Air-breathing ecology of Arapaima sp.: Conservation implications for an imperilled fish
Funding information: Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: #313183/2014-7; Instituto Piagaçu, Grant/Award Number: "Peixes da Floresta" project sponsored by Petrobras Environmental Program
Abstract
- Arapaima (Arapaima sp.) are highly overexploited fish endemic to the Amazon basin. Because the fish are obligate air-breathers, it is possible to use surface-breathing events to count individuals visually for population censuses important for conservation, yet uncertainties remain about body size and environmental influences on air-breathing intervals, and thus count accuracy.
- This study examined relationships between breathing intervals and environmental parameters (e.g. water temperature and transparency) and body size for radio-tagged arapaima (n = 12) in an upland river-floodplain (Lake Ayapuá, Amazonas, Brazil). Generalized additive mixed models were used to evaluate environmental, size, and behavioural correlates of breathing intervals.
- Temperature was the most influential predictor of air-breathing intervals, followed by body size. The shortest breathing intervals were associated with consecutive ‘aggressive’ breaths while the longest breathing intervals had consecutive ‘calm’ breaths. Type of breath, size, and temperature predictors revealed that breathing intervals ranged from 4 to 46 min and were not significantly different among life stages (
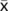 = 15.9 min for sub-adults and adults (>1 m);
= 15.9 min for sub-adults and adults (>1 m);
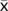 = 14.8 min for juveniles (<1 m)).
= 14.8 min for juveniles (<1 m)). - Whereas the current population census method uses fish counts in 20-min intervals, this study found that 15% of observed breaths, and two thirds of fish, took longer than 20 min to breathe. These findings were obtained in relatively cool-water environments, so it is recommended that future population census methods consider water temperature (e.g. extend intervals used for counts in cooler waters), which may improve the accuracy of census counts and thus further enhance arapaima conservation efforts.
- This study demonstrated an effective method in which fundamental biological information is used to inform and improve population census methods for an imperilled fish in a region where traditional stock assessment is ineffective. Similar approaches for adaptive stock assessments could be applied to improve conservation of other air-breathing fishes (e.g. lungfishes) globally.
1 INTRODUCTION
Many of the estimated 450 species of air-breathing fishes occur in the tropics (e.g. Arapaima sp. and Channa sp.), where aquatic habitats are being subjected to some of the most rapid rates of environmental degradation on the planet (Graham, 1997; Barlow et al., 2018). The surfacing behaviour of many air-breathing fishes offers unique opportunities to assess their populations for conservation management purposes. Although not commonly applied to air-breathing fishes, surfacing counts can be used to estimate their abundance as is routinely done for other aquatic species (e.g. marine mammals and sea turtles; Seber, 1982; Pollock et al., 2006; Seminoff et al., 2014). Robust visual census techniques require knowledge of surfacing behaviour and the responses of species to environmental conditions. Whereas individual activity patterns and metabolism drive the demand for oxygen and thus the frequency of surfacing events to acquire air, external factors (such as water temperature) may influence the air-breathing interval of fishes (Fry, 1971; Graham, 1997). An understanding of the factors that affect the breathing behaviour of air-breathing fishes is necessary to improve count accuracy and thus improve population assessments and conservation actions.
The most important environmental factor influencing metabolism in fishes is water temperature (Brett, 1971; Fry, 1971). Increased metabolism, and probably frequent respiration and decreased breathing intervals in air-breathing fishes, is positively associated with water temperature (Fry, 1971). In the case of visual predators, which are often more active at dawn and dusk, reduced air-breathing intervals may occur in response to increased energy expenditures for foraging during low light (Graham, 1983; Graham & Baird, 1984; Guthrie, 1986). High turbidity is known to reduce prey capture efficiency and increase energy expenditure for feeding activities of visual predators (Gregory & Levings, 1998; Reid, Fox & Whillans, 1999), so it too could reduce breathing intervals. Air-breathing is also influenced by body size, as oxygen consumption is a positive function of body mass (Winberg, 1956). Among continuous, obligate air-breathing fishes, which require surface respiration for normal function, the way surface breathing occurs can also vary (Graham, 1997). Surfacing may occur rapidly to avoid predation threats, aggressively as a defence or warning mechanism, or synchronously as a means of communication (de Lima Filho et al., 2012). Higher energy demands required for vigorous or aggressive surfacing can be expected to require higher metabolism, and thus more frequent breathing.
Although previous studies have established that the most notable determinant of oxygen demand, and therefore breathing interval, is metabolic function (Graham, 1997), the extent to which other environmental and intrinsic factors influence air-breathing intervals in fish remains largely unstudied. Despite the occurrence of air-breathing in many fishes, most studies on air-breathing of fishes to date have been done in laboratory conditions (e.g. Stevens & Holeton, 1978; Seymour et al., 2004; Fernandes et al., 2012), have evaluated the effect of only one factor at a time (e.g. Tuong et al., 2018) and have tended to focus on small-bodied species or small specimens of large-bodied taxa (e.g. Randle & Chapman, 2005). The general applicability of many such findings to wild populations has yet to be established.
Here, in contribution to the conservation of air-breathing fishes, the influence of environmental characteristics and body size on the air-breathing behaviour of one of the most imperilled air-breathing species were examined in natural environments. Arapaima spp. (Teleostei, Arapaimidae) primarily inhabit the Amazon River basin, growing to up to 3 m in length and 200 kg in weight (Arantes et al., 2010). They are mostly piscivorous but display some omnivory, relying on vision to locate prey (Queiroz, 2000; Watson, Stewart & Teece, 2013; Carvalho et al., 2018). They typically inhabit floodplain lakes in the dry season, move to the flooded forest to feed and reproduce as waters rise, and migrate back to the main channel and lakes as waters recede (Castello, 2008a).
Owing to their large size and high economic value, arapaima are one of the Amazon's most overexploited fishes (Castello et al., 2015). Overfishing led Arapaima gigas to be listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in 1975 and as ‘data deficient’ by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (World Conservation Monitoring Centre, 1996). Although many populations of arapaima are overexploited, declining or even considered locally extinct, some are recovering as a result of a conservation management system implemented by fishers over the past 20 years (Campos-Silva & Peres, 2016; Petersen et al., 2016). In this system, fishers use visual and acoustic cues to count the number of individuals at the time of their surfacing (air-breathing) for 20-min periods in lakes during the dry season (Castello, 2004). Counts of arapaima are not made during windy or rainy conditions and require fishers to remain silent without disturbing the fish (Castello, 2004). In collaboration with local governmental and non-governmental organizations, including the regional fisheries agency (IBAMA), fishers and associated stakeholders then jointly use the count data to determine conservative harvest quotas. Nearly 500 fishing communities in the State of Amazonas, Brazil, are currently managing arapaima using this approach (Castello et al., 2009), but it was developed in a single ecosystem (a seasonally flooded whitewater floodplain) with near-constant water temperatures (mean, 30.1°C; Castello, 2004) and without consideration of environmental influences (such as temperature variability) on count accuracy. The widespread use of a method developed from a single study suggests that there is an urgent need to assess the extent to which external and internal influences (e.g. environmental conditions and body size) affect the air-breathing behaviour (e.g. breathing intervals) of arapaima in natural environments.
Arapaima are adapted to the oxygen-poor waters and dynamic flooding cycles of the Amazon River basin by relying on continuous surface breathing (Graham, 1997). Field observations suggest that adult arapaima surface every 10–15 min (not longer than 40 min) and that breathing intervals increase with size (e.g. larvae < 7 min and juveniles 8–9 min) (Fontenele, 1948; Romero, 1960; Lüling, 1964; Bard & Imbiriba, 1986). Beginning 8–9 days after hatching, arapaima rely primarily on atmospheric air for respiration using a highly vascularized swim bladder for oxygen attainment and small gill structures for carbon dioxide excretion (Lüling, 1964; Farrell & Randall, 1978; Brauner & Val, 1996; Brauner et al., 2004). As such, air-breathing in arapaima is likely to be influenced primarily by physicochemical factors other than dissolved oxygen (e.g. temperature), or by swimming activity (e.g. the way in which they surface). Such expected influences may explain distinct surfacing characteristics of arapaima, which have been described as calm, normal and aggressive (Pezo, 2015; Organización de Manejo de Recursos Naturales [ORMARENA], 2010). Surfacing descriptions reflect the movement of a fish to exchange air, including the way in which the body breaks the surface, its behaviour at the surface and the way in which it submerges back into the water. The relationship, if any, between these surfacing types and air-breathing intervals remains unknown.
This study was motivated by local fishers who manage arapaima in a headwater stream with a gradient of water temperatures and believe that the fish take longer than 20 min to breathe when found in relatively cooler pools located in the upper sections of the stream. Thus, they requested an evaluation of whether arapaima breathing exceeds the 20-min counting protocol and if so, how they should adapt their counting method to ensure accuracy. Accordingly, this study aimed to: (i) examine the effects of water temperature, transparency, body size, time of day, and day of year on arapaima air-breathing intervals; and (ii) examine the effect of surfacing characteristics on air-breathing intervals. It was expected that (i) breathing intervals would be positively associated with body size and water transparency, negatively associated with temperature and, as a result of temperature associations, would be correlated with both time of day and day of year; and (ii) breathing intervals would be positively associated with calm breathing and negatively associated with aggressive breathing.
2 METHODS
2.1 Study area
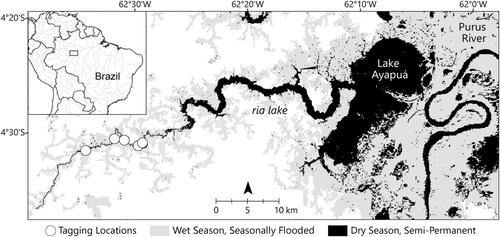
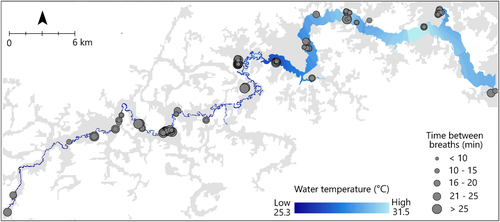
This study was conducted in the Piagaçu-Purus Sustainable Development Reserve (State of Amazonas, Brazil), which is located south of the confluence of the Solimões and Purus rivers (Figure 1). Lake Ayapuá is situated on the floodplain of the lower Purus River, a whitewater river characterized by high turbidity (20–40 NTU), nearly neutral pH (6–7), high productivity, warm waters, and abundant surface vegetation (Junk et al., 2012; Ríos-Villamizar et al., 2017). The lake is an oblong, shallow, turbid water body bordered by seasonally flooded lowland forest (várzea). It is connected to the Purus River downstream and is partially filled by slow-flowing streams characteristic of a blackwater river (e.g. acidic, less turbid, and cooler waters than typical whitewater rivers); such is representative of environments common to Amazonian lowlands (Venticinque et al., 2016). The area forms a narrow valley (ria) lake with upland habitat (terra firme) on both sides of the main channel and many connective side channels (igarapes; Figure 1). Ria lakes are found throughout low-sloping, blocked valleys of the Amazon (e.g. Tapajos, Xingu, Tefé, and Coarí) (Latrubesse, 2012). All data in this study were collected in a stretch of approximately 85–110 km (dry–flood season) of the ria lake between 11 January and 29 November 2015 (Figure 1).
2.2 Study taxa
To date, five nominal species of arapaima have been described based on morphological differentiation, which is probably a result of geographically distinct areas in tributaries of the Solimões and Amazon rivers (Stewart, 2013). How many species exist and the precise distribution of each is not known, although it is suspected that there may be additional undescribed species (Stewart, 2013). For this reason, and because no genetic or taxonomic work on arapaima has been done where this study took place, the study taxon will be referred to simply as Arapaima sp.
2.3 Fish tagging
Sixteen arapaima (total length, TL = 119 ± 26 cm; range = 80–170 cm) were captured using a seine net (140 × 6 m) in three pools distributed along the headwaters of Lake Ayapuá in November 2014 (Figure 1). Upon capture, arapaima were placed to recover in a partially flooded canoe and were monitored until air-breathing intervals normalized to approximately every 10–15 min. Total length was measured from the tip of the lower jaw along the lateral curvature of the body to the nearest 1 cm. Two to four scales were removed from the ventro-lateral flank slightly anterior to the anus and a local anaesthetic (topical benzocaine 20%) was applied. Two tags were placed on each fish: one radio transmitter (MCFT2-3EM (Lotek Wireless, Canada) at 20 pings per minute or Pisces (Sigma Eight, Canada) at 60 pings per minute) was inserted into the peritoneal cavity following standardized tagging procedures (Wagner et al., 2011) and one anchor tag (FT-1-94, Floy Tag & Mfg. Inc.) with a unique fish identification number was inserted proximal to the base of the dorsal fin. The mass of the radio tag relative to the body mass of fish was negligible and much less than the generally used 2% rule (estimate was <0.1%). After tagging, each fish was monitored until it regained sufficient strength to swim away (typically 5–10 min), at which point it was released. Tagged fish were closely monitored after release by tracking for approximately 60 min. All capture and tagging methods were approved by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University (IACUC #14-085) and the Sistema de Autorização e Informação em Biodiversidade (SISBIO #6289511).
2.4 Radio telemetry
Individually radio-tagged arapaima were monitored using active tracking performed daily by boat using a 3-element Yagi antenna connected to a portable VHF radio receiver (BioTracker, Lotek Wireless Ltd, Canada). Each month during the study period (January–November, 2015), a minimum of 150 h and 15 days of active tracking of all tagged fish were completed. The entire navigable study area upstream of Lake Ayapuá was searched at least twice monthly.
The expansive study area required the use of a motorized boat to locate the general area of the fish. As this could introduce disturbance during the approach, once a tagged fish was detected via telemetry the field assistant disengaged the motor to minimize disturbance and paddled silently, ideally within a distance enabling visual detection. Each tagged fish that was encountered was observed for a minimum of 30 min or three breathing events, whichever came first. Observations were made until the fish swam out of reliable detection range or was no longer able to be detected. If any noise was created by the field team, or if other human disturbance occurred, all subsequent breathing data were discarded and counts for breathing events began again, starting at zero min, at least 20 min after disturbance. At the time of each surfacing event, water temperatures (°C; Professional Plus Multiparameter, YSI Ltd, USA) were recorded at 0, 2, and 4 m (Table 1). Such depths are representative of the <3.8 m depths most commonly used by arapaima (Castello, 2008a). Water transparency (cm) was measured at the same time as temperature with a Secchi disk. Breathing counts were performed according to established conditions (i.e. under silent conditions, with no disturbance; Castello, 2004) with the exception of the required use of a motorized boat as the only feasible method to search for a tagged fish. An attempt to relocate the fish later in the day and on consecutive days was made until it was no longer detected. All observations occurred between 0800 and 2200 h daily. To ensure reliability of fish detection and breathing events, observations made in low-light conditions required that no other fish be observed in the area. If no other fish were observed after 20 min, data for the tagged fish were recorded.
| Predictor | Description | Range | Mean ± SD |
|---|---|---|---|
| Temperature | Mean of measures at 0, 2 & 4 m | 22.8–35.5°C | 27.67 ± 1.79°C |
| Day of year | Day of calendar year from 01/01/2015 | 13–310 | 131.42 ± 86.49 |
| Time of day | Hour of day | 8:03–21:43 | 12:39 ± 2.91 min |
| Transparency | Water clarity from Secchi disk | 31–153 cm | 95.89 ± 22.57 cm |
| Total length | Total length of tagged fish | 80–145 cm | 103.77 ± 18.83 cm |
| Type of breath | Identified by local classification system | Calm–aggressive | -- |
One primary knowledgeable fisher assisted in the confirmation of a breathing event for the duration of the study. A breathing event was detected by audible or visual observation using a telemetry fix and the fisher's knowledge of breathing characteristics. Time of breath was recorded as the time the fish was visually or aurally determined to have broken the surface of the water. Breathing interval was calculated as the time between consecutive surfacing events. A measure of confidence was recorded (0 = uncertain, 1 = certain) to indicate whether the observers felt certain that a breathing event was associated with the tagged individual fish under observation. If there was uncertainty caused by a large number of other fish in the area, high amounts of fish movement, weather interference or other factors, observers recorded the breathing event as uncertain (0). All uncertain events were excluded from the analysis.
Type of breath was recorded using the surfacing characteristics of arapaima: calm (local name: mansa), normal (normal), and aggressive (braba). For the purposes of this study and owing to difficulties associated with distinguishing between them, normal and calm breath types were considered together as a single breathing type. A calm or normal breath referred to a small, tame surfacing event where the fish broke the surface in a gentle manner for a gulp of air. The head and dorsal area surfaced briefly before the fish resubmerged. An aggressive breath was distinguishable by a loud smacking noise made by the tail slapping the water after surfacing. Aggressive breathing events are often associated with a visible large splash and are heard from a distance. This study examined a change (or lack thereof) of breathing intervals as a function of surfacing characteristics of consecutive breaths. As such, the data were organized as observations consisting of the combination of consecutive breaths. This resulted in four possible categories for two consecutive breathing events: ‘aa’ (aggressive–aggressive); ‘ac’, (aggressive–calm); ‘ca’ (calm–aggressive): and ‘cc’ (calm–calm).
2.5 Data analysis
Data exploration included examination of Cleveland and box-and-whisker plots to identify outliers and influential points and a visual assessment for the assumed data distribution (Zuur, Ieno & Elphick, 2010). Variance inflation factors were examined to check for collinearity, and variograms were used to assess spatiotemporal autocorrelation in model residuals. No collinearity or autocorrelation issues were found based on, respectively, variance inflation factors <5 and visual examination of correlation and semi-variogram plots.
2.5.1 Environmental and body size effects on breathing intervals
 ()
()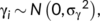

All possible subsets of the global model were fitted to the data and ranked by corrected Akaike information criterion (AICc) values. All models included in the 95% confidence set for the best model were used for generating model-averaged predictions and unconditional 95% confidence intervals (Burnham & Anderson, 2002). Marginal and conditional R2 values were calculated to determine the amount of variability explained by fixed and random effects in each of the models in the 95% confidence set (Nakagawa & Schielzeth, 2013). The ‘zero method’ was used for model averaging, which assumes that a given variable is included in every model but sets coefficients to 0 if not (Grueber et al., 2011). Data for each prediction were generated using the minimum and maximum observed values for the covariate of interest while holding all other values at a constant mean. The exception to this was total length, where the maximum was set beyond the measured limit within the dataset in order to predict breathing interval over a larger range of sizes.
2.5.2 Influence of surfacing characteristics on breathing interval
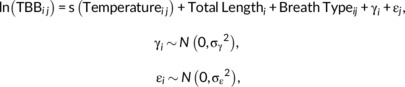 ()
()All statistical analyses and computations were performed using R (version 3.3.0) software (R Core Team, 2016). Models were fitted using the package ‘mgcv’ (mixed generalized additive model computation vehicle) and model selection and model averaging were conducted using package ‘MuMIn’ (Barton, 2016).
3 RESULTS
In total, 281 breathing events (201 breathing intervals) from 12 individual fish were observed during 1,425 h of telemetry effort conducted over 180 days throughout the 11-month study. The median number of total breathing events per fish was 13 (range = 2–65 breaths per fish). The median time spent with each fish per encounter was 27 min (range = 15–139 min). The median breathing interval across all individuals was 14.0 ± 7.1 min (range = 4–46 min). Mean breathing interval was 15.9 ± 8.3 min (range = 5–46 min) for juveniles (<1 m total length, n = 117) and 14.8 ± 6.0 min (range = 4–41 min) for sub-adults and adults (>1 m total length, n = 84). Of all observed breathing intervals, 15% (n = 31) were longer than 20 min and were observed for eight of the 12 fish tracked in the study. Longer breathing intervals (>20 min) occurred throughout the day (range = 9:06–21:26). There was no distinct size association for fish that breathed longer than 20 min (range = 87–145 cm).
Temperature and total length were the most important factors associated with breathing interval (Table 2). Temperature was included in all the models in the 95% confidence set, and the addition of total length only added a small weight relative to the second-ranked model (Table 2). Temperature, total length, and time of day contributed at least 0.10 of the model weights; collectively the top three models with these predictors contributed more than 50% of cumulative model weights (Table 2). Conditional R2 and marginal R2 values differed by less than 0.005 and thus only marginal R2 values are reported. Marginal R2 values of top models ranged from 0.059–0.105 and indicated that only 6–10% of the variation is explained by the covariates (a similar amount of variability was explained by variability among individuals). An estimated 84% of the variability was unexplained by the models.
| Candidate models | k | Loglik | AICc | ∆i | wi | acc wi | ER | Mar. R2 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | s(TM) + TL | 6 | −113.7 | 239.9 | 0.00 | 0.22 | 0.22 | 1.00 | 0.084 |
| 2 | s(TM) | 5 | −115.0 | 240.4 | 0.52 | 0.17 | 0.39 | 1.29 | 0.059 |
| 3 | s(TM) + s(TD) | 7 | −113.1 | 240.9 | 1.00 | 0.14 | 0.53 | 1.57 | 0.074 |
| 4 | s(TM) + s(TR) + TL | 7 | −113.6 | 241.8 | 1.98 | 0.08 | 0.61 | 2.75 | 0.083 |
| 5 | s(TM) + s(TD) + TL | 8 | −112.6 | 241.9 | 2.08 | 0.08 | 0.69 | 2.75 | 0.078 |
| 6 | s(TM) + s(DY) + TL | 8 | −112.8 | 242.2 | 2.39 | 0.07 | 0.76 | 3.14 | 0.093 |
| 7 | s(TM) + s(TR) | 6 | −115.0 | 242.5 | 2.64 | 0.06 | 0.82 | 3.67 | 0.059 |
| 8 | s(TM) + s(TD) + s(TR) | 8 | −113.2 | 243.1 | 3.21 | 0.05 | 0.87 | 4.40 | 0.072 |
| 9 | s(TM) + s(TD) + s(TR) + s(TD) × s(TM)) | 11 | −110.1 | 243.7 | 3.80 | 0.03 | 0.90 | 7.33 | 0.105 |
| 10 | s(TM) + s(TD) + s(TR) | 9 | −112.6 | 244.1 | 4.21 | 0.03 | 0.93 | 7.33 | 0.072 |
| 11 | s(TM) + s(TD) + s(TR) + TL | 9 | −112.8 | 244.4 | 4.57 | 0.02 | 0.95 | 11.00 | 0.078 |
- Note: The covariates are temperature (TM), total length (TL), time of day (TD), day of year (DY), and transparency (TR); k is the number of parameters in the model, Loglik is the log-likelihood, AICc is the Akaike information criterion corrected for small samples, ∆i is the difference in AIC between model i and the top ranked model, wi is the AICc weight, acc wi is the cumulative AICc weight, ER is the evidence ratio computed as wj/wi where wj is the weight of the top model (1) and wi the weights of the consecutive models (2–11), and Mar. R2 is the marginal R2 values. The smoothing function s() is denoted when utilized for covariates.
Of all measured covariates, temperature had the greatest effect on breathing interval and was described by a non-linear relationship (Figure 2a). After a small increase between 22.5 and 25°C, breathing intervals decreased between 25 and 35°C (Figure 2a). Breathing interval was predicted to decrease by 4.2 min between 25 and 30°C, and 2.4 min between 30 and 35°C for fish of average size in the study. Predicted breathing intervals were 16 min at 25°C to 9.4 min at 35°C for fish of average size in the study (Figure 2a). Visual examination of the temperature gradient in the study area showed longer average breathing intervals in colder upstream waters and a higher number of shorter intervals nearer to the mouth where the warm lake waters mix with the headwaters (Figure 3).
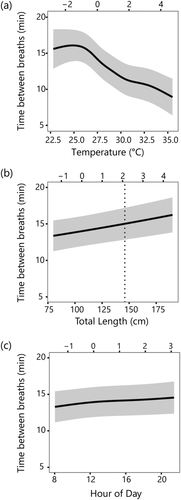
Predictions for breathing interval showed a positive relationship with total length of fish (Figure 2b). Breathing interval was predicted to increase by 2.5 min between fish of lengths 80 cm (13.5 min) and 180 cm (16 min). The length relationship predicted that for every increase of 0.25 m, breathing interval would increase by approximately 0.6 min. Results also showed a very small decrease in breathing interval during the early morning hours, followed by a small increase in breathing intervals throughout the rest of the day and evening (Figure 2c). No obvious associations were observed for water transparency and seasonality (day of the year; see Supporting Information). The model was further improved by the addition of type of breath, with an AICc value (163.45) that was 3.85 lower than the model with only temperature and total length (AICc = 167.30). The addition of type of breath in the model increased the marginal R2 from 0.12 to 0.16. Like the first analysis, the conditional R2 value was very similar to the marginal R2 value in both models.
The data used to examine the effects of type of breath on breathing intervals contained 85% of surfacing events characterized by calm breathing (n = 153) and 15% (n = 28) characterized by aggressive breathing. Predicted breathing interval were the shortest (mean = 12 min, range = 6–22 min) for sequential aggressive breaths, whereas sequential calm breaths yielded the longest (mean = 18 min, range = 10–34 min) predicted breathing intervals (Figure 4a,b). A change in breath from calm to aggressive or vice-versa had the widest range of breathing intervals (8–30 min; Figure 4a,b). For example, a 1.5-m fish at 30°C is predicted to breathe every 12 min when consistently aggressive (aa), every 18 min when consistently calm (cc), and every 16 min when breath type changes (ac or ca; Figure 4a).

4 DISCUSSION
This study was conducted in a near-pristine tropical river environment on a sample of individuals with a wide range of body sizes. Six environmental variables were measured concurrently during fish observations and spanned a full annual fluvial flooding cycle. The results therefore provide a baseline for understanding the influence of environmental variables and body size on breathing intervals of a wild arapaima population. Results indicated that, in addition to previously understood metabolic influences, environment is an important determinant of air-breathing intervals. More specifically, this study showed that (i) of the considered variables, water temperature and body size had the greatest effect on the air-breathing intervals of arapaima; (ii) consecutive aggressive surfacing events were associated with shorter breathing intervals, whereas consecutive calm surfacing events were associated with longer breathing intervals; and (iii) whereas time of day had a small effect on air-breathing interval, water transparency and time of year exhibited no relationship with air-breathing intervals. Importantly, results support the predictions of local fishers: temperature has the greatest influence on breathing intervals, some fish take longer than 20 min to breathe, and, as such, adaptive counts should be considered.
The non-linear relationship found between temperature and air-breathing intervals showed intervals increasing slightly to a maximum near 25°C, then decreasing sharply. One possible explanation for this non-linear relationship is the association of physiological exertion (e.g. breathing intervals) and conditions outside the bounds of the normal range (e.g. very high or very low temperatures). Water temperature of this study area (average = 27.7°C, 2014–2015) was at the lower end of the typical seasonal temperature range (27–31°C) for floodplain lakes, probably because of coldwater inputs from upland streams in the headwaters of the lake (Melack & Forsberg, 2001). Increased breathing intervals in cooler waters, particularly in the far upstream reaches, is consistent with reductions in oxygen demand, as temperature decreases metabolically driven oxygen demand, thus resulting in longer breathing intervals than seen elsewhere in the Amazon (Val, de Almeida-Val & Randall, 2006).
The positive relationship found between breathing interval and body size may be supported by the known scaling relationship between oxygen consumption and body mass of fish or that larger fish are capable of gulping proportionately more air per breath than juveniles (Tytler & Calow, 1985; Clarke & Johnston, 1999). Although an overall linear trend is seen, this study reveals highly variable breathing intervals for individual fish. A very small change in R2 when considering interindividual variability implies that there is large within-individual variability. For example, breathing intervals for one fish spanned a 41-min difference between the shortest and the longest breaths (TL = 87 cm), suggesting the influence of other factors. Additional considerations for size-related breathing variability include synchronous breathing in juveniles (<1 m) as a predation reduction strategy (Olsen, 2014) and reproductive investment strategies (i.e. gonadal maturation, digging nests, guarding eggs, and protecting young) in mature adults (>1.5 m) in the late dry season (~Nov-Dec) (Castello, 2008b).
Model-averaged predictions suggest that time of day had a small effect on breathing intervals, with breathing intervals slightly decreasing in the early morning hours, possibly as a result of increased foraging or energetic costs of digestion (specific dynamic action). This result is consistent with previously reported feeding activity of arapaima in the pre-dawn hours (Watson, Stewart & Teece, 2013). Indeed, as predators, it may be more efficient for arapaima to hunt when low light conditions in the early morning reduce the chance of detection and evasion by prey.
An additional 4% of variability was explained by the addition of the type of breath. This indicates that the time between surfacing events can be influenced by the behavioural state of the fish. Fish with two consecutive aggressive breaths were observed breathing more rapidly. This may be explained in one of three ways: (i) the fish may expend more energy to break the surface and slap its tail; (ii) the fish is breathing aggressively from a stimulus, which increased metabolic rates; or (iii) the fish gulps less air per breath when breathing aggressively, which could force the individual to come to the surface sooner. In many cases, aggressive breathing began suddenly, perhaps because of the onset of human presence, predators (in the case of juvenile arapaima), or boat traffic. In such cases where an aggressive breath results from being startled or frightened, the total amount of air consumed may be reduced. The large decrease in mean breathing intervals (18 to 12 min) from consecutive calm to consecutive aggressive breaths indicates that external influences (e.g. boat noise and fishing activity) may cause rapid and highly dynamic changes in behaviour and metabolism of arapaima.
Although some of the variability in breathing interval is explained by covariates considered in this study, much of the variability remains unexplained (up to 84%). The small portion of variability explained by the models warrants further study of other important variables that influence air-breathing intervals. One possible explanation for this is the influence of other correlates that may (i.e. other environmental correlates such as habitat structure; behavioural correlates such as cycles of foraging activity) or may not (i.e. interactions such as social behaviour between fish, human disturbance and rapidly changing environmental conditions) be measurable under field conditions. Future studies could consider collecting data from the shore or by accelerometer biologgers (see Lennox et al., 2018, for an example on arapaima), or targeting specific length classes, to measure environmental and interaction effects more precisely.
4.1 Conservation implications
The results of this study yield two important conservation implications: (i) the consideration of water temperature and surfacing type for increased accuracy in arapaima population censuses, and (ii) an example of how the environmental influences on biological traits can be used for adaptive management of an imperilled species. First, results imply that accounting for temperature during arapaima population counts can improve the accuracy of the resulting estimates of abundance. As such, the effect of temperature on breathing intervals should be considered in the future. In Lake Ayapuá, there is a difference of 8–10°C from the mouth of the lake to the furthest upstream reaches. In this study, 15% of fish took longer than 20 min to breathe, with breathing intervals being shorter closer to the lake and longer in the furthest upstream reaches. When using the 20-min protocol, fishers may be more likely to accurately count fish nearer to the lake and more likely to under-count fish in the upstream reaches of the study area. Under-counting would result in lower quotas, resulting in a precautionary approach to harvest limits and lost economic opportunity. Based on the results of this study, if average water temperature near the surface is less than 28°C, fishers could increase the duration of breathing counts. An increase of 5 min would be inclusive of as many as 93% of fish, according to the data from this study, where, on average, 6.8% of fish (n = 18) took longer than 25 min to breathe. As such, and as discussed with local fishers in the area, counts performed in the farthest upstream reaches could be made over a 25-min period, to improve count accuracy.
In addition, the results imply that surfacing characteristics could be considered in population counts, as this study shows them to have some influence on breathing intervals. Consecutive aggressive breaths were associated with shorter breathing intervals. Thus, if aggressive breathing is linked to human disturbance, the most reliable counts would come from undisturbed conditions (i.e. without talking, boat traffic, fishing activity, or entry into water). Similarly, if any change in breathing behaviour is seen (e.g. calm to aggressive), breathing intervals may shorten, thereby increasing the likelihood that fishers cannot distinguish single individuals that surface multiple times during the 20-min counting period. Such situations could inflate population estimates, resulting in higher harvest quotas and hence an increased risk of overharvest. It is thus important that counts be conducted with only minimal disturbance.
Second, results of this study demonstrate that understanding environmental influences on biological traits can improve conservation management strategies for air-breathing fishes. To the authors' knowledge, no other air-breathing fishes are managed using surfacing events, but such approaches may be feasible. Although few obligate air-breathing fishes (e.g. lungfishes, polypterids, electric eels, and snakeheads) are commercially important, many involve conservation concerns and population assessments. If the effects of environmental factors and body size on other species were examined as was done in this study, a management protocol using abundance indices based on air-breathing events could be developed. Accurate counts for facultative air-breathers may require a comprehensive assessment of local environmental conditions, including oxygen concentration. In North America, two air-breathing species of relevance include gar (family Lepisosteidae) and bowfin (Amia calva). Studies of air-breathing intervals for bowfin suggest a similarly positive relationship with temperature and that shorter breathing intervals occur at night (Horn & Riggs, 1973). Similar to arapaima, gar have a vascularized swim bladder for respiration, although their facultative air-breathing behaviour has not been widely studied (Smatresk & Azizi, 1987). Globally, the results of this study could be useful in the case of other imperilled air-breathing fishes such as Clarias magur (wagur), Megalops atlanticus (tarpon), and Channa diplogramma (Malabar snakehead).
Most of the estimated 450 species and 49 families of air-breathing fishes occur in the tropics, which are undergoing some of the most rapid changes on Earth from human influences. In the face of accelerated climate change with predictions for warming in most fresh waters where fish reside (Ficke, Myrick & Hansen, 2007), effective fish conservation requires knowledge of physiological traits, such as air-breathing behaviour, and their relationship to environmental characteristics. It is of growing importance, therefore, to design and implement conservation strategies for tropical air-breathing fishes while considering the knowledge that local fishers can contribute to the process. To that goal, this study provided fundamental ecological knowledge useful in developing conservation monitoring schemes based on air-breathing behaviour as was done for arapaima. Such an approach remains largely under-utilized, despite its potential to suppress overharvesting while supporting fishing communities and sustainable fish populations.
ACKNOWLEDGEMENTS
Gerson Pinheiro, the local field assistant, provided essential support for the entirety of the study. Instituto Piagaçu staff members F. Rossoni and S. Brum, and community leaders I. Bastos (Padre), S. Guerreiro de Brito (Assis), and M. Jorge assisted in field coordination and monitoring support. Many local fishers assisted in data collection and tagging efforts. This study was funded by Instituto Piagaçu's ‘Peixes da Floresta’ project via Petrobras Environmental Program. J.Z. received a productivity grant from CNPq (#313183/2014-7).
CONFLICT OF INTEREST
The authors declare no conflict of interest.




