increased likelihood that it was chosen. Critically, an interaction between AQ and gaze proportion was also detected χ2 (1) = 4.09, p = 0.040, indicating that the relationship between gaze and preference was modulated by AQ. These data are depicted in Figure 3(A). Inspection of this figure reveals a weaker relationship between gaze and choice behavior in high AQ individuals.
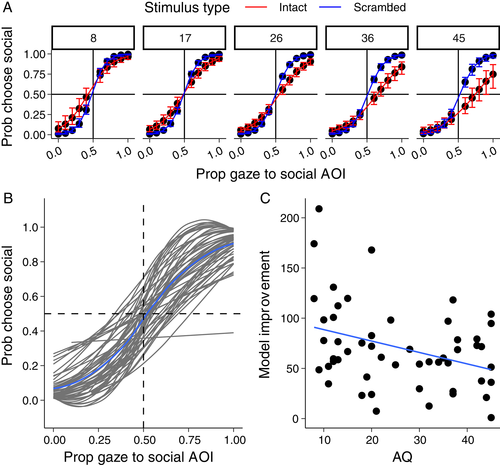
No higher-level interaction between AQ, gaze proportion and stimulus type was detected, indicating that the dissociation between gaze and choice was not specific to socially-relevant decisions χ2 (1) = 0.08, p = 0.780. To further investigate these varying gaze–choice relationships, we proceeded to fit individual-level models for each observer (Figure 3(B)). We then used Akaike's information criteria to compare the fit of an empty (intercept only) model to a model with gaze proportion as a predictor of choice probability. Fifty-two of 53 observers data were better explained by the gaze model. There was, however, substantial individual variation in the degree of model improvement (Figure 3(C)). Consistent with the above analyses, AQ was negatively associated with model improvement—higher AQ was associated with a reduced influence of gaze on choice behavior r(51) = −0.351, p = 0.009.
Temporal relationship between gaze and choice
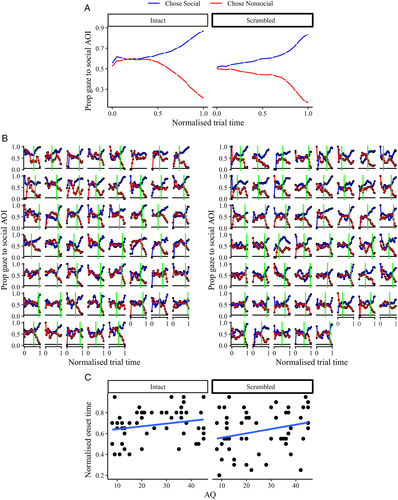
To determine how this relationship between gaze and choice behavior evolved over the duration of the trial, we first normalized time series data relative to stimulus onset. The data were then partitioned into 20 linearly spaced time bins from stimulus onset (0) to the time of the response (1). Figure 4(A), which depicts the group-averaged data, reveals a standard “gaze cascade” effect (Shimojo et al., 2003). At the start of the trial, both images are inspected roughly equally, but in the moments leading up to making a choice, the proportion of gaze towards the chosen image increases. To establish the onset of this “cascade” effect for each observer, we used a bootstrapping procedure to determine the earliest point at which gaze remained reliably predictive of the ensuing choices until the choice was made (see Supporting Information Section S4). These estimated onsets, which are shown by the vertical lines in Figure 4(B), capture the points of divergence quite well. There was substantial variability in onsets, ranging from prior to half way through the normalized trial duration (0.40) to the final portion of the trial (0.95). Critically, we detected a main effect of AQ on onset latency χ2 (1) = 4.12, p = 0.040. Consistent with the weaker gaze–choice relationship for high AQ observers, this indicates that higher AQ also tended to predict a later gaze cascade onset (Figure 4(C)). We observed no interaction between stimulus type and AQ χ2 (1) = 0.73, p = 0.390 on onsets. Again, this indicates that the effect of AQ on the interaction between gaze and choice behavior was not restricted to socially relevant decisions.
Discussion
In this study, we observed that autistic traits predict differences in social choice behaviors, and the role of gaze in influencing choices. We found that a higher level of autistic traits predict later and less frequent selection of social rewards. This is consistent with behavioral paradigms that have indicated that individuals with high autistic traits invest less effort to receive social engagement (Dubey et al., 2015; Dubey et al., 2017). Critically, we found no evidence to indicate that differences in choice latencies were driven by generalized motoric influences, or an impulsive decision-making process. This suggests that the mechanism for selection of social stimuli results from a higher-level evaluation of the stimuli, rather than on low-level influences or domain-general differences in task performance. Additionally, our analyses of gaze switches revealed greater uncertainty in selecting social stimuli for high AQ observers—an effect that was not replicated with scrambled stimuli.
Extending recent reports (Smith & Krajbich, 2018), we found substantial individual differences in the relationship between gaze and choice behavior. This finding emphasises the importance of modeling these individual differences, in line with recent proposals (Molter, Thomas, Heekeren, & Mohr, 2019). Critically, we found that AQ predicted a weaker relationship between gaze and choice behavior. In high AQ observers, an increase in gaze towards a stimulus predicted a smaller increase in the probability it would be chosen. Moreover, we found that gaze became predictive of choice later in the trial for high AQ observers.
What could be the mechanism that underlies these effects? One plausible interpretation is that in high AQ individuals, more sampling of, or exposure to a stimulus is required to support positive evaluations. This is consistent with observations from diverse strands of literature that indicate wider atypicalities in exposure-affect coding in ASD. For instance, psychophysiological studies indicate delayed habituation to novel stimuli in ASD—relative to neurotypicals, more exposure to stimuli is required before negative arousal is attenuated (Guiraud et al., 2011; Perry, Minassian, Lopez, Maron, & Lincoln, 2007). A related finding is that high autistic traits are also associated with an increased preference for familiar stimuli (Gustafsson & Papliński, 2004; Pellicano & Burr, 2012). Moreover, neuroimaging work has indicated reduced activity in the ventral striatum (implicated in temporal aspects of value coding) in response to rewards in ASD (Scott-Van Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010). At the behavioral level, some initial work has also indicated delayed “mere exposure” effects in ASD—liking ratings of individuals with ASD have not been found to scale with repeated exposures in the same way as NT individuals (Filliter, 2014).
One important thing to note is that the observed interactions between AQ and gaze on choice behavior were not modulated by stimulus type—indicating that the effects generalize beyond socially relevant stimuli. The generality of this effect may be consistent with neuroimaging data, which indicate that differences in reward coding in ASD are not restricted to social stimuli, and are more generalized than initially suspected (Dawson, Webb, & McPartland, 2005; Schultz, 2005). Why might a domain-general alteration in exposure-affect coding be associated with traits that index atypical social behaviors? The explanation may be simple. Borrowing concepts from classical conditioning, exposure to a stimulus alone may lead to the formation of a preference due to the simple absence of aversive consequences. Such a mechanism likely provides a primitive, fundamental basis for promoting engagement with social stimuli (Zajonc, 2001). The clearest demonstrations of this are phenomena such as imprinting effects, where neonates narrow their social preferences as a simple consequence of exposure (Bateson, 1966). Atypical, or delayed exposure-affect coding may therefore disrupt preferences for engaging with social stimuli. By extension, this reduced engagement has been proposed to impede learning of skills vital to successful for social functioning, such as language acquisition and face/emotion recognition (Johnson, 2005; Schultz, 2005).
In discussing diminished preferences for social stimuli, it is important not to neglect discussion of preferences for nonsocial stimuli—and to acknowledge that these are ‘two sides of the same coin.” For instance, another related strand of literature is concerned with the restricted and repetitive behaviors and interests associated with autism. Such behaviors, which involve an insistence on sameness and preoccupying restricted interests are well documented—but the neurobiological basis of such behaviors are still emerging (Kohls, Yerys, & Schultz, 2014). The rewarding effect of restricted-interest stimuli is thought to reflect a preference for predictability in the environment. Notably, such restricted interest stimuli are typically more stable and predictable than social stimuli, which are typically diverse and volatile (Kohls et al., 2014). Because such stimuli are more circumscribed, and possess more immediate and reliable reinforcement contingencies than social stimuli, preferences for these stimuli may be formed more readily in individuals with disrupted exposure-affect coding. Consequently, such restricted interest stimuli may co-opt the reward circuitry typically allocated to social stimuli (Watson et al., 2015) and hijack the typical trajectory of social-seeking behaviors. Thus, an important goal of future studies is to examine this inherent interplay between social and nonsocial reward responsivity throughout development.
In interpreting our findings, we reiterate that gaze both reflects and influences choice behavior in a mutually interactive manner. Although some authors have posited that gaze drives choice (Armel et al., 2008; Pärnamets et al., 2015), a challenge to this claim is that attention may merely reflect emerging preferences (Shimojo et al., 2003). There is good evidence, however, that gaze does not merely reflect preference, but itself is actively involved in forming preferences. For instance, directly manipulating gaze duration (not just exposure duration) can bias choice to the fixated location (Shimojo et al., 2003). Moreover, computational models wherein fixations guide the choice process can provide unique, precise predictions about idiosyncratic properties of choice behavior. For instance, modeling the role of fixations in the choice process can predict why preferences tend to be biased towards the first fixated item, why choice behaviors sometimes conflict with previous valuations of the stimuli (Krajbich, Armel, & Rangel, 2010) and why choice latencies are inversely proportional to the overall valuation of the two choice options (Smith & Krajbich, 2019). Thus, the gaze–choice relationship is bidirectional. It is therefore most sensible to interpret our data as reflecting individual variation in a positive feedback loop, within which gaze and preference are intrinsically linked.
Methodologically, our data emphasize the importance of including controls for low-level confounds in studies of social attention—we found that a measurable portion of observer preferences were driven by low-level properties of the stimuli (Figure 2A). This accords with an observation from a recent free-viewing experiment, wherein gaze bias toward social images was partly explained by low-level properties of the stimuli (Hedger, Haffey, McSorley, & Chakrabarti, 2018). Future studies of social attention should include control stimuli that allow simple low-level influences on behavior to be characterized.
It is noteworthy that our data appear inconsistent with an earlier eye-tracking study that observed no differences between ASD and NT groups in terms of gaze cascade trajectories or social choice behaviors (Gharib, Mier, Adolphs, & Shimojo, 2015). In context, however, the differences between this study and our own may outweigh the similarities. First, our study involved trials with direct competition between two complex social and nonsocial stimuli, whereas Gharib et al. implemented a comparison between two face stimuli. In addition, it is possible that our larger sample size and dimensional approach to the analyses may have had additional sensitivity to detect individual differences.
In interpreting our findings, it is important to do so in the context of several limitations. First, the choice behaviors in our experiment are a symbolic, and imperfect analogue of the relevant real-world social behaviors. For instance, if observers actually received what is represented by the preferred image (e.g., joining the interaction depicted in the image) then this would likely alter choice behavior. This is important in the context of a recent, novel study that indexed forced choices between actually engaging in (as opposed to simply observing) social and nonsocial activities, which found no difference between ASD and NT observers (Goldberg et al., 2017). By extension, it is important to acknowledge that the choice data presented here may be better described as reflecting responsivity to social rewards, rather than social motivation per se. As highlighted by recent reviews, behavioral studies indicating a lack of social motivation often conflict with the testimony of the individuals themselves (Jaswal and Akhtar, 2018).
It is also important to note that in addition to social reward responsivity, autistic traits are associated with other more generalized differences in perceptual and information-processing styles. Those relevant to our task may include a sharper attentional gradient (Robertson, Kravitz, Freyberg, Baron-Cohen, & Baker, 2013), impaired temporal integration (Nakano, Ota, Kato, & Kitazawa, 2010), and a precedence of a local over global processing style (Lowe, Stevenson, Barense, Cant, & Ferber, 2018). In the absence of robust measures of these domains, we were unable to formally characterize their role on gaze and choice behaviors. Finally, it is important to note the inherent social context that might color the valuation of nominally “nonsocial” stimuli. Stimulus categories such as food and money have their own inherent value—but part of their valuation is linked to social interactions. Part of the valuation of money may relate to social exchanges, and food valuations may relate to the association with social gatherings. Despite these interpretive cautions, the data indicate that the responsivity to these reward categories and the relation between gaze and choice behavior vary as a function of autistic traits.
In summary, our data demonstrate that autistic traits predict reduced and delayed selection of social stimuli during choice. Critically, during such choices, autistic traits predicted a weaker and delayed relationship between gaze and preference. Considered with previous findings, this result implies that domain-general atypicalities in exposure-affect coding may contribute to atypical social functioning observed in individuals with high autistic traits.
Acknowledgments
The authors declare that they have no conflict of interest related to the work reported in this manuscript. The authors would like to acknowledge support from the Leverhulme Trust for this work. The first author was funded by a Philip Leverhulme prize awarded to the second author and by an early career fellowship (ECF-2019-305). The authors would like to thank Dr Anthony Haffey for his role in maintaining the database of volunteers for autism research. We would also like to thank Dr Martina Fusaro and Dr Valentina Fanti for administering the IQ tests to the participants in this study.




