Influence of pozzolans on plant oil-sulfur polymer cements: More sustainable and chemically-resistant alternatives to Portland cement
Funding information: National Science Foundation, Grant/Award Number: CHE-2203669
Abstract
Low cost and high durability have made Portland cement the most widely-used building material, but benefits are offset by environmental harm of cement production contributing 8–10% of total anthropogenic CO2 gas emissions. High sulfur-content materials (HSMs) are an alternative that can perform the binding roles as cements with a smaller carbon footprint, and possibly superior chemical, physical, and mechanical properties. Inverse vulcanization of 90 wt% sulfur with 10 wt% canola oil or sunflower oil to yield CanS or SunS, respectively. Notably, these HSMs prepared at temperatures ≤180 °C compared to >1200 °C hours for Portland cement CanS was combined with 5 wt% fly ash (FA), silica fume (SF), ground granulated blast furnace slag (GGBFS), or metakaolin (MK) to give composites CanS-FA, CanS-SF, CanS-GGBFS, and CanS-MK, respectively. The analogous protocol with SunS likewise yielded SunS-FA, SunS-SF, SunS-GGBFS, and SunS-MK. Each of these HSMs exhibit high compressive mechanical strength, low water uptake values, and exceptional resistance to acid-induced corrosion. All of the composites also exhibit superior compressive strength retention after exposure to acidic solutions, conditions under which Portland cement undergoes dissolution. The polymer cement-pozzolan composites reported herein may thus serve as greener alternatives to traditional Portland cement in some applications.
1 INTRODUCTION
Ordinary Portland cement (OPC) production contributes 8–10% of the total anthropogenic CO2 greenhouse gas emissions every year,1 reflecting the requisite thermal decomposition of carbonates directly into CO2(g) as well as the CO2(g) indirectly produced by the power consumption associated with heating to the necessary temperatures (>1200°C) to achieve this transformation. Alternatives to OPC that do not involve conversion of carbonates to CO2(g) and can be accessed at lower temperatures would thus significantly reduce a major contributor to anthropogenic greenhouse gas emissions. High sulfur-content materials (HSMs), in contrast, can be prepared using temperatures as low as 159°C and, impressively, can be thermally-recycled over dozens of cycles without any losses in mechanical strength.2, 3 These intrinsically “greener” thermal features of HSMs reflect the thermally-labile SS bonds present in the polysulfide (–Sn–) catenates that comprise the bulk of the HSMs. Multiple processing methods are effective for HSMs, including hot-pressing,4 melt-casting,5 and nucleophile-induced interfacial bonding,6 which make HSMs particularly attractive in additive manufacturing applications. HSMs have shown promise in applications ranging from lithium-sulfur batteries7-16 and IR transparent lenses,17-23 to adhesives,24 fertilizers,5-11 and adsorbents for environmental remediation.25-33
Elemental sulfur, comprising principally cyclo-S8, is a low-value coproduct of petrochemical industrial processes that chemically remove S-atoms from petrochemicals to prevent poisoning of the noble metal catalysts employed in later steps and to prevent acid rain that results from burning sulfur-containing fuels. Truly staggering quantities of elemental sulfur, in excess of 70 megatons, are generated each year, the vast majority of which finds no productive use and ends up as solid waste.34-36
Consequently, HSMs represent a productive use of elemental sulfur that actively reduces petrochemical wastes in the environment, in addition to constituting a sustainable OPC alternative. By itself, elemental sulfur possesses poor mechanical properties that render it unsuitable for many structural materials applications. When heated above 159°C, elemental sulfur undergoes homolytic ring-opening to afford radical-terminated polysulfide chains, which can then add to carbon–carbon π-bonds through a process termed inverse vulcanization (Scheme 1). In this manner, the polysulfide chains are anchored to organic fragments that function as crosslinkers. Recent work has demonstrated that inverse vulcanization can be facilitated mechanochemically as well as by thermal and catalytic routes.37-40
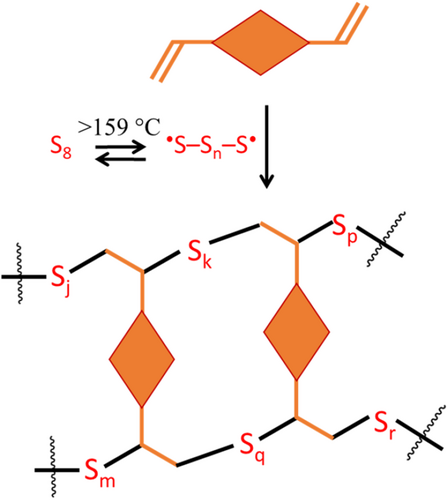
First-generation HSMs employed petroleum-derived olefins,41-44 but the scope of CC bond-containing molecules has expanded massively to include a wide variety of biologically-produced olefins. HSMs comprising fatty acids,45-47 terpenoids,23, 48-53 starch,54-56 garlic extracts,57 amino acids,58 lignin,59-63 cellulose,48, 64 and lignocellulosic biomass derivatives,62, 65 as sustainable organic crosslinkers have all been demonstrated. In fact, some classes of bio-olefins are produced from atmospheric CO2(g) as the carbon feedstock, thus the HSMs which incorporate them formally have negative carbon-footprints in terms of materials incorporated. Bio-olefins afford HSMs without any losses in mechanical strength compared to petroleum-derived olefins, and, impressively, HSMs comprising plant oils66 and animal fats as the organic crosslinkers exhibit greater compressive strength values than OPC itself.
In practice, OPC is rarely used in isolation, and is instead combined with fines, predominantly in the form of sand. The particular type of sand used in such applications is becoming scarce and its collection often involves environmentally detrimental practices such as dredging riverbeds. These deleterious aspects have led to recent efforts not only to replace OPC but also the sand. Attractive surrogates include pozzolans, generally industrial waste or more sustainably-obtained clay products.67-69 Our group has previously shown that pozzolans produced as wastes by other industries – fly ash (FA), silica fume (SF), ground granulated blast furnace slag (GGBFS), and metakaolin clay (MK) – can be combined with our sustainable HSMs to yield composites which exhibited greater compressive strength and dramatically improved corrosion resistance compared to OPC.70
Herein we evaluate the impact of combining pozzolanic mineral additives with plant oil-based HSMs on the mechanical properties and chemical resistance of the resulting composites. The HSMs CanS and SunS were synthesized by reacting 90 wt% elemental sulfur with 10 wt% canola oil or sunflower oil, respectively. Combination of 95 wt% CanS or SunS with 5 wt% of pozzolan FA, SF, GGBFS, or MK afforded the concrete-like composites CanS-FA, CanS-SF, CanS-GGBFS CanS-MK, SunS-FA, SunS-SF, SunS-GGBFS, or SunS-MK. All composites exhibited water uptake values below 0.3 wt%, compared to 28 wt% observed with OPC, and thus these composites will be far less sensitive to freeze–thaw damage. Compressive strength values for these HSM-based composites were observed within the range of 6.1–19.1 MPa, comparable to the value of 17 MPa for OPC. Perhaps the most impressive property of the HSM-based composites is exceptional resistance to chemical corrosion. Whereas 100% of OPC disintegrated upon 24 h challenge with H2SO4(aq) acid, each of the HSM-based composites retained >50% of its initial compressive strength, with 7 of the 10 composites exhibiting zero loss in compressive strength.
2 RESULTS AND DISCUSSION
Canola oil and sunflower oil were selected as the triglyceride crosslinkers for HSMs based on their high abundance of oleic acid and linoleic acid, respectively (Table 1), as well as the prior detailed characterization of the sulfur cements prepared with these oils.66 Analysis by FAMES71 revealed the canola oil and sunflower oil triglycerides comprised 92 wt% and 87 wt% unsaturated fatty acid, with average unsaturation numbers of 1.3 and 1.5 unsaturated units per esterified fatty acid chain, respectively. Heating canola oil or sunflower oil with 90 wt% sulfur at 180°C for 35 min yielded CanS and SunS, respectively, according to the reported procedure.66 Thermogravimetric analysis (TGA) revealed 5% decomposition temperatures of 227°C for CanS and 229°C for SunS. Differential scanning calorimetry (DSC) elucidated a cold crystallization peak at 81°C and crystallinity of 5.0% for CanS, with SunS exhibiting cold crystallization peaks at 24.8 and 39.8°C and crystallinity of 25%, as summarized in Table 2.66
| Canola oil | Sunflower oil | |
|---|---|---|
| Saturated Chains (%) | 8 | 13 |
| Oleic and other monounsaturated (%)a | 60 | 22 |
| Linoleic and other diunsaturated (%)a | 22 | 65 |
| Linolenic and other triunsaturated (%)a | 10 | <1 |
| Unsaturated bonds per chain (average) | 1.3 | 1.5 |
- a In each category of unsaturated chains, <2% of the oils are components other than oleic, linoleic or linolenic acid chains.
| Materials | Td/°Ca | Tcc/°Cb | Tm/°Cc | ∆Hmd J/g | ∆Hcce J/g | Percent crystallinityf |
|---|---|---|---|---|---|---|
| S8 | 229 | NA | 118 | 45 | NA | 100 |
| CanS | 227 | 81.0 | 117 | −38.0 | 35.8 | 5.0 |
| SunS | 226 | 24.8, 39.8 | 117 | −35.3 | 18.3, 5.9 | 25 |
- a Decomposition temperature, here defined as the temperature at which 5% mass loss was observed.
- b Cold crystallization temperature.
- c Melting temperature.
- d Integrated heat of melting for the orthorhombic sulfur melt transition.
- e Integrated area for all cold crystallization transitions.
- f The reduction of percent crystallinity of each samples was calculated with respect to sulfur (normalized to 100%).
Several mineral pozzolans were selected for incorporation into the CanS and SunS cements based on their availability in large quantities, low cost, and established use as OPC additives.
Specifically, fly ash (FA), silica fume (SF), ground granulated blast furnace slag (GGBFS), and metakaolin (MK) were used in this study. These pozzolans are minerals comprised by oxides or silicates of Ca, Si, Al or Fe (Table 3). The fineness moduli for these materials were determined according to the reported procedure as summarized in Table 3.70
| Materials | Primary constituents | Fineness modulus |
|---|---|---|
| Silica Fume | SiO2 | 3.78 |
| Fly Ash | SiO2, CaO, Al2O3, Fe3O2 | 2.53 |
| Ground Granulated Blast Furnace Slag | 2CaO·SiO2, CaAl2Si2O8 | 3.35 |
| Metakaolin | Al2Si2O7 | 4.63 |
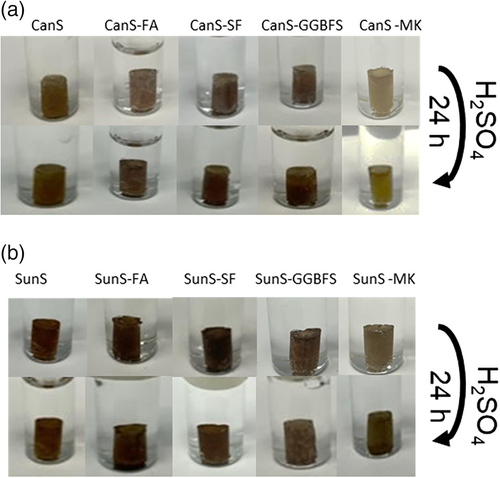
Preparation of composites was accomplished by modifying a reported procedure for preparing composites of ZOS90 (a polymer cement made from oleic acid and 90 wt % sulfur) with FA, GGBFS, MK, OPC, or SF to give FAOS, GGBFOS, MKOS, PCOS, and SFOS, respectively.70 In this work, each pozzolan-containing composite was prepared by heating CanS or SunS with 5 wt% of the pozzolan, aided by rapid mechanical stirring until the mixture had homogenized (1 hour). Attempts to incorporate 10 wt% of pozzolans led to materials that were too viscous in the molten state to pour effectively into molds. In this way, CanS was combined with fly ash (FA), silica fume (SF), ground granulated blast furnace slag (GGBFS), or metakaolin (MK) to give the final materials CanS, CanS-FA, CanS-SF, CanS-GGBFS, CanS-MK, respectively. In the case of SunS, its reaction with the pozzolans yielded composites SunS-FA, SunS-SF, SunS-GGBFS, and SunS-MK. Each of the materials was remeltable and brown in color (Figure 1).
The density (ρ) of the polymer cements and derivative composites were calculated from the dimensions and masses of the same cylinders used for other physical and mechanical tests. These calculations reveal that all materials meet American Concrete Institute (ACI) standard ACI-213R and ASTM 169C density guidelines to qualify them as lightweight structural materials (ρ = 1500–1800 kg/m3, Table 4). These densities agree well with those of a sulfur polymer cement made from oleic acid, ZnO, and 90 wt% sulfur (ZOS90) and its composites containing FA (giving FAOS), SF (giving SFOS), GGBFS (giving GGBFSOS) and metakaolin (giving MKOS).70 With ρ = 1500 kg/m3, lightweight ordinary Portland cement (OPC) has a density toward the lower end of the range for the current materials.
| Compressive strengthc | |||||
|---|---|---|---|---|---|
| Materials | Density (kg/m3)a | Water uptake (wt%)b | As-prepared (MPa) | After acid (MPa) | Retained strength (% of pre-acid) |
| CanS | 1700 | 0.26 ± 0.1 | 17.5 ± 0.6 | 9.0 ± 0.5 | 52 |
| CanS-MK | 1800 | 0.04 ± 0.1 | 10.2 ± 0.2 | 9.7 ± 0.3 | 97 |
| CanS-SF | 1800 | 0.04 ± 0.1 | 10.3 ± 2.3 | 9.9 ± 2.1 | 97 |
| CanS-GGBFS | 1900 | 0.15 ± 0.1 | 17.2 ± 1.1 | 18.0 ± 1.1 | 100 |
| CanS-FA | 1700 | 0.26 ± 0.1 | 16.5 ± 0.4 | 12.4 ± 2.9 | 75 |
| SunS | 1700 | 0.19 ± 0.1 | 14.0 ± 0.7 | 10.5 ± 1.9 | 74 |
| SunS-MK | 1700 | 0.07 ± 0.1 | 6.1 ± 0.2 | 7.0 ± 0.9 | 100 |
| SunS-SF | 1700 | 0.04 ± 0.1 | 19.1 ± 0.8 | 17.7 ± 0.8 | 93 |
| SunS-GGBFS | 1500 | 0.07 + 0.1 | 18.2 ± 0.4 | 17.7 ± 0.7 | 97 |
| SunS-FA | 1600 | 0.21 ± 0.1 | 15.4 ± 2.9 | 15.0 ± 0.5 | 98 |
| OPCd | 1500 | Up to 28% | 17 | Decomposed | 0 |
| LinS | 1800 | 0.0 | 22.9 ± 3.5 | 23.8 | 100 |
| ZOS90 | 1700 | 0.0 | 19.4 ± 1.8 | ND | ND |
| FAOS | 1700 | 0.0 | 20.6 ± 5.7 | ND | ND |
| GGBFSOS | 1700 | 0.0 | 8.50 ± 0.1 | ND | ND |
| MKOS | 1700 | 0.0 | 9.1 ± 1.2 | ND | ND |
| PCOS | 1600 | 0.1 | 22.0 ± 0.1 | ND | ND |
| SFOS | 1600 | 0.0 | 12.4 ± 4.4 | ND | ND |
- a Density is reported as the average of three measurements on cylinders used for mechanical testing.
- b Water uptake was calculated by measuring the mass before and after the cylinder was submerged in deionized water for 24 h (average of three trials).
- c Average of three measurements with standard deviations.
- d Values for residential building-grade ordinary Portland cement for foundations and footings.
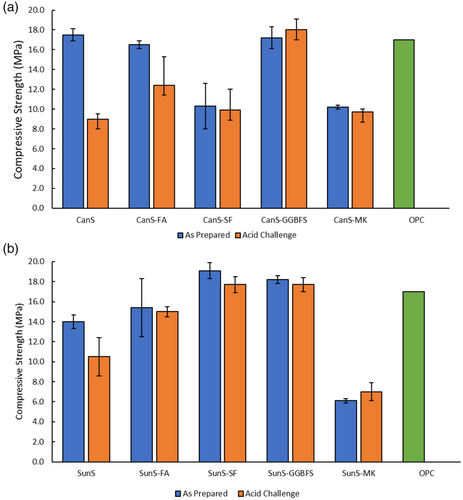
The hallmark property of OPC for structural, load-bearing applications is its high compressive strength. Building regulations for cement foundations and footings for residential buildings such as ACI specification 332.1R-06 indicate a minimum compressive strength of 17 MPa for qualified materials. The highest compressive strength previously reported for sulfur polymer cements employing plant oil cements was for a 5 wt% linseed oil/95 wt% sulfur material (23 MPa).66 In contrast, a compressive strength of over twice that required for foundations was accomplished in materials comprising 5 wt% brown grease (a high fatty acid animal fat product), 5 wt% sunflower oil, and 90 wt % sulfur.72 The compressive strengths of the canola oil and sunflower oil cements and their derivative pozzolan composites are displayed graphically in Figure 2 and summarized in Table 4. The lowest compressive strength for each series of composites is observed for metakaolin-containing composites, likely a result of the significantly coarser nature of the metakaolin powder (fineness modulus, F = 4.63) compared to those of the other pozzolans employed (F ≤ 3.78). The influence of particle size on properties has been similarly noted for HSMs comprising peanut oil, sulfur, and variably-sized ground biomass particles (ground peanut hulls, primarily lignocellulosic in composition).73 In contrast, however, reported compressive strengths of composites of ZOS90 did not show such a predictable trend with respect to fineness moduli (Table 4).70 This was attributed to the potential for reaction between basic mineral pozzolans and oleic acid used. The triglyceride plant oils used in this study are not acidic and thus do not show such reactivity. Overall, the plant oil-sulfur polymer cements and derivative composites exhibit high compressive strengths, with those of SunS-SF (19.1 ± 0.8 MPa) and SunS-GGBFS (18.2 ± 0.4 MPa) having superior strength exceeding the standard for residential building foundations.
Another advantageous property of sulfur polymer cements is their retention of mechanical integrity and strength in acidic conditions that rapidly degrade OPC. Sulfur is nonpolar and hydrophobic, and thus resists either penetration by aqueous acid or reactions with electrophilic protons. In contrast, mineral additives like pozzolans are composed of highly polar SiO or metalO bonds that are highly susceptible to degradation by acid. It was therefore of interest to assess whether the current composites would be susceptible to acid degradation or if the polymer-cement-encapsulated pozzolans would be protected from acidic solutions. Cylinders of each composite were thus submerged in 0.50 M H2SO4 for 24 h and their compressive strengths were then measures after this acid challenge (Table 4). Under these conditions a cylinder of OPC loses its shape and undergoes significant dissolution so that its compressive strength cannot be measured. After acid challenge, all of the composites retained their shape, color, and 52%–100% of their compressive strength (Figure 1). Similar results have been reported for sulfur polymer cements comprising vulcanized linseed oil (i.e., LinS, Table 4),66 cellulose derivatives and terpenoids.
3 CONCLUSIONS
Polymer cements prepared from industrial waste sulfur and plant oils via 100% atom-economical inverse vulcanization were combined with pozzolans to yield eight new polymer composites. Several of the composites exhibit density, water uptake and compressive strength that exceed those required of commercial materials used in foundations and footings of residential buildings. The composites also demonstrated superior acid resistance compared to that of traditional mineral cements. The composites reported herein thus represent more sustainably-produced alternatives to mineral cements and are superior options for acid-contact contexts such as wastewater conduits or in some industrial settings.
4 EXPERIMENTAL PROCEDURES
4.1 General considerations
Compressive strength measurements of test cylinders were carried out using a Mark-10 ES30 Manual Test Stand equipped with a Mark 10 M3-200 Force Gauge by a modified ASTM C39 standard. Materials were aged for 4 d prior to compressive strength testing to match the standard cure times reported for other triglyceride HSMs.66 CAUTION: Heating elemental sulfur with organics can result in the formation of H2S gas. H2S is toxic, foul-smelling, and corrosive. Metal salts added to these mixtures can suppress H2S generation in some such cases.46
4.2 Chemical precursor sources
All materials were used as received: canola oil (Crisco, USA), high linoleic sunflower oil (Maple Holistics, USA), elemental sulfur (Dugas Diesel, USA), fly ash (FA), silica fume (SF) and ground granulated blast furnace slag (GGBFS) pozzolanic cement (Diversified Minerals, Inc., USA) and metakaolin (MK, Opptipozz, USA). Portland cement test specimens for comparison were prepared by mixing sifted residential Portland cement (Quikcrete, USA) with twice the mass of water and allowing it to cure according to manufacturer instructions for residential building purposes.
4.3 General synthesis of triglyceride-sulfur polymer cements CanS and SunS
This procedure is adapted from the reported method.49 A 180.0 g sample of elemental sulfur and 20.00 g of the requisite plant oil (canola oil to prepare CanS or sunflower oil to prepare SunS) were added to a 2.5 L Erlenmeyer flask. The vessel was placed in a thermostat-controlled oil bath set to 180°C and stirred with an overhead mechanical stirrer equipped with a stainless-steel stir rod and vane. As the mixture heated, it initially melted to form a yellow-orange liquid at about 120°C. As the temperature of the mixture exceeded 160°C, the viscosity increased and the liquid took on a deep red color characteristic of polymeric sulfur radicals. After 35 min the color had changed to a deep brown color. After cooling to room temperature, the materials were hard, non-sticky, remeltable brown solids. Materials were shaped into compressive test cylinders by pouring the molten liquids into silicone molds.
4.4 General procedure for addition of pozzolans to polymer cements to give derivative composites
This procedure follows a reported method for adding pozzolans to fatty acid-sulfur polymer cements.70 The pozzolan-containing composites were prepared by heating 50.0 g of the requisite plant oil-sulfur polymer cement material with 2.6 g of the pozzolan to a temperature of 180°C with stirring for 1 h, after which the mixture was removed from the heat and cylinders for compressive analysis were prepared by pouring the molten mixture into silicone molds.
4.5 Treatment of samples to assess acid resistance
Acid challenge was performed by submerging samples into 0.50 M H2SO4 for 24 h after which they were removed, rinsed gently with DI water, and blotted dry. The compressive strength of the cylinders were then immediately tested. A minimum of three cylinders were subjected to this process for each material evaluated and the errors reported are the standard deviations of these tests.
AUTHOR CONTRIBUTIONS
Matthew J. Graham: Data curation (lead); formal analysis (lead); investigation (lead); writing – original draft (supporting); writing – review and editing (supporting). Claudia V. Lopez: Data curation (supporting); formal analysis (supporting); writing – review and editing (supporting). Charini P. Maladeniya: Data curation (supporting); writing – review and editing (supporting). Andrew G. Tennyson: Formal analysis (supporting); resources (lead); supervision (supporting); writing – review and editing (supporting). Rhett C Smith: Resources (supporting); supervision (lead); writing – original draft (supporting); writing – review and editing (lead).
ACKNOWLEDGMENTS
This research was funded by The National Science Foundation grant number CHE-2203669.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.