Effect of pro-oxidants on the aerobic biodegradation, disintegration, and physio-mechanical properties of compostable polymers
Abstract
Biodegradable polymers are gaining momentum to resolve the globally acknowledged plastic waste problem. Understanding, characterizing, and developing new generations of biodegradable plastics is crucial to provide industries with alternative green materials that can fully satisfy biodegradation rates and lifetime specifications. This study evaluates the influence of metal pro-oxidant additives on the degradation properties of various biodegradable polymer systems. For this purpose, iron (III) stearate (FeSt3) and bismuth oxide (Bi2O3), as oxidant agents, were incorporated into poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(butylene adipate-co-terephthalate) (PBAT), cellulose acetate (CA), poly(lactic acid) (PLA), and thermoplastic starch (TPS) bioplastics. The material performances and biodegradability properties due to the additives on the resulting bioplastic formulations were investigated. A mechanism was proposed in which both pro-oxidant additives can accelerate the thermo-oxidation processes under composting conditions and cleave the polymer chains into smaller fragments to stimulate the biodegradation rate through microorganisms' activity. The study revealed that both pro-oxidant additives, FeSt3 and Bi2O3, effectively improved the biodegradation process for all tested polymers except TPS, which already had a very high biodegradation rate. The observed change in the barrier and mechanical properties due to the additives were within tolerable limits of corresponding neat polymers.
1 INTRODUCTION
The use of traditional petrochemical-based synthetic plastics has led to many challenges associated with municipal end-of-life disposal and environmental pollution concerns.1, 2 As a result, sustainable and biodegradable packaging and containment materials are gaining extensive attention in the last two decades as a promising technique to reduce plastic waste management challenges.3-5 The development of affordable biodegradable single-use material alternatives with short degradation time and appropriate performance properties has been regarded as a paramount goal to replace traditional petrochemical-based polymers. Such achievements could further empower our societies to cope with environmental challenges and responsibly use appropriate resources to secure sustainable development targets.6
Biodegradability and compostability have become imperative for assessing material suitability in packaging industries.3 One of the essential qualities in evaluating the biodegradability of renewable packaging materials is the total time required to break the materials into smaller natural components, such as carbon dioxide (CO2), water (H2O), and compostable biomass.7, 8 Depending on the materials' chemical structure and composition, temperature, humidity, and microorganism type, the time frames for biodegradable polymers to assimilate under compost conditions can vary from a few weeks to a few months.9 Thus, biodegradation engineering for packaging materials has recently provided many prominent research interests, encouraging various technological developments to improve further and accelerate the biodegradation process as expected from sustainable polymers.3, 8 The incorporation of biodegradation-promoting compounds inside polymer films/coatings, especially those of pro-oxidant additives, is currently incentivized as a pragmatic strategy to accelerate the biodegradation pace without compromising the mechanical performance of polymeric packaging materials.10
Pro-oxidant additives containing transition metal ions, such as Mn2+, Co2+, and Fe3+stearates, are often used in polyolefin-based blends to accelerate carbon-chain conversion into H2O and CO2.11, 12 Incorporating these metal pro-oxidant additives can be utilized to accelerate the biodegradation process in biodegradable polymer films/coatings. Iron (Fe3+) stearate (FeSt3) can chemically promote fracture points of polymer carbon chains under heating via thermo-oxidation processes, which helps to break down polymer macromolecules and speeds up the degradation processes partially.13-16 Iron (Fe3+) ions, under the effects of heat and/or sunlight, can decompose the hydroperoxides (RCOO) formed during the polymer degradation, leading to chain scission of polymers without the need for metal catalysts (Scheme 1a).17-19 In addition, the polymer's chain disassembly due to chain scissions results in scattering oligomer fragments with hydroxyl and carbonyl functional groups, increasing the hydrophilicity of the polymer surfaces and promoting their interactions with microorganisms to enhance the biodegradation process.10, 11 At the same time, FeSt3 could be utilized as a reinforcing additive to improve the barrier properties of packaging films.17-19 Abrusci et al. reported higher hydrophobicity for FeSt3-containing polymers and observed improvement in the water vapor permeability properties of the polymer membranes.11
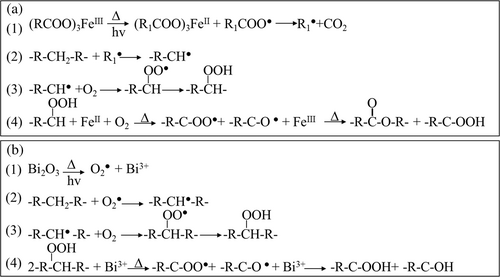
Another metal oxide, Bismuth (III) oxide (Bi2O3), is also a promising pro-oxidant additive that could be utilized to accelerate the biodegradation process, which has been used in different cosmetic and pharmaceutical applications due to its low toxicity.20 Bi2O3, an oxidant agent, has been used extensively in photo- and thermo-oxidation processes to degrade inorganic structures, such as poly(ethylene) (PE) or carbon nitride.10, 16 The incorporation of Bi2O3 could affect the biodegradation rate of renewable packaging materials in many ways. The presence of Bi2O3 under heating and/or sunlight accelerates the rupture in polymer chains by incorporating oxygen atoms in the carbon skeleton of polymers and converting the structure into carbon dioxide (CO2) (Scheme 1b).20, 21 Moreover, it has been proven that Bi2O3 has higher exothermic characteristics and photocatalytic activities than other pro-oxidant metal oxides, such as Fe2O3, CuO, and MnO2, which could contribute significantly to the photos- and thermos-oxidation processes of the polymers' heterostructure under visible light irritation.16, 20, 21
Moreover, Bi2O3 possesses many other workability characteristics as filler additives for packaging applications, including low density, low toxicity, high water vapor permeability, exceptional mechanical properties, bending resistance, and excellent x-ray shielding properties.13, 14 El-Khatib et al. reported that the filler structure of Bi2O3 particles could improve the morphological and mechanical properties of polypropylene (PP) films by reducing the voids in the polymers.15 Additionally, the high reactivity of Bi2O3 with released carbon dioxide (CO2) to produce bismuth carbonate could reduce the amount of carbon emission from biodegradation processes which add up to its competitiveness.22
In this research, the impacts of both FeSt3 and Bi2O3 as additives on the biodegradation properties of various renewable polymers [poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(butylene adipate-co-terephthalate) (PBAT), cellulose acetate (CA), poly(lactic acid) (PLA), and thermoplastic starch (TPS)] were investigated under aerobic degradation and soil burial environments. As an essential aspect of developing sustainable materials, the effect of prooxidant additives' in promoting biodegradation was assessed by incorporating selected additives at varying concentrations. In this study, the effect of prooxidants was evaluated by preparing CA and PLA films containing three different levels of iron stearate (FeSt3). The thermo-oxidation percentage data were determined by measuring the CO2 evolution from aerobic biodegradation under controlled composting conditions defined by ASTM D5988-18, which was previously employed by Chinaglia et al.23, 24 Additionally, disintegration characterization tests in soil were investigated for up to 13 weeks, to evaluate the physically compostability of renewable films with additives over times.25, 26 Results of this study provide valuable scientific insight into the biodegradation/compostable behaviors of renewable packaging materials under the influence of applied additives.
2 EXPERIMENTAL
2.1 Materials
Corn starch (containing 73% amylopectin and 27% amylose, >99% purity), potassium hydroxide pellets (KOH), hydrochloric acid (HCl) (ACS reagent grade, 37%), oleic acid, bismuth (III) oxide (Bi2O3) (>99.9%) were obtained from Sigma-Aldrich. ThermoFisher Scientific, Canada, supplied glycerol (99%). Iron (III) stearate (FeSt3) was supplied by VWR. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) (99/1 wt%) was obtained from Ningbo Tianan Biologic Material Co. (TianAn, China) in powder form. Poly(butylene adipate-co-terephthalate) (PBAT) resins were obtained by T&T Industry Group Ltd. (Shenzhen, China) in pellets form. Cellulose acetate (CA) in pellets form was obtained from CTK Bio Canada (Port Coquitlam, BC, Canada). Poly(lactic acid) (PLA) was supplied by Total Corbion (Luminy LX 175). Commercial pots of soil, compost, perlite, and ammonium phosphate were obtained from a local store (Home Depot, Waterloo, Canada). All chemicals were used as received.
2.2 Methods
2.2.1 Thermoplastic starch preparation
Corn starch powder was weighed and dried (at 75°C) overnight in a convection oven to obtain a moisture level below 1 wt%. Dried starch (69 wt%) was mixed with 30 wt% glycerol plasticizer and 1 wt% oleic acid and stored in a sealed container for 24 h. TPS pellets were prepared by processing the powdered mixture with a twin-screw extruder (TSE) (Process 11 Parallel Twin-Screw Extruder, Thermo Fisher Scientific) with an 11 mm barrel diameter and 40 L/D ratios. The screw speed of the extruder was set at 150 rpm and the melt temperature at 120°C. Then, TPS strands were air chilled, pelletized, and stored in air-tight bags.5, 26, 27
2.2.2 Incorporation of additives in polymer matrices
The polymers were processed using a Process 11 twin-screw extruder (TSE), and temperatures were varied based on their melting points. Polymers were blended with a certain amount of FeSt3 or Bi2O3 additives. The biodegradation effect of the additives was assessed by preparing CA and PLA films with three different concentrations of FeSt3. The sample formulations and processing temperatures used for extrusion are listed in Table 1. Extrusion temperatures for each polymer were determined through a combination of factors, including a literature review and consideration of their unique thermal characteristics, with preliminary optimization experiments conducted within ±5 degrees Celsius of the respective polymer's melting point. All additives-filled sample strands were pelletized, stored, and conditioned at room temperature.
| Sample | PHBV (%) | PBAT (%) | TPS (%) | CA (%) | PLA (%) | Iron stearate (%) | Bismuth oxide (%) | Extrusion temperature (°C) |
|---|---|---|---|---|---|---|---|---|
| PHBV | 100 | – | – | – | – | – | – | 180 |
| PHBV-Bi2O3(1%) | 99 | – | – | – | – | – | 1 | 180 |
| PHBV-FeSt3(1%) | 99 | – | – | – | – | 1 | – | 180 |
| PBAT | – | 100 | – | – | – | – | – | 120 |
| PBAT-Bi2O3(1%) | – | 99 | – | – | – | – | 1 | 120 |
| PBAT-FeSt3(1%) | – | 99 | – | – | – | 1 | – | 120 |
| TPS | – | – | 100 | – | – | – | – | 120 |
| TPS-Bi2O3(1%) | – | – | 99 | – | – | – | 1 | 120 |
| TPS-FeSt3(1%) | – | – | 99 | – | – | 1 | – | 120 |
| CA | – | – | – | 100 | – | – | 220 | |
| CA-Bi2O3(1%) | – | – | – | 99 | – | 1 | 220 | |
| CA-FeSt3(1%) | – | – | – | 97 | 1 | – | 220 | |
| CA-FeSt3(3%) | – | – | – | 95 | 3 | – | 220 | |
| CA-FeSt3(5%) | – | – | – | 99 | 5 | – | 220 | |
| PLA | – | – | – | – | 100 | – | – | 220 |
| PLA-FeSt3(1%) | – | – | – | – | 99 | 1 | – | 220 |
| PLA-FeSt3(3%) | – | – | – | – | 97 | 3 | – | 220 |
| PLA-FeSt3(5%) | – | – | – | – | 95 | 5 | – | 220 |
2.2.3 Cast film preparation
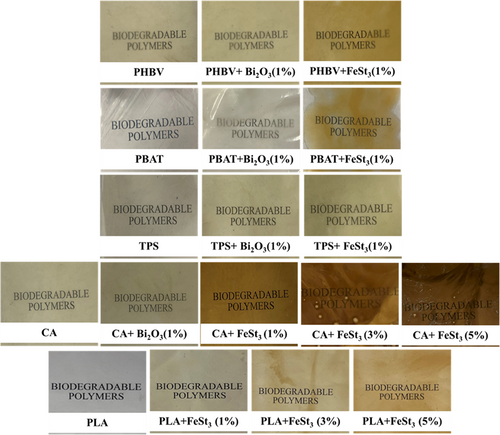
The flat sheets of all biodegradable polymer samples were prepared using a compression molding process under a temperature-controlled hydraulic press (Carver, Inc., Wabash, IN, USA). The films were melt-pressed using a 0.15 mm mold spacer for 5 min at the same extrusion melt-processing temperature. Figure 1 presents the prepared films after the compression molding process. After cooling to room temperature, the films were cut into appropriate sizes for further analysis.
2.2.4 Biodegradation characterization
The aerobic biodegradation of the samples was evaluated as per ASTM D5988-18. The composting soil used for the study comprised 86% (by mass) commercial pot soil, 4% compost, 9% perlite, and 1% ammonium phosphate. The mixture was thoroughly ground and filtrated through a 2 mm mesh. The free moisture percentage of the mass solids was set to 65 wt% by adding water to the soil. Triplicates of each sample and two benchmark samples [filter paper as positive and poly(ethylene) (PE) as negative] were cut into 0.3–0.5 cm2-sized pieces and mixed with the compost soil. Glassware containing KOH (0.5 N, 20 mL) and distilled water (30 mL) was added to the soil and sample mixture and replaced after every reading (Figure S1). The initial weight of each sample's container was recorded and placed in a temperature-controlled chamber (58°C). The KOH reacts with free CO2 released from the sample and soil in each container, and the KOH solution of each container was titrated with HCl (0.25 N) every 2 weeks for up to 3 months to quantify the amount of released carbon © from each sample by subtracting the CO2 released in each vessel with the negative reference.14, 19
2.2.5 Disintegration characterization
2.2.6 Mechanical properties
The stress–strain measurement was performed under tensile tension using universal tensile testing equipment (AGS-X, Shimadzu, Japan), as per ASTM D882-18. After compression molding, the prepared films were cut into 70 × 20 mm strips. The tensile test was then conducted at room temperature with a load cell capacity of 500 N and strain rate of 5 mm min−1. A minimum of five replicates were tested for each specimen, and their averages and standard deviations were reported.
2.2.7 Dynamic mechanical analysis
Dynamic mechanical thermal analysis (DMA) was conducted through DMA multifrequency—strain (Q800 V21.3 Build 96). The polymer films were subjected to DMA experiments at a stress amplitude of 15%, frequency of 1 Hz, and a temperature range from −90°C to 80°C with a heat rate of 3°C min−1. The film dimensions were set at 15.0 mm × 6.0 mm × 0.2 mm, and their thickness and width were measured using a digital caliper. The Tg was determined by observing the abrupt change in storage modulus slope and the corresponding loss modulus maximum, commonly referred to as a polymer's alpha relaxation.
2.2.8 Permeability testing
2.2.9 Water absorption characterization
2.2.10 Surface morphology analysis
The surface morphology and dispersion of FeSt3 and Bi2O3 on the surface of polymer sheets were monitored through field emission scanning electron microscopy (FE-SEM) images at ×500 magnification. SEM images were captured using an environmental scanning electron microscope (ESEM; Quanta FEG-SEM 250, Oxford Instrument) coupled with an energy-dispersive X-ray system (EDX) capability (Abingdon, Oxon, UK) without any sputter coating. An Olympus BX53M polarizing optical microscope (USA) with a built-in SC 100 camera system was used for optical microscopy images at 20× magnification of polymer sheets.
2.2.11 Statistical analysis
Replicate data in this work are presented as average ± standard deviation. Analysis of variance (ANOVA) with a significance level of α > 0.05 was used to evaluate the findings' statistical significance.
2.3 Results and discussion
2.3.1 Biodegradation analysis
To investigate the pro-oxidant additives' impact on polymers' biodegradability, the released CO2 of each sample was compared to those of polyethylene (as the negative control for nondegradable polymer) and cellulose paper (as the positive control for degradable materials). For each combination of polymer and additive-containing polymers, three replicates were conditioned over 24 weeks (~ 6 months), and the released CO2 was measured after 1, 2, 4, 6, 8, 10, 12, 14, 16, 20, and 24 weeks. The changes in the appearance of the sample mixed in the solid were noted, collecting images every 2 weeks over 6 months (Figure S2). The biodegradation percentage of all samples was calculated, and two-factor ANOVA tests were performed to evaluate each polymer's combined impact of elapsed time and additive type (total of 12 tests; presented in Table S1). Moreover, the root means squared deviation (RMSE) of all the samples was calculated and compared with the positive control (paper sample) and normalized on the interquartile range (RMSD-IQR) (Table S1).
Adding Bi2O3 and FeSt3 caused statistically significant (p < 0.05) improvement in the biodegradation rate of all the studied biopolymers (PHBV, PBAT, CA, and PLA polymers), as illustrated in Figures 2, S3, and Table S3. However, the FeSt3 pro-oxidant additive was slightly more effective with a higher biodegradation rate and final biodegradation percentage.
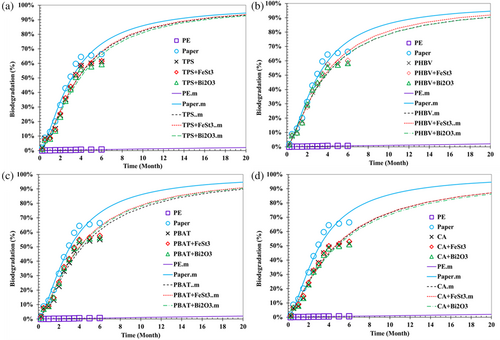
Generally, lower biodegradability was observed for CA compared to the other samples (Figure 2). The CA films containing Bi2O3 and FeSt3 indicated similar degradation profiles. For TPS samples with pro-oxidant additives, no improvement in the biodegradation profile as measured by CO2 release was noted by incorporating either pro-oxidant additives. This is likely because TPS is highly hydrophilic and displays extensive and fast biodegradation without the need for external additives. The employed plasticizers to generate the TPS (glycerol and oleic acid) are also bio-biobased and biodegradable. It is interesting to note that the incorporation of FeSt3 pro-oxidant caused a slightly detrimental impact on TPS degradation (Table S2 and Figure 2). This could be associated with the hydrophobicity of FeSt3, which inhibits moisture migration and microbial growth.
2.3.2 Disintegration analysis
The gradual fragmentation and disintegration of the polymer films in the composting soils were evaluated by monitoring their percentage weight loss and recording the morphology change of the samples. For this, the visual observation of polymer film disintegration mixed with soils was recorded biweekly (Figures S4 and 3). As presented in Figure 3a, the changes in the polymer films' integrity are shown to compare the disintegration rate of PHBV, PBAT, and TPS films with time as well as with the incorporation of Bi2O3 and FeSt3 additives. Additionally, the impacts of additives concentration were also assessed by evaluating the disintegration rate of CA and PLA films with varying FeSt3 concentrations (1, 3, and 5 wt%; Figure 3b). Figure 4 presents the sample's weight loss (%) over time, as well as assess the impact of FeSt3 and Bi2O3 as pro-oxidant agents.
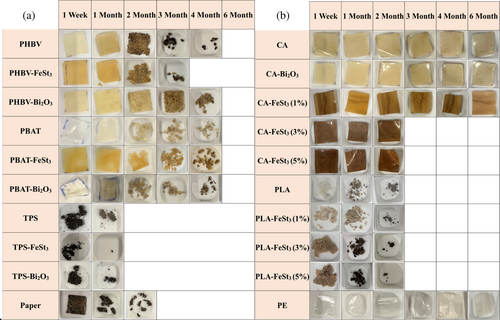
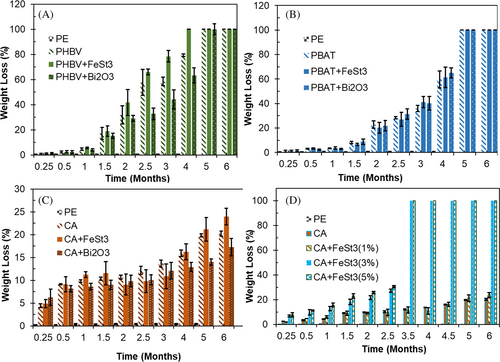
The results showed that adding FeSt3 has improved the fragmentation rate of PHBV. At the same time, Bi2O3 presented a slower disintegration rate than neat PHBV, possibly because Bi2O3 functioned primarily as a filler rather than as an oxidation accelerator. On the other hand, adding FeSt3 and Bi2O3 seems to accelerate the disintegration rate of PBAT samples slightly, but it was not statistically significant (α < 0.05). The TPS samples (with and without additives) presented substantially faster disintegration than all the other polymers, including cellulose paper and disappeared entirely after 1 month of evaluation. CA and CA-Bi2O3, with a final weight loss of about 20%, presented the slowest disintegration rate among all other samples, excluding PE.
Since FeSt3 provided better oxidative disintegration at 1 wt% loading levels, the impact of loading concentration variations (1, 3, and 5 wt%) of FeSt3 on the disintegration of selected polymers (CA and PLA films) was further assessed (Figure 4d). The overall increase in weight loss in comparison with all samples indicated that after 3.5 months, the CA films containing higher concentrations at 3 and 5 wt% are capable of being totally disintegrated under composting conditions. The results indicated that 3 wt% is the critical concentration for FeSt3 to be effective as a biodegradation accelerator in CA polymer matrices. As for PLA, the incorporation of FeSt3 was also evaluated at 1, 3, and 5 wt%, respectively. The disintegration analysis showed that PLA experienced more weight loss with increasing FeSt3 contents, as displayed in Figure S5. The effect of polymer crystallinity on the degradation kinetics is important, which can be the subject of a follow up study.
2.3.3 Mechanical properties
The impacts of the two additives (FeSt3 vs. Bi2O3) at 1 wt% composite loading on the mechanical properties of each polymer by evaluating the elastic modulus, tensile strength, elongation at break, and rupture energy from tensile test, as illustrated in Figure 5. The ANOVA single factor statistical analysis with a significance level of α > 0.05 was performed on the result of each measured parameter (neat polymer vs. FeSt3, neat polymer vs. Bi2O3, and FeSt3 vs. Bi2O3; Figure 6).
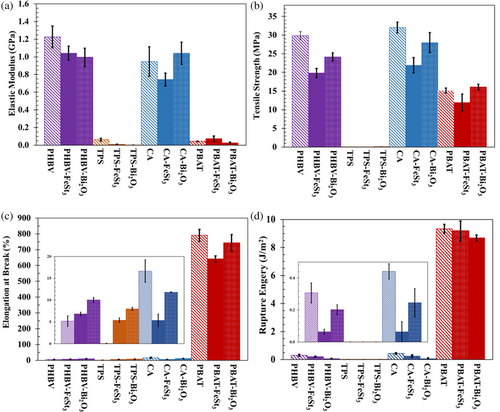
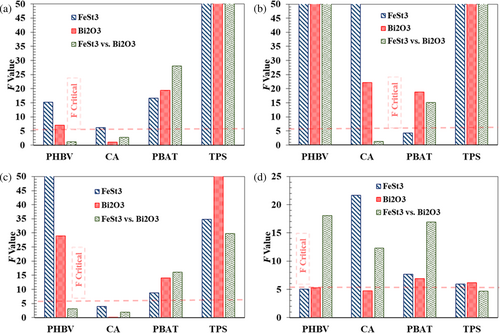
In the case of PHBV, both additives significantly reduced the elastic modulus and tensile strength, as indicated in Figure 5 and Table S2. However, comparing the impact of the additives in PHBV films, their additive influences on elastic modulus are statistically similar within a 95% confidence level; the addition of FeSt3 caused significantly more reduction in the tensile strength than those with Bi2O3. On the other hand, Bi2O3 showed plasticizing effects on the PHBV polymer, as indicated by the significantly increased elongation at break within the predetermined confidence level. At the same time, the addition of FeSt3 is statistically insignificant, indicating no effects on the elasticity of PHBV. The reinforcing and plasticizing effects of Bi2O3 compared to FeSt3 were more evident, as indicated by all of the composite samples exhibiting higher tensile strength, elongation at break, and rupture energy.
In the case of CA, Bi2O3 had less of an impact on the mechanical properties in comparison to FeSt3. The statistical analysis indicated that only the elongation at break and tensile strength had been affected by Bi2O3, which was at a lower intensity level compared to those containing FeSt3 additives, as shown in Figure 6 and Table S2.
FeSt3 and Bi2O3 exhibited contrasting impacts on the mechanical properties of PBAT. Particularly, the main impact of FeSt3 on PBAT's mechanical properties is the elongation at break. On the other hand, the effects of Bi2O3 on elongation at break were virtually insignificant. However, the additives had a more significant impact on the improvement of tensile strength, which was determined to be statistically significant. At the same time, the observed reduction in tensile strength by adding FeSt3 is not statistically significant within a 0.05% significance level.
Overall, the two additives positively impact all evaluated mechanical properties of TPS. Both additives have been shown to significantly reduce the elastic modulus while causing an increase in the tensile strength and elongation at break. As shown in Figure 6 and Table S2, Bi2O3 has a slight edge in terms of improvement in the mechanical properties of TPS as compared to samples containing FeSt3.
2.3.4 DMA analysis
The thermomechanical properties of different polymer films and their respective biocomposites containing FeSt3 were evaluated with DMA and presented in Figure 7. The storage modulus of neat polymer of PHBV, PBAT, CA, and TPS and their respective composites containing FeSt3 was presented in Figure 7. Overall, composites of PHBV, CA, and TPS containing FeSt3 all exhibited lower storage modulus than their neat film samples, indicating that the stiffness of the composites decreased with the addition of the additive FeSt3. This observation agrees with the reduction in elastic modulus results obtained from tensile testing, in which the incorporation of FeSt3 may have plasticizing effects on the polymer matrices. On the other hand, the composite PBAT-FeSt3 displayed higher storage modulus compared to neat PBAT, illustrating excellent interaction and compatibility between PBAT and FeSt3 matrices. The increase in storage modulus indicated FeSt3 could also have a reinforcing effect on the film of PBAT, which makes the films much more stronger and stiffer.
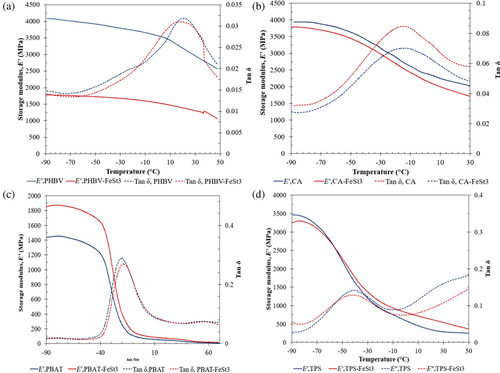
The peaks tan delta (δ), as a function of temperatures, can obtain the glass transition temperatures (Tg) of polymer films, as well as obtain information about the mobility of the polymer chain segments from the intensity of tan δ peaks. Typically, higher intensity of tan δ peaks indicates higher energy dissipation and better molecular chain movement, while lower peaks represent hindrance in the mobility of polymer chains. As anticipated, the additions of FeSt3 in various polymer systems of PHBV, PBAT, CA, and TPS decreased in tan δ intensity, which was attributed to the restricted sliding motions of polymer chains caused by the metal oxide filler. The higher Tg shift, as well as the narrower tan δ peak, of PBAT-FeSt3 compared to PBAT indicated that FeSt3 has improved compositional distribution and interaction with PBAT polymer. On the other hand, other polymers such as PHBV, CA, and TPS with FeSt3 all exhibited slightly broader tan δ as compared to the neat counterparts, indicating broader polydispersity due to the poor interaction with polymer chains with the additives and reduction in the inherent intermolecular forces.
2.3.5 Permeability testing
The various films' water vapor permeability (WVP) was evaluated at constant temperature and humidity. As anticipated, TPS exhibited the highest water weight loss through the film (from the cup) due to its inherent hydrophilicity, indicating poor water vapor barrier properties. After only 24 h, fractures were identified on the surface of all TPS films; thus, the data collection for TPS and TPS-Bi2O3 samples was aborted after 24 h (Figure S4B). No barrier property data was reported for TPS-FeSt3, as all films were torn apart after 30 min exposure to the barrier cup (Figure S4B). On the other hand, PHBV displayed the lowest WVP values, as was expected from the hydrophobicity of the polymer.26 As shown in Figure 8, CA films with and without additives presented inferior barrier properties (higher weight loss) than PBAT and PHBV. This is attributed to the polarity of CA as it is inherently a cellulosic material with β-1,4-glucosidic bonds connecting the anhydroglucose units and hydroxyl groups.31
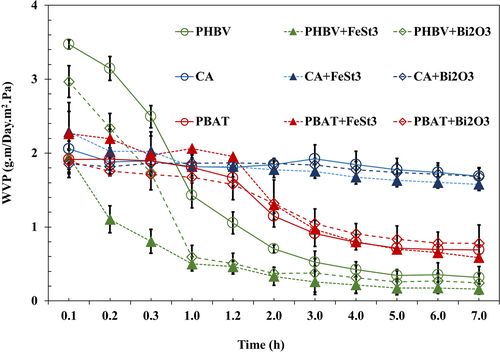
Moreover, the WVP results indicated the superior water barrier properties of PHBV and PBAT compared to CA-based films. Overall, adding FeSt3 provided higher moisture barrier properties for the film samples, while incorporating Bi2O3 only impacted the water vapor permeability of tested polymers. This is because of the hydrophobicity of FeSt3 from the stearate moieties. The rapid reduction in water vapor permeability (WVP) of polymer films during the initial hours of exposure is primarily due to the quick saturation of the film's surface and the availability of sorption sites. As moisture penetrates deeper into the film, the rate of WVP slows down, influenced by factors, such as film swelling, barrier layer formation, and the approach to an equilibrium state between moisture concentrations on both sides of the film.
2.3.6 Water absorption analysis
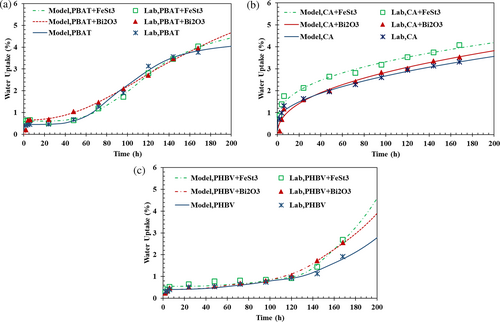
The effects of FeSt3 and Bi2O3 additives on the water absorption of the biodegradable composite films compared to neat polymeric films can be observed in Figure 9. It should be noted that the TPS sample completely dissolves and breaks down into many fragments when submerged in water due to its extreme hydrophilicity, making the water absorption test virtually impossible. As shown in Figure 9a, PBAT films showed low water absorption and substantially increased over 3 days. The PBAT sample containing FeSt3 and Bi2O3 showed a slight increase in water uptake over 7 days. Figure 9b illustrates a drastic water absorption of CA films in the first 2 days, and graphs slowly plateaued out in the last 5 days. CA-FeSt3 presented higher water uptake percentages. As shown in Figure 9c, PHBV films generally had the lowest water adsorption rate in the first 5 days, indicating higher hydrophobicity, consistent with the water vapor permeability test finding. On the sixth day of water submersion, PHBV films' uptake increased significantly. Notably, the PHBV containing FeSt3 and Bi2O3 showed a rapid increase in water uptake after 122 h, indicating that the additives increased the water absorption capability of the sample. After 8 days, PBAT and CA exhibited the highest and lowest water adsorption, respectively.
2.3.7 Dispersion morphology
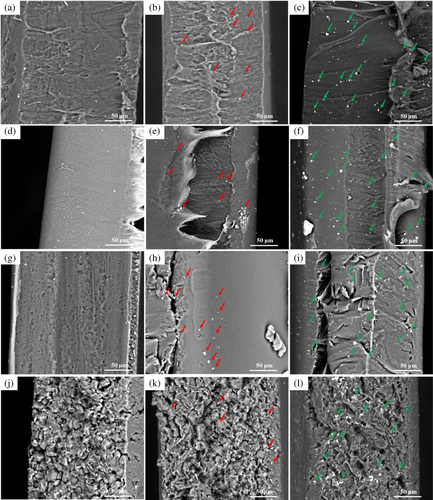
Figure 10 presents the SEM images of all compression-molded films of the samples. The location of dispersed Iron stearate and bismuth oxide on the surface of polymer sheets are marked with red and green arrows, respectively. As presented in Figure 10, due to the larger molecular weight of Bismuth oxide particles, a larger number of bismuth oxide particles are indicated than Iron stearate ones. Comparing all polymer surfaces, the porous surface of TPS, TPS-FeSt3, and TPS-Bi2O3 sheets confirms the reason for their lower tensile strength. The results indicated that all samples containing FeSt3 and Bi2O3 are finely dispersed. The microscopy image of the films is presented in Figure S5.
3 CONCLUSIONS
The primary objective of this work was to evaluate the effect of FeSt3 and Bi2O3 oxidative additives in improving the biodegradability properties of PHBV, PBAT, CA, PLA, and TPS. Also, the performance of modified and non-modified polymers was evaluated according to their mechanical, stress–strain behavior, and water-oxygen permeability properties. The varying effect of additives on the mechanical properties of the host polymer was reasonable, and the statistical analysis indicated that the impact of additives on mechanical properties depends on their interaction with the specific polymers. Although the permeability properties of the polymers were improved through introducing both additives, FeSt3 modifications have provided more improvement. Compared to all polymer films, TPS showed the weakest water adsorption resistance, which limits its application for products in contact with water. With the lowest water adsorption, CA can be the best candidate for applications that require water contact or barrier performance. PHBV-FeSt3 samples showed significantly lower permeability and water absorption, with the lowest biodegradation rate compared to other samples. PHBV is an appealing material choice for materials that require rigidity due to its highest elastic modulus. Disintegration rate analysis of CA and PLA containing different additive concentrations indicated the presence of higher concentrations of additives significantly enhanced the degradation rate of samples, as demonstrated by disintegration rate improvement.
AUTHOR CONTRIBUTIONS
Tizazu Mekonnen: Formal analysis (equal); funding acquisition (lead); investigation (equal); project administration (lead); writing – review and editing (lead). Parinaz Ataeian: Conceptualization (supporting); data curation (lead); investigation (equal); methodology (lead); validation (lead); writing – original draft (lead). Binh Minh Trinh: Data curation (equal); methodology (equal); validation (lead); writing – original draft (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
The financial support of CTK Bio Canada and MITACS is greatly appreciated.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available from the corresponding author on reasonable request.




