A critical reflection on the success of rearing and culturing juvenile freshwater mussels with a focus on the endangered freshwater pearl mussel (Margaritifera margaritifera L.)
ABSTRACT
- Freshwater mussels are in decline throughout their range. The lack of natural recruitment in freshwater pearl mussel populations and other freshwater molluscs has led to controversies about the usefulness and applicability of captive breeding techniques for their conservation.
- The current state of rearing and culturing programmes for freshwater mussels in Europe and North America is reviewed here with a focus on the endangered freshwater pearl mussel (Margaritifera margaritifera). Different strategies of propagation and the key factors of success are addressed and conservation management decisions are discussed with respect to risk reduction and the intensity of the culturing system.
- Based on the rearing techniques applied for multiple species in North America, and for the genus Margaritifera in European countries, sufficient numbers of juveniles can be produced to sustain selected populations. However, captive breeding and stocking should be carefully documented and must not replace the restoration of functional stream habitats.
-
From a conservation point of view, captive breeding of endangered mussel species can be a last-minute rescue tool in order to retain the evolutionary potential of priority populations which would not persist long enough to benefit from habitat restoration practices.
Copyright © 2011 John Wiley & Sons, Ltd.
INTRODUCTION
Integrated concepts for the conservation of freshwater biodiversity are urgently needed (Geist, 2011). Endangered freshwater molluscs, particularly freshwater pearl mussels, are considered target species for the conservation of stream ecosystems since they simultaneously fulfil the criteria of flagship, indicator, keystone and umbrella species concepts (Geist, 2010). A lack of recruitment in freshwater pearl mussel (Margaritifera margaritifera L.) and other bivalve species has led to controversies about the usefulness of (semi-)artificial propagation and captive breeding in the context of their conservation (Barnhart, 2006; Preston et al., 2007). In a recent publication, Schmidt and Vandré (2010) investigated the survival and growth of captive-bred juvenile pearl mussels exposed to different types of cages and holding systems. They observed low survival rates and inconsistent variations in survival, questioning the suitability of these rearing approaches for conservation and bioindication purposes. On the other hand, there are several successful examples of mussel rearing and culturing from various countries and species.
The objective of this contribution is to review the current state of rearing and culturing programmes of freshwater pearl mussels in Europe and related freshwater bivalve molluscs in North America, and to discuss the key factors that distinguish successful from less successful approaches. Based on this information, conservation management decisions of captive breeding and habitat restoration are addressed with respect to risk reduction and the intensity of the culturing system.
EXISTING REARING AND CULTURING PROGRAMMES FOR FRESHWATER MUSSELS
Over the last two decades a large increase in initiatives and programmes of captive breeding for highly threatened mollusc species has been observed worldwide (Strayer et al., 2004; Barnhart, 2006; Thomas et al., 2010). Tables 1 and 2 provide a summary of past and present examples of (successful) artificial rearing of freshwater mussel species in North America and Europe both with regard to the applied propagation methods as well as to rearing success measured by the number of reared specimens released into the wild.
| Country | Region / institution | Species | Years / since | Rearing success | Method | Reference |
|---|---|---|---|---|---|---|
| USA, Virginia | Virginia Tech, Freshwater Mollusk Conservation Center (FMCC) at Blacksburg | ~ 25 species (Epioblasma capsaeformis, E. brevidens,E. f. walkeri, E. triquetra, Villosa perpurpurea, Villosa iris,Potamilus alatus, Lampsilis ovate, Lemiox rimosus, Hemistena lata, Dromus dromas, Cyprogenia stegaria and others) | Early 1990s, first releases of juveniles in 1997 | ~ 250 000 juveniles produced each year of 10–12 species;~10 000 juveniles or more from 6–10 species released each year since 2001. | Intensive cultivation systems: juveniles propagated in the laboratory and kept in baskets or raceways in flow-through or re-circulation pond water systems: new knowledge of mussel species life-history traits, dietary needs and habitat requirements leads to improved culture techniques. | http://fishwild.vt.edu/mussel/ e.g. Jones and Neves (2002), Neves (2004), Beaty and Neves (2004), Jones et al. (2005) |
| USA, Virginia | Virginia Department of Game and Inland Fisheries, Aquatic Wildlife Conservation Center, AWCC, at Marion | ~ 30 species (Actinonaias ligamentina, Actinonaia spectorosa, Cyprogenia stegaria, Dromus dromas, Elliptio dilatata, Epioblasma brevidens, E.capsaeformis, E. florentina walkeri, E. triquetra, Fusconaia cor, Hemistena lata, Lampsilis abrupta, Lampsilis fasciola, Lampsilis ovate, Lasmigona costata, Lasmigona holstonia, Lemiox rimosus, Ligumia recta, Medionidus conradicus, Pleurobema oviforme, Ptychobranchus fasciolaris, Ptychobranchus sebtentum, Strophitus undulates, Villosa iris, Villosa perpurpurea, Villosa vanuxemensis) | 1998 | 2003–2010: > 4 million juveniles produced and ~ 650 000 released into the wild (from 1 week to 4 years old). | Intensive cultivation systems: juveniles propagated in the laboratory and kept in culture baskets or raceways in flow-through or re-circulation systems; e.g. at AWCC rearing systems include an array of 5 L tanks that are supplied with filtered water from the river. AWCC is constantly developing and testing new systems and methods. | see AWCC's propagation and release summaries 2003–2010 at: http://www.dgif.virginia.gov/awcc/freshwater-mussel-restoration/ e.g. Gatenby (2000) |
| USA, Missouri | SW Missouri State University | Epioblasma triquetra, Lampsilis siliquoidea, L. reeveiana, L. rafinesqueana, Leptodea leptodon, Ligumia recta, Villosa iris iris | 1998 | e.g. by 2001: release of ~ 50 000 juveniles of L. rafinesqueana. | Intensive cultivation system; early culture of juveniles in bucket systems; ~ 2000 juveniles per chamber. Barnhart (2006): survival > 75%; 6 to 10 weeks of growth. | Barnhart (2006) |
| USA, W. Virginia, | Numerous state and federal facilities, e.g.USFWS, Dept of Fish & Wildlife Resources, or universities (e.g. University of Minnesota, Tennessee Tech, Ohio State University) | > 15 species (Epioblasma torulosa rangiana, Pleurobema clava, Quadrula fragosa, Lampsilis higginsii, Ligumia recta, Actinonaias pectorosa, Cyprogenia stegaria, Leptodea leptodon, Lemiox rimosus, Cumberlandia monodonta, Quadrula cylindrica strigillata, Potamilus capax, Lasmigona sp., Villosa sp., Fusconaia sp)., for target species see: http://www.fws.gov/midwest/endangered/clams/index.html | Late 1990s | Several 10 000 juveniles yr-1 of different species; recently major advances in propagation technology for large-scale production and cultivation; release of larger tagged juvenile mussels back into rivers. | Construction of separate freshwater mussel facilities and/or raceways within section of existing fish hatcheries for purpose of culturing endangered mussel species (e.g. White Sulphur Springs National Fish Hatchery, WV, Genoa National Fish Hatchery in LaCrosse, WI; Center for Mollusk Conservation in Frankfort, KY) as well as new construction of specific mussel propagation facilities along candidate rivers (e.g. St. Croix River, MN); after successful determination of suitable host fish in preliminary studies application of different (intensive) cultivation systems such as recirculating aquaculture systems or flow-through systems supplied with river or lake water. | e.g. Gatenby et al. (1996, 2009); Neves (2004); O'Beirn et al. (1998); see also: http://www.fws.gov/midwest/mussel/projects.html and technical reports of the US Fish and Wildlife Service and the US Geological Service. |
| Tennessee, | ||||||
| Kentucky, | ||||||
| Arkansas, | ||||||
| Minnesota, | ||||||
| Wisconsin, | ||||||
| Georgia, | ||||||
| Ontario, | ||||||
| Ohio, | ||||||
| others |
| Country | Region /institution | Species | Years / since | Rearing success | Method | Reference |
|---|---|---|---|---|---|---|
| Czech Republic | River Blanice, mussel hatchery; since 2007 team Bohumil Dort | Margaritifera margaritifera | 1990 (Hruška)2007 (Spisar) | ~ 30 000 juveniles ( > 3 years old) max. size of 5- year old: 24 mm | Hruška's method, labour intensive, initially held in tanks without flow, then transferred into special cages in rivers or in small wetland channels connected to rivers; provision of organic detritus as food for juveniles. | Hruška (1992, 1999, 2001); O. Spisar (pers. comm. 2011) |
| Germany, Lower Saxony | University of Hannover | Margaritifera margaritifera | 1989 | Survival rate declining 5–20% after 1–2 years and < 5% after 52 months. | Buddensiek sheet cages consisting of a thick perspex plate with holes drilled through, and a mesh covering on either side. ~5 juveniles placed into each hole; plates placed in rivers fastened above the bottom so that they were facing the current. | Buddensiek (1995) |
| Germany, Saxony | Vogtland, mussel hatchery; Interreg Project SN-01-I1-3-C0203-EEV | Margaritifera margaritifera | 2001-2007 | > 9000 produced; reach 6 mm in 3 growth seasons; reach ~20 mm after 5 years | Modified method of Hruška and Buddensiek; initial laboratory cultivation in tanks, fed detritus from wetland ditches and animal protein; cage system checked and cleaned regularly; ~ 4000 released up to 2010. | Lange (2005, 2009); Lange and Selheim (2011) |
| Germany, Bavaria | Oberpfalz, Fish hatchery Kleeberg | Margaritifera margaritifera | 1997-2007 | < 100; survival rates < 1% | Modified Buddensiek method by testing a variety of sheet cages, sediment boxes and mesh baskets using spring and river water. | Schmidt and Vandré (2010) |
| Germany, Bavaria | Lower Bavaria/LPV Passau | Margaritifera margaritifera | 2007 | 500–1000 several-year-old juveniles (cohorts 2007, 2008) | Modified method of Hruška and Buddensiek; initial laboratory cultivation in tanks with detritus from FPM river water; cage system checked and cleaned regularly. | F. Elender and R. Mayr (pers. comm. 2011) |
| Germany, Nord-Rhine-Westphalia | River Perlenbach Biological Station Aachen | Margaritifera margaritifera | 2008 | (to be determined) | Modified method of Hruška and Buddensiek; initial laboratory cultivation in tanks, fed detritus and animal protein; cage system checked and cleaned regularly. | H. Selheim (pers. comm. 2011) |
| Ireland | River Nore, Fish Farm Aherlow, Coomhola Salmon Trust | Margaritifera durrovensis | 2005 | 13 600 juveniles from cohorts 2009/2010 | Since 2009: new design for non-intensive rearing introduced; juvenile vivarium facility: provision of silt-free, high quality water to long or circular tanks of gravel holding juvenile mussels. | Moorkens (2011) |
| Luxembourg | River Our, Mussel hatchery
EU Life-Nature Project |
Margaritifera margaritifera | 2008 | ~ 1500 juveniles from cohorts 2008–2010 | Since 2009: initial laboratory cultivation in buckets (Barnhart, 2006), fed detritus and algae; juveniles > 3 months kept in sheet cages and sediment boxes in natural river; cage systems checked and cleaned regularly. | F. Thielen (pers. comm. 2011) |
| UK, Northern Ireland | Ballinderry River, Ballindery Fish Hatchery | Margaritifera margaritifera | 1998 | 3600 (1999); 13 000 (2000); 2010: ~ 6500 juveniles (1–9 years) in vivarium tanks. (~ 700 released up to 2010) | Semi-natural flow-through system (low-intensity approach) with 140 parental mussels; juvenile mussels excyst from fish directly into river gravel sediment in an artificial stream: 12 m × 1 m long raceways. | Preston et al. (2007); Wilson (2010) |
| UK, Scotland | River Moidart, River Dee | Margaritifera margaritifera | 2001 | < 100 several-year-old juveniles produced;
1–11% survival rate < 10 months, later 80% loss of cage systems |
Different semi-intensive and intensive methods (mussel cages and baskets) tested, including a culture system with juveniles in cages similar to those used by Buddensiek as well as sediment baskets supplied with flowing river water. | Hastie and Young (2003) |
| UK, England, Cumbria | FPM Ark Project Freshwater Biological Association (FBA), Natural England, Environment Agency | Margaritifera margaritifera | 2007 | ~ 5000 1–3 year old juveniles (1–3 mm length) from 8 different populations; over 40 000 juveniles collected in 2010 and 2011; high juvenile mortality | Adult mussels from 9 English rivers kept separately in tanks permanently supplied with lake water in a flow-through system (low-intensity approach); infection of trout, salmon or charr strains; collection of excysted mussels and transfer into modified salmonid egg incubation boxes filled with sand and supplied with lake water, no addition of extra food. | R. Sweeting, L. Miles, (pers. comm. 2011) FBA, Windermere |
| UK, Wales | Mawddach Fish Hatchery, Dolgellau | Margaritifera margaritifera | 2005 | 2008: <100 several-year-old juveniles produced: > 80% survival rate in the first year; low survival for older juveniles (0.12%) | Diversity of methods tried at the different hatcheries and sites; juveniles transferred into incubation trays; for the first 7 months juveniles were reared in floating mesh trays with a sparse layer of fine gravel, in flowing tanks with spray bars above; juveniles older than 7 months transferred into larger salmonid ova incubation trays with a 1 cm layer of gravel. | McIvor and Aldridge (2008) |
| Scriven et al. (2011) | ||||||
| France, Bretagne | EU Life Project | Margaritifera margaritifera | 2010-2016 | To be determined | Planned captive breeding intended to be part of the programme but currently not in effect. | M. Capoulade (pers. comm. 2010) |
| Spain, Galicia | EU Life Project | Margaritifera margaritifera | 2011 | To be determined | Planned captive breeding intended to be part of the programme but currently not in effect. | S. Lois (pers. comm. 2011) |
| Austria | Upper Austria | Margaritifera margaritifera | 2011 | To be determined | Captive breeding has started. | C. Scheder (pers. comm. 2011) |
Culturing programmes in North America
Over decades of research, the scientific basis of freshwater mussel culture in the USA was mainly developed by Richard Neves and co-workers at Virginia Tech (Freshwater Mollusk Conservation Center, FMCC). Methods for freshwater mussel rearing, especially with regard to technical solutions for intensive cultivation systems and propagation scale-up, were then developed further by universities (Beaty and Neves, 2004 at Virginia Tech; Barnhart, 2006 at Missouri State University) as well as several government organizations specializing in aquatic conservation such as the Aquatic Wildlife Conservation Center (AWCC) at Marion, Virginia. Today, numerous state and federal facilities in the USA are involved in propagation and culture of the most critically endangered mollusc fauna at regional scales (Gatenby et al., 2009) and new rearing methods are being developed further (Center for Mollusk Conservation in Frankfort, KY; Genoa National Fish Hatchery, WV).
Numerous rearing protocols and propagation techniques for more than 40 endangered mussel species have been developed, particularly in south-eastern North America, which is considered the most prominent hotspot of freshwater bivalve biodiversity (> 250 species; Neves, 2004; Barnhart, 2006; Gatenby et al., 2009). Since the end of the 1990s, more than 6 million juvenile mussels have been produced by the two major freshwater mussel research and rearing institutions AWCC and FMCC and more than 1 million specimens had been released into the wild by 2010. Almost all freshwater mussel culturing efforts have a core focus on conservation, but at least one species, the pink heelsplitter (Potamilus alatus), is also a target for pearl culture (Hua and Neves, 2007). Typically, conservation and propagation programmes are initiated after catastrophic population declines, e.g. caused by sewage or mining impacts on extant freshwater mussel populations. North American mussel cultivation systems can mostly be considered intensive (i.e. with permanent surveillance), with juveniles being propagated in laboratory cultures and bucket and/or raceway systems, partially with water recirculation. In most cases, cultured algae, commercial shellfish diets or re-circulated pond water is used for feeding. In contrast to European approaches, representative numbers of juvenile mussels (sub-adults, usually 1–2 years old) are tagged for monitoring purposes before their release into the wild. Consequently, information on survival and growth rates is available (e.g. see guidelines by Virginia Department of Game and Inland Fisheries, 2010), which is typically not the case for most releases in Europe. In parallel with propagation, translocation of adult mussels is considered an appropriate tool for population restoration in the USA.
Culturing programmes in Europe
In contrast to North America, all European efforts in freshwater mussel propagation and captive breeding techniques have exclusively focused on Margaritifera spp. because of the dramatic and accelerating decline of this genus throughout its distribution. To our knowledge, Table 2 considers all major past and present European freshwater pearl mussel (FPM) rearing activities and summarizes the different methods applied since Hruška's first approach (Hruška, 1992). In addition, it provides information on the FPM rearing success in terms of the overall number of juveniles produced and the survival rates based on both the available peer-reviewed literature as well as published technical reports and direct correspondence with the respective institutions in the period from January to May 2011. In the Czech Republic, Germany, the UK, Northern Ireland, and Luxembourg, a total number of about 80 000 pearl mussels have been reared, with an estimated release of 35 000 several-year-old specimens into the wild. New propagation projects have recently started in Spain, France, and Austria.
Since many European rearing and culturing programmes involve the direct release of young mussels (from early post-parasitic phase to a few years) into receiving streams without tagging, little is known about the success of these projects. Only recently, Wilson (2010) released a sample of tagged juveniles into the wild to document survival and growth. In many European countries, culturing programmes have not been in place for a long time and juvenile mussels are mostly still being kept in controlled systems.
Strategies of FPM glochidia harvesting and captive breeding
Generally, two different strategies can be distinguished with regard to the time-span of parental mussels kept outside their natural habitat and the manipulation and handling of mussels for harvesting viable glochidia and subsequent host fish infection.
The first approach follows the idea of maintaining adult mussels in their natural environment. For collection of glochidia, mussels need to be inspected to determine the time of completion of glochidial development. They are then only removed for a few minutes to hours when they are placed into small containers to influence glochidial release by temperature shock and/or oxygen depletion. The released mussel larvae (usually from several females) can be stored for a few days until infection of fish hosts takes place under controlled conditions in hatcheries (Taeubert et al., 2010). With slight modifications, this method has been carried out in the Czech Republic, Germany and Luxembourg (Hruška, 1999; Schmidt and Vandré, 2010; Thielen, pers. comm. 2011).
With the intention to safeguard the regionally most threatened populations from local extinction, the second strategy is fundamentally different from the first one in transferring either some or all of a mussel population permanently into captivity (so-called ‘ark’ projects). Typically, about 50 adult mussels of target populations are kept in gravel baskets within flow-through systems supplied with river or lake water (e.g. Freshwater Biological Association Station, Windermere, UK) or are transferred into artificial streams/raceways filled with gravel in indoor or outdoor hatchery facilities (e.g. Ballinderry River and Fish Hatchery, Northern Ireland). Infection of host fish species is achieved by maintaining host fish populations either directly within the mussel tanks or in the effluent water of the mussel containers. This approach is mainly used in the UK (Northern Ireland, Wales, England) and Ireland (Preston et al., 2007; Moorkens, 2011).
The next stage after completion of mussel larvae development, i.e. glochidia excystment and harvesting, is rearing of juvenile mussels, and this again involves two strategies differing in the intensity of mussel care involved (Table 2).
The original, and still the most successful method of FPM propagation in respect to the overall number of several-year-old juveniles reared, is the method developed by Hruška (1999, 2001). Starting in the 1980s, J. Hruška established methods for semi-natural breeding of FPM and studied the effect of temperature on reproduction, growth, and age structure of the population from the Blanice River in South Bohemia (Hruška, 1992). In brief, the Hruška method can be described as follows: (i) maintenance of infected host fish under controlled conditions; (ii) daily collection of freshly excysted juveniles; (iii) laboratory pre-culture in small containers without flow and feeding of organic detrital suspension; and (iv) transfer of juveniles into cages or containers adapted to mussel size and placed in natural rivers or semi-natural flow channels. By application of this relatively labour-intensive method, 30 000 juveniles more than 3 years old were produced in small wetland ditches (Hruška, 1999; Spisar, pers. comm. 2011).
The Hruška method has been successfully adopted by M. Lange from Saxony, Germany, with a few modifications to the initial diet in the laboratory pre-cultivation of juveniles for a few months (by adding animal protein components due to lower quality detritus to achieve high initial growth rates) but mainly by the use of mussel field cage systems as previously described by Buddensiek (1995). Subsequently, for cultivating juveniles older than 2 years, gravel boxes with 300 µm mesh gauze are used and placed upright in the free-flowing water of local streams. Approximately 35 000 several-year-old juveniles have been released (most of them in the Czech Republic and Saxony) up to 2010; thus the number of semi-naturally reared FPM probably exceeds the number of naturally recruited juveniles in Central Europe. Following the principles of the modified Hruška method, FPM rearing programmes started a few years ago in Luxembourg (EU Life Project) and in federal states of Germany (Bavaria, NRW). However, these projects have not been running for long enough to evaluate their overall rearing success.
In contrast to the relatively labour-intensive methods developed in the Czech Republic and Saxony, Preston et al. (2007) followed a low-intensity approach in Northern Ireland. The method is based on a semi-natural flow-through system supplied by river water with juvenile mussels excysting from the host fish directly into the gravel substratum of a hatchery raceway. Successful rearing of up to 13 000 individuals for single cohorts has been achieved. Approximately 700 juveniles ranging in age from 4 to 9 years were released during a pilot reintroduction study with about 6 500 juvenile mussels (from 1 to 9 years old) remaining in the vivarium raceway (Wilson, 2010). However, it is yet unclear whether this method can be scaled up for long-term propagation (Thomas et al., 2010).
Key factors for success
First, as indicated by the results of FPM propagation studies carried out so far, overall rearing success seems to be strongly determined by the survival rates during the first months following excystment, which appears to be the most critical phase. During this period and especially over the first winter, mortality rates are highest (Buddensiek, 1995; Schmidt and Vandré, 2010). Second, if outdoor sheet cages and sediment boxes are to be used, the following factors are considered crucial, based on the authors' practical experience and those of colleagues (Buddensiek, 1995, F. Elender, pers. comm. 2011): (i) positioning of field mussel cages; (ii) their regular maintenance to maintain exchange with ambient water; (iii) the temperature regime and food/detritus quality of the selected stream (Lange and Selheim, 2011); as well as (iv) the security and recovery rate of cages which strongly depends on the size and flow dynamics of the respective stream (Hastie and Young, 2003).
Initial growth and survival was found to be most crucial at the stage of freshly excysted mussels (Buddensiek, 1995) and often is not addressed sufficiently by studies and experiments carried out so far (Schmidt and Vandré, 2010). According to Lange and Selheim (2011) early growth to a minimum total shell length of about 1 mm can be considered a threshold minimum size for survival during the first winter. Based on practical experience, we assume that one of the main reasons for the lower rate of mortality and the higher rearing success in Saxony or the Czech Republic compared with the low success observed by Schmidt and Vandré (2010) in Bavaria is the use of an initial laboratory phase (Hruška, 1992). In the study by Schmidt and Vandré (2010), only freshly excysted juveniles were directly transferred into different types of experimental mussel field cages such as Buddensiek cages or sediment baskets without initial feeding of a detritus suspension. In contrast, the approach by Lange (2005) largely prevents young mussels in weak condition from being transferred into the sheet cages, which are usually highly susceptible to infection by fungi. At the same time, the proposed preparatory culture (i.e. generating young mussels of ~ 1 mm size) allows the earlier application of a gauze with larger mesh width (≥ 300 µm) already in the first field cage phase. This provides a more effective food and oxygen supply for the caged juvenile pearl mussels, leading to a direct beneficial influence on growth and survival.
As is evident from the rearing of juvenile pearl mussels over the last 10 years, the success or failure regarding the overall survival of 1+ FPM juveniles (after initial laboratory culture) depends largely on the experimental setup and on the exact positioning of sheet cages and gravel boxes in the stream flow, as well as their regular maintenance and cleaning. In the present trials, a fixed vertical position of sheet cages at a 90° angle to the flow direction resulted in the highest survival rates. In contrast to the approach by Schmidt and Vandré (2010), burying cages or boxes in the substratum was not performed. Our results show that the distribution patterns of older juveniles (> 3 summer periods) kept in gravel boxes indicate a preference for the gravel surface and the side-wall areas within the cages near the gauze (Figure 1). In these interface areas, detrimental effects of siltation are lowest and fresh water, oxygen, and food supply is highest. Indeed, stream siltation with suspended fine sediments is a fundamental problem for freshwater pearl mussels during their early life stages (Geist and Auerswald, 2007). In Saxony, Lower Bavaria, and Luxembourg, cages or boxes are cleaned weekly from the outside, removing any clogged material and fine sediments. These observations are also supported by McIvor and Aldridge (2008) who report that the high early survival (> 90%) of juveniles in the Welsh FPM rearing programme was probably the result of careful cleaning of the mesh and increasing the mesh sizes to maintain the flow of water past the juveniles. Therefore, technical maintenance of cages and boxes at regular intervals is essential for early survival of juveniles. Moreover, before placing juveniles into the cages, all types of boxes should be placed in the respective streams at least for 1 week (or longer) in order to allow the establishment of a biofilm.
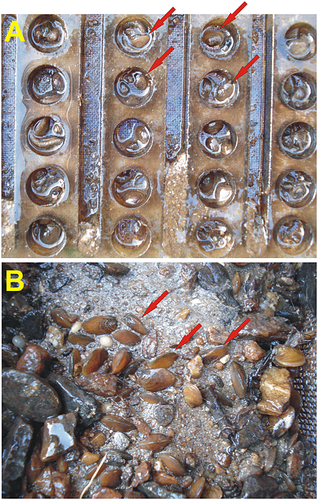
In summary, seemingly minor details may determine the overall success for semi-natural propagation of freshwater mussels in different types of cage systems. In Saxony, about 4000 several-year-old mussels reared in Buddensiek sheet cages and gravel boxes (as described above) were released to the wild in 2009/2010 and viable individuals were found 1 year later at the point of release (M. Lange, pers. observation 2011).
Conservation and management decisions
‘Risk’ is the first crucial management decision that should be considered in the context of FPM rearing and conservation: for example, the risk of keeping the last remaining individuals of relict populations in degraded habitats or transferring them into captivity in order to establish ‘arks’; or the risk of releasing captive-bred juveniles into (still) unsuitable habitats. Geist (2010) discussed risk in the context of developing conservation strategies for the FPM by simultaneously considering genetic and ecological traits. Risk can be reduced by distributing infected host fishes and juvenile mussels to different laboratories and by a spread of juvenile mussels over different stream reaches or streams.
Further important decisions concern the input intensity of the rearing strategy. Whereas most freshwater mussel propagation programmes in North America are carried out in specific rearing facilities (e.g. former fish hatcheries), the propagation of Margaritifera in Europe is more diverse, ranging from intense culture (e.g. Luxembourg) to low-intensity and field-based approaches (Preston et al., 2007; Schmidt and Vandré, 2010). In contrast to the low-intensity approach followed by Preston et al. (2007), semi-natural captive breeding of M. margaritifera following the Hruška method is relatively labour intensive. However, captive breeding of juvenile FPM in mussel field cage systems provides essential baseline data and will contribute knowledge on factors that determine FPM habitat quality. Such information will also greatly enhance recovery programmes which depend on the identification and suitability of locations for the intended release of the reared mussels.
Ultimately, there remain considerable challenges in FPM rearing such as potential loss of fitness of parental mussels and juveniles reared in artificial environments, the lack of knowledge concerning the optimal starting diet (detritus, algae), the development of efficient rearing systems for long-term propagation, the genetic integrity of cultured mussels, and the maintenance of mussel cages in large quantities until juvenile release.
CONCLUSIONS
In conclusion, the successful use of cage systems and the rearing of juvenile freshwater pearl mussels and other species is possible. However, it must not replace the restoration of stream habitats where natural reproduction can occur. Captive breeding of juvenile mussels can be a last-minute rescue tool to retain the evolutionary potential of highly valued populations that would not persist long enough to benefit from habitat restoration. In addition to this ‘time-bridging-effect’, captive reared mussels themselves are probably the best bioindicators to test the spatio-temporal suitability of habitats and of restoration efforts for sensitive target species. Both the long-term restoration of habitats and short-term captive breeding require large inputs of time and money and are only useful where clear conservation priorities have been set (Geist, 2010). In any case, records should be kept of any reared and captive-bred mussels released into the wild.
Cultivation of juveniles without proper maintenance of cage systems, or the release of juveniles without accompanying monitoring, is clearly not an appropriate conservation measure. Instead, current knowledge on successful culturing and release of juvenile mussels should be widely available and collaboration between existing and newly founded rearing stations in Luxembourg, Saxony, Bavaria, the Czech Republic, France, Spain and Austria should be considered good practice.
In general, both successful and unsuccessful attempts to rear and culture freshwater mussels should be published in order to increase the efficiency of future approaches. This information is necessary both for understanding the impact of these conservation measures on wild populations as well as for improving the efficiency of these conservation actions.
ACKNOWLEDGEMENTS
We are grateful to all mussel researchers who contributed information concerning the rearing and culturing facilities for freshwater mussels in their countries: Franz Elender, Robert Mayer, Ondrej Spisar, Heidi Selheim, Frankie Thielen, Roger Sweeting, Louise Miles, Marie Capoulade, Sabela Lois, Conor Wilson, and Evelyn Moorkens.




