Dispersion of hydroxyapatite nanoparticles in natural rubber latex and poly lactic acid based matrices
Funding information: This work was supported by FAPESP (grant numbers 2016/09736–8, 2017/19603–8, 2017/16324–0, and 2018/20089–0). Author H.P. acknowledges financial support from the Australian Government: RTP scholarship.
Abstract
Hydroxyapatite (HAp)-polymer composite materials are of interest for materials that interface with or substitute bone tissue. HAp nanoparticles (length 290 nm, width 56 nm) synthesized by a wet chemical method were incorporated (5 wt%) into natural rubber latex (NRL), poly lactic acid (PLA) and NRL/PLA (75/25) solvent cast films using three different methods: dry nanoparticles; a dispersion prepared from dry nanoparticles; a dispersion prepared through solvent exchange. Common to all composite materials were an improvement in the contact angle by 10%–13% and a reduction in the elongation at break from 430% to 330%–370%. Furthermore, the method of HAp incorporation influenced the properties of the material with the use of dioxane suspension improving the homogeneity of the films as evidenced from visual appearance and SEM. This led to slower water uptake and higher thermal stability with a shift in the PLA melting peak from 296°C to 334°C when compared to pure polymer and composites with poorly dispersed particle.
1 INTRODUCTION
Hydroxyapatite (HAp) has applications in bone regeneration due to its high affinity for biopolymers and high osteogenic activity. It is a calcium phosphate based mineral similar to that found in bone where it makes up about 65% of total bone mass.1 There is a large variety of methods developed to synthetize nano-sized HAp including dry methods, wet methods, high-temperature processes, methods based on biogenic sources and combination procedures. The wet method called chemical precipitation or co-precipitation is frequently used due to the ability to control the nanoparticle characteristics.1, 2
HAp-polymer composites have been widely explored for development of biomaterials that are intended to be in contact with or substitute bone tissue.3 The incorporation of HAp into a polymer matrix has previously been shown to improve hydrophilicity,4, 5 the modulus and strength,4-6 in vitro mineralization,6 in vitro degradation,4, 7, 8 in vitro cell adhesion8, 9 as well as in vivo performance.7, 8 The choice of polymer in such composites depends on the intended application and is to some extent driven by the desired mechanical properties and desired rate of degradation.3, 10
Natural rubber latex (NRL) and poly lactic acid (PLA) have gained considerable interest for the use as biomaterials. NRL has been used in medical prostheses and grafts due to its ability to stimulate angiogenesis and cell adhesion,11 in addition to its good film forming capability. It is a natural polymer extracted from the rubber tree Hevea brasiliensis and is a colloidal system which is composed of 50% water, 30–45% rubber (cis-1,4-polyisoprene), and 4%–5% nonrubber components (phospholipids and proteins).12 Furthermore, it has shown promise for guided bone regeneration.12-14 PLA is a bioresorbable synthetic thermoplastic polymer with current applications in orthopedics because of its high mechanical strength and high modulus of elasticity, as well as low toxicity.15 NRL and PLA blends have been developed for bone regeneration and a previous study showed that a film with a NRL:PLA mass composition of 3:1 had the best properties for this application.16 The blend film had improved degradation properties and no hemolytic effects.16 To further improve its properties for application in bone regeneration, the incorporation of HAp is an attractive approach, which has been explored previously in the literature for both NRL and PLA,4, 8, 17-22 but not their blends.
To maximize the benefit of incorporating HAp, the use of nanosized particles offers a larger surface area and potential for greater benefit at lower filler loading.3 However, it can be challenging to achieve good dispersion of HAp nanoparticles in a polymer matrix (required for enhanced bulk properties) as well as good presentation of HAp at the surface (required for enhanced bioactivity). This is particularly challenging when the polymers in the material are hydrophobic and require dissolution in an organic solvent. Poor dispersion and formation of agglomerates can act as nucleation sites for creation of defects leading to fracture planes in the material. One strategy to minimize agglomeration in organic solvents involved the use of chemically modified HAp nanoparticles.3 In some cases, enhanced dispersion of the HAp nanoparticles results in enhanced mechanical properties5 while in other cases no improvements are observed.23
The first aim of this study was to evaluate the incorporation of HAp nanoparticles into NRL/PLA blend films and compare to that of the pure PLA and pure NRL. This included evaluating the dispersion of the nanoparticles within the polymer and their presentation at the surface to enhance the hydrophilicity of the material based on visual appearance, Fourier transform infrared (FTIR) and contact angle measurements. HAp nanoparticles were synthetized by a wet chemical method, characterized by FTIR and transmission electron microscopy (TEM), and incorporated into the materials using three different methods: in a dry form, in a dispersion prepared from dry nanoparticles, and in a dispersion prepared through solvent exchange. The second aim of this study was to characterize in detail the composite films of the blend and this was achieved using scanning electron microscopy (SEM), FTIR spectroscopy, swelling, thermogravimetric analysis (TGA), and mechanical evaluation.
2 EXPERIMENTAL
2.1 Material
NRL was purchased from BDF Latex (Guarantã, Brazil) as a suspension in ammonia. The rubber particles are composed of a mixture of the two clones RRIM 600 and PB 235. Prior to use, the NRL solution was centrifuged at 8000g to reduce the NRL protein content resulting in a final solution concentration of 60% dry rubber content.12 Poly lactic acid (PLA) was purchased from Zhejiang Hisun Biomaterials Co. (China) (density of 1.25 ± 0.05 g cm−3, melting point of 135–150°C, glass transition temperature of 54–60°C). Dichloromethane (DCM), 1,4-dioxane (DO), calcium nitrate tetra hydrate (99%), diammonium hydrogen phosphate (99%), and ammonia solution (28%–30%) were purchased from Sigma-Aldrich. Ultra-pure (UP) water was obtained from a Veolia PURELAB flex system.
2.2 Methods
2.2.1 HAp nanoparticle synthesis
The synthesis of HAp nanoparticles followed a published procedure.24 In short, calcium nitrate tetra hydrate (1.174 g) dissolved in water (45 ml) was heated to 40°C. Diammonium hydrogen phosphate (0.394 g) dissolved in water (35 ml) was slowly added to the calcium nitrate solution with vigorous stirring. The resulting suspension was brought to pH 11 by addition of concentrated ammonia (28%–30%). To reduce the carbonate content, the mixture was brought to the boil and immediately removed from the heat, then kept under stirring overnight.
Three different HAp preparations were made as outlined in the Supporting Information (Figure S1). First, to produce dried nanoparticles, the as-prepared HAp suspension was centrifuged, and the HAp pellet thoroughly washed five times with UP water followed by oven drying overnight at 80°C. The resulting HAp was ground into a fine powder using an agate mortar and pestle.24 Second, dry HAp nanoparticles were used to produce dispersions in UP water or in DO. Third, HAp dispersions were produced through solvent exchange from the as-prepared HAp suspension. The HAp water suspensions were prepared directly from the as-prepared suspension by washing as described above. To prepare a DO suspension, the washed HAp nanoparticles were transferred from the aqueous dispersion into DO by iterative solvent exchange (25%, 50%, 75%, and 100%). All HAp dispersions had a concentration of 0.025 g/ml.
The dispersion stability of the HAp nanoparticles in DCM, DO and water was evaluated by dispersing 0.0125 g of dry HAp nanoparticles in 3 ml of the solvent by manual mixing and observing the dispersion 10 min after mixing.
2.2.2 Fabrication of polymer and composite films
NRL, PLA, and NRL/PLA blend films were prepared by casting method according to Cesar et al.16 using a Petri dish mold and leaving at ambient conditions to dry. In short, the NRL suspension was used to prepare NRL films while PLA granules dissolved in DCM at room temperature were used to prepare PLA films. To prepare the NRL/PLA blend films, solid PLA and NRL solution were stirred with DCM at an NRL:PLA mass ratio of 3:1 until the polymers had fully dissolved. The final mass of all films was 0.25 g.
Composite films incorporating 5 wt% of HAp were prepared using three different methods and polymer contents of 0.25 g. The first method used dried HAp nanoparticles (0.0125 g) which were added to the polymer solutions. The second method used dispersions of the dried HAp nanoparticles in DO or water (0.025 g/ml, described in Section 2.2.1). The DO dispersion was added to the PLA and NRL/PLA solutions, while the water dispersion was added to the NRL solution. The third method used the HAp dispersions in UP water and DO (0.025 g/ml) which were prepared through solvent exchange (described in Section 2.2.1). For this method, the DO dispersion was added to solutions of PLA and NRL/PLA, while the water dispersion was added to the NRL solution. The dispersions were left in a sonication bath for 5 min before casting to form composite films as described above. The detailed composition of all samples as well as the sample names are described in Table 1 and the surface which was in contact with glass during the casting process is represented as the glass surface (GS), while the opposite surface is represented as the air surface (AS).
| Sample | HAp type | m(HAp) (g) | m(NRL) (g) | m(PLA) (g) |
|---|---|---|---|---|
| NRL-D-HAp | Dry HAp | 0.0125 | 0.25 | – |
| NRL-D-Aq-HAp | Dried HAp dispersion in water | 0.0125 | 0.25 | – |
| NRL-Aq-HAp | HAp dispersion in watera | 0.0125 | 0.25 | – |
| PLA-D-HAp | Dry HAp | 0.0125 | – | 0.25 |
| PLA-D-DO-HAp | Dried HAp dispersion in DO | 0.0125 | – | 0.25 |
| PLA-DO-HAp | HAp dispersion in DOb | 0.0125 | – | 0.25 |
| NRL/PLA-D-HAp | Dry HAp | 0.0125 | 0.1875 | 0.0625 |
| NRL/PLA-D-DO-HAp | Dried HAp dispersion in DO | 0.0125 | 0.1875 | 0.0625 |
| NRL/PLA-DO-HAp | HAp dispersion in DOb | 0.0125 | 0.1875 | 0.0625 |
- a As prepared HAp.
- b As prepared HAp solvent exchanged into DO.
2.3 Characterization
2.3.1 Fourier transform infrared spectroscopy
2.3.2 Scanning electron microscopy
SEM was used to observe the surfaces morphology of the NRL/PLA blend films. SEM was performed in a high-vacuum mode at 6 kV on a SU3500 (Hitachi) microscope on samples coated with iridium. The images obtained at 4000 times magnification were analyzed on freeware ImageJ v1.53a to calculate the percentage area of HAp coverage. The images were defined as 8-bit and in black and white filter. The threshold was set as default, first auto adjusted, and then fine adjusted manually to guarantee that the areas of interest were contrasted.
2.3.3 Transmission electron microscopy
TEM was performed on a Hitachi HT7700 transmission electron microscope equipped with a tungsten filament operation at 80 kV. Oven-dried and solvent exchanged samples were dispersed in ethanol from the as-prepared suspension and diluted to 1:100 in filtered ethanol. The 8 μL aliquots of each sample was dispersed onto formvar coated, plasma cleaned, copper TEM grids (200 mesh) and left to dry for at least 2 h in a desiccator before analysis. Bright field images were analyzed in ImageJ (ver. 1.53) to measure size and morphology of HAp nanoparticles.
2.3.4 Water contact angle
Water contact angle was measured on both surfaces (AS and GS) of the films by placing a drop (5 μL) of UP water on three different spots of the film using an OCA 20 (DataPhysics). The angle between the drop and the surface of the material was calculated immediately after placing the drop on the material by an ellipse fitting method using the DataPhysics SCA202 software. The following symbols, θAS and θGS, are used to denote contact angles measured on the AS and the GS, respectively.
2.3.5 Thermogravimetric analysis
Thermogravimetric analysis was measured on samples (approximately 10 mg) from 35 to 700°C, at a heating rate of 10°C/min, using a thermal analyzer PerkinElmer TG4000 under nitrogen flow to obtain temperature curves of weight and derivative weight versus temperature. The loading of HAp estimated using triplicate samples was 4.4% ± 0.8% in agreement with the theoretical loading.
2.3.6 The swelling ratio
2.3.7 Mechanical properties
2.4 Statistical analysis
Experiments performed in replicates were expressed as a mean ± SD. Statistical analysis was performed for contact angle measurements, swelling ratio and mechanical properties in software OriginPro 8.5 by normality test followed by one-way analysis of variance (ANOVA) and Tukey test. Differences were considered significant at a level of p < 0.05. For IR values, samples were considered significantly different when two values were more than one SD apart.
3 RESULTS AND DISCUSSION
3.1 Fabrication of composite films
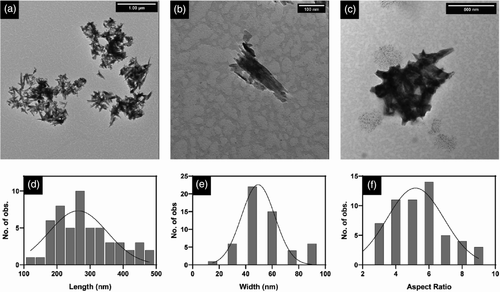
The mineral filler, HAp (Ca10(PO4)6(OH)2), used in the current study was produced via a chemical precipitation method using calcium nitrate as the Ca2+ source and diammonium hydrogen phosphate as the PO43− source. This method is a simple and low-cost procedure that results in nano-sized HAp particles.24, 25 Nanoparticles isolated by gradual solvent exchange with DO were imaged with TEM (Figure 1a,b) where the average particle length and width were 290 ± 13 nm and 56 ± 2 nm, respectively (n = 55) (Figure 1d, e). This morphology was considered ‘needle-like’ with an average aspect ratio of 5.5 ± 0.2 nm (Figure 1f). The size is larger than that previously reported25 and this is attributed to a longer curing time in the current study (overnight stirring compared to 1 h). The oven-dried particles appeared to agglomerate and fuse to create >500 nm clusters (Figure 1c). Confirmation of the chemical composition of HAp prepared in the current study was achieved from FTIR analysis as described in the Supporting Information (Figure S2).
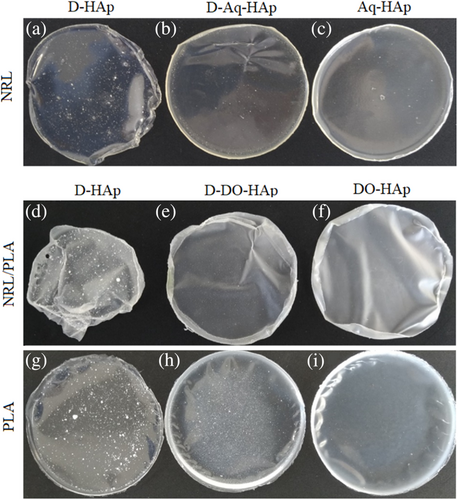
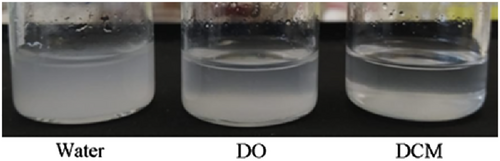
Three different approaches were used to incorporate the HAp nanoparticles into the polymer films at 5 wt% loading for production of the composite films as outlined in Section 2.2.2 and Table 1. The homogeneity of the composite films at the macroscopic scale was affected by the method of HAp addition during the material fabrication as shown in the photographs in Figure 2. In the first approach, the nanoparticles were added in a dry form to solutions of the polymer prior to casting (Figure 2a, d, and g), and poor HAp dispersion was observed. For all three composite films made this way, large mineral clusters can be clearly observed indicating large-scale agglomeration and/or aggregation, however, this appears to be less pronounced in the NRL film (NRL-D-HAp). Other studies have likewise found poor dispersion of calcium-phosphate based nanoparticles in polymer composites when using dried inorganic particles added directly to a polymer solution.3 The second approach made use of a HAp dispersion created from dry HAp nanoparticles prior to mixing with the polymer solutions and this greatly improved the nanoparticle dispersion in the films (Figure 2b, e, and h). The poorest dispersion amongst these samples was observed for the PLA-D-DO-HAp composite displaying nanoparticle clusters. The presence of large HAp clusters in these films is related to the clusters observed in the TEM images of dried nanoparticles (Figure 1c). In contrast, using the third approach of solvent exchange, thereby avoiding drying the nanoparticles prior to composite fabrication meant that the HAp nanoparticles remained dispersed (Figure 1a,b) and this resulted in films with no visible HAp clusters (Figures 2c, f and i). Overall, the appearance of the films (Figure 2) can furthermore be correlated to the relative dispersion stability of the HAp nanoparticles in different solvents. DO and water were chosen as solvents for HAp incorporation because of the miscibility with DCM and with NRL solutions, respectively. The dispersion stability of the nanoparticles shown in Figure 3 revealed that the water dispersion remained cloudy for 10 min and that there was evidence of nanoparticle sedimentation in the DO suspension. For the DCM suspension, the nanoparticles fully sedimented within 10 min. Thus, the dispersion stability for HAp particles in different solvents is; water > DO > DCM. Poor dispersion stability of HAp in DCM has been reported previously.26 The good particle dispersion in the NRL composite films compared to the PLA and NRL/PLA films, thus correlates with the use of a water dispersion for its preparation. Furthermore, the PLA composite films displayed the poorest dispersion of the HAp nanoparticles regardless of how they were added to the polymer solution. This correlates with the poor dispersion stability of the HAp nanoparticles in DCM. Even when adding the DO-nanoparticle suspension to the polymer solution, the majority of the solvent was DCM. Previous studies have likewise found a correlation between HAp particle dispersion in solution and in a polymer matrix. Chemically modified particles that showed higher dispersion stability in DMF, also had superior dispersion in a PHBV film which resulted in improved mechanical properties.5 Likewise, a direct correlation was observed between mechanical properties of HAp PCL composites and the dispersion stability of chemically modified HAp preparations in the solvent DCM.26
3.2 Evaluation of particle dispersion through surface analysis
The polymers NRL and PLA are considered hydrophobic15, 27 and the incorporation of the hydrophilic filler HAp in the current study was done with the overall aim of reducing the hydrophobicity which was evaluated through contact angle measurements. Since this method evaluates only the outmost molecular layer, it allows evaluation of the surface presentation of HAp. The water contact angle data of the pure films (Figure S3) are in agreement with those previously reported.15, 27, 28 The water contact angle of the NRL/PLA film fall between those of the pure NRL and PLA films, but closer to the NRL contact angle which correlates with NRL being the dominant polymer in the blends (75%). For PLA, a value of 94 ± 3° for the AS is observed and this is a larger value compared to θGS which is attributed to the higher surface roughness of the AS due to solvent evaporation. For the NRL and NRL/PLA films, the θGS values are higher (89 ± 2°) than θAS which can be attributed to the presence of nonrubber constituents, such as proteins and lipids (4%–5%) which migrate to the AS of the film during the drying process reducing the hydrophobicity.28
The addition of HAp into the films significantly decreased the contact angle of all samples (Figure S4), except θAS of the NRL-D-Aq-HAp film, making the materials more hydrophilic as previously reported.4, 5 There was, however, no clear trends observed in the contact angle values and the method of HAp incorporation. A similar observation was made for HAp/PLA composites prepared either by dry mixing or solvent dispersion.21 For the PLA films incorporating HAp all θAS values were statistically insignificant as were all θGS values. The average reduction was 18° for θAS and 10° for θGS. For one sample, PLA-D-DO-HAp, the θGS value was significantly lower than θAS. In this sample, it would therefore appear that a larger proportion of HAp agglomerates are protruding from the GS. For the NRL composite films, all θGS values were statistically insignificant; however, the θAS value of the NRL-D-HAp sample was significantly lower than that of the other composite films. For these samples, a significantly lower contact angle value was observed for the AS compared to the GS of the NRL-D-HAp and NRL-D-Aq-HAp films, while for the other composite film, the values for the AS and GS were not significantly different. The average reduction of the contact angle for the NRL films was 7° for the AS and 16° for the GS. For the NRL/PLA films incorporating HAp, the θAS values were not statistically different, however, the θGS value of sample NRL/PLA-DO-HAp was significantly lower than for the other composite films. All the NRL/PLA composite films display a significantly lower θAS value compared to θGS. The average reduction of the contact angle for the NRL/PLA films was similar (11–12°) for both the AS and GS.
The chemical composition of the surface of the films was evaluated using ATR-FTIR spectroscopy. This method measures to a depth of approximately one micron in polymeric materials. The FTIR spectrum of the dry HAp nanoparticles is provided in the Supporting Information (Figure S2) together with band assignments. Most importantly, for the current work are the bands at 560, 600 and 1025 cm−1 attributed to various PO4 vibrational modes. The spectra of the polymer films are included in Figure 4. The PLA film (Figure 4a) has characteristic bands that appear at 1750 cm−1 (C═O stretch), 1185 cm−1 (asymmetrical COC stretch) and 1080 cm−1 (symmetrical COC stretch).29 The NRL polymer (Figure 4b) displays characteristic bands 1446 cm−1 (CH2 deformation), 1375 cm−1 (CH3 deformation), 1661 cm−1 (C═C deformation), and 836 cm−1 (out of plane C-H deformation) signifying the R2C═CHR (cis-1,4) moiety.14 The NRL/PLA films (Figure 4c) display bands from both components. In previous studies, the detection limit of HAp in polymer composites has been observed to be 10%–50% depending on spectral overlap.6, 7, 30 Considering that the composite films produced in the current study contained 5% of HAp, it is expected that bands from this component are observed only if enriched in the outmost one micron of the film. For the PLA-D-HAp sample (Figure 4a) bands at 560 and 600 cm−1 are clearly observed and this is accompanied with an increase in the intensity at 1025 cm−1. For the other PLA composites, there is, however, no clear evidence of the presence of HAp and it appears that there is an impurity peak at 600 cm−1. The NRL/PLA films (Figure 4c) display a shoulder/band at 600 cm−1 and again this is accompanied with a change in the intensity at 1025 cm−1. For the NRL films (Figure 4b) there is not any clear evidence of the HAp nanoparticles.
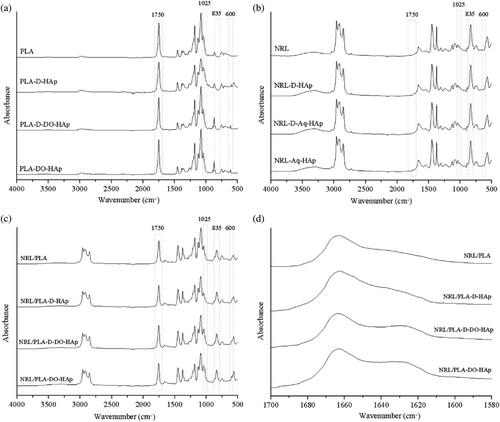
In order to evaluate the relative surface presentation of HAp on the AS versus GS and in different films, specific bands were selected to represent the different components. Since the absolute intensity of a band in an ATR-FTIR spectrum cannot be correlated to concentration/amount of a component, intensity ratio (IR) values were used in this evaluation. The intensity at 1750 cm−1 related to PLA (I1750) and 600 cm−1 related to HAp (I600) were used to calculate IRHAp (outlined in Section 2.3). For the PLA composites, these bands uniquely represent each of the components with minimal absorbance from the other component as clearly seen in the figure comparing the FTIR spectra of each of the components provided in the Supporting Information (Figure S5). For the NRL/PLA-based materials, the main bands of HAp (560, 600 and 1025 cm−1) all overlap with bands from NRL (Figure S5). Since IR values are compared to the pure polymer material this may still give valuable information about the relative HAp content and it was found that similar trends were observed using either of the main HAp bands. The IRHAp values are given in Table 2 and are defined such that the larger the value, the greater is the PLA component at the surface. It should be noted that the use of the IRHAp values is only valid if the relative presentation of the two polymers at the surface in the blend remain constant. To validate this hypothesis, the segregation of the two polymers NRL and PLA depth-wise in the films was assessed by calculating the IR based on the unique PLA band at 1750 cm−1 and the unique NRL band at 835 cm−1. The IRPLA/NRL values are listed in Table 2 and are defined such that a larger value corresponds to a higher PLA presentation at the surface. For the NRL/PLA-DO-HAp sample, both the GS and AS values are significantly different to the pure polymer blend. This means that the evaluation based on the IRHAp values for this sample is not valid. However, for the other two NRL/PLA samples, there are no significant difference in the IRPLA/NRL values between the pure NRL/PLA film and the composite films and the evaluation based on the IRHAp values is considered valid.
| Sample | IRHAp | IRPLA/NRL | ||
|---|---|---|---|---|
| GS | AS | GS | AS | |
| PLA | 22 | – | – | |
| PLA-D-HAp | 3.7 ± 0.2 | 4.3 ± 0.6 | – | – |
| NRL/PLA | 4 | 1.7 | ||
| NRL/PLA-D-HAp | 3.4 ± 0.7 | 3.4 ± 0.3 | 2.1 ± 0.2 | 1.5 ± 0.1 |
| NRL/PLA-D-DO-HAp | 2.8 ± 0.5 | 2.84 ± 0.03 | 1.7 ± 0.4 | 1.6 ± 0.1 |
| NRL/PLA-DO-HAp | – | – | 3.4 ± 0.2 | 1.45 ± 0.04 |
- Note: Measurements were done in triplicate and the SD is given. For the pure polymer samples, only a single value was obtained.
The IRHAp values (Table 2) were used to gain a relative measure of the presentation of HAp at the two surfaces of selected films. PLA-D-HAp (prepared by adding oven dried HAp nanoparticles to a PLA solution) displayed a significantly reduced IRHAp value for both the GS and AS compared to the pure PLA film indicating that there was HAp present in the first micron of the film on both surfaces. The values for the two surfaces were, however, not considered significantly different. For the NRL/PLA-D-HAp sample (prepared adding oven dried nanoparticles into the polymer solution), the values for AS and GS were the same and not considered significantly different from the polymer film. However, for the NRL/PLA-D-DO-HAp sample (prepared by adding a suspension of dried HAp nanoparticles to the polymer solution), a reduction in the IRHAp value compared to the polymer film was observed for both AS and GS. Based on this evaluation, the relative surface presentation of HAp appears to be NRL/PLA-D-HAp < NRL/PLA-D-DO-HAp.
3.3 Comparison of particle dispersion
The dispersion of HAp nanoparticles in the different composite films was evaluated in terms of visual appearance, FITR and contact angle measurements in order to compare the effect of HAp incorporation and the effect of polymer type. For the PLA materials, the visual appearance indicated that the use of HAp dispersed in DO improved the HAp dispersion within the polymer film and this was most pronounced for the material where the dispersion was prepared using solvent exchange. Only for one sample (PLA-D-HAp prepared by adding dry HAp nanoparticles into the PLA solution) was HAp detectable by FTIR indicating that this material had the largest amount of HAp present in the outmost one micron of the film, in agreement with the visual appearance. The contact angle values for all PLA composite samples were significantly lower than for the pure PLA film, however, not different between the different composite films indicating that at the outmost molecular layer, the same proportion of HAp was exposed. This in turn indicates that for the PLA-D-HAp film, a large portion of the nanoparticles that are present in the outmost micron (as detected by FTIR) are not exposed to the outmost surface. In addition, for the PLA-DO-HAp film where no visible clusters can be observed, it can be inferred that agglomerates are less than one micron or that the HAp particles remained on the nanoscale.
For the NRL materials, no bands from HAp could be conclusively detected in the FTIR spectra. Since the spectral overlap of the HAp and NRL components are greater than for HAp and PLA this lack of bands from HAp in the NRL materials does not necessary equate to a lower amount of HAp at the surface of these materials. From the visual inspection, both NRL-D-Aq-HAp and NRL-Aq-HAp contained well dispersed with HAp nanoparticles in the polymer matrix, indicating that the method of using a HAp dispersion is advantageous. Contact angle values were lower for all composite materials compared to the control NRL film. The NRL-D-HAp film had a significantly larger reduction in the θAS value compared to the other composite NRL films indicating a larger proportion of HAp clusters formed and were exposed on the surface in agreement with the visual inspection.
For the NRL/PLA films, the visual appearance indicated good dispersion of HAp in the films where HAp was added as a DO dispersion. A more detailed picture could be obtained from the SEM analysis which is discussed in Section 3.5. The quantitative FTIR analysis indicated that there was more HAp in the outer one micron of the film for NRL/PLA-D-DO-HAp compared to NRL/PLA-D-HAp. Contact angle values were lower for all composite materials compared to the control NRL/PLA film. The wettability of the AS was the same for all materials and lower than the GS which can be attributed to the presence of proteins and lipids at this interface.
Overall, the poorest dispersion of the HAp nanoparticles were seen for the PLA composite materials and the best dispersion was seen for the NRL composite materials. This observation correlates with the particle dispersion stability in relevant solvents. Furthermore, these results indicate that the method of solvent exchange can be considered a good method to incorporate HAp leading to homogeneous materials which can be attributed to the limited agglomeration of HAp during the formation of the composite and this aspect is discussed further below.
3.4 Detailed evaluation of NRL/PLA composite films
FTIR data have previously been used to assess interactions between components in a mixture and changes in band positions attributed to strong intramolecular interactions. The FTIR transmittance spectra comparing the NRL/PLA films with and without HAp are displayed in Figure S6. Based on this, there is no clear evidence of intermolecular interactions between HAp and NRL or between HAp and PLA. However, previous research has suggested that the calcium ions (Ca2+) in the mineral phase directly interact with proteins and phospholipids present in NRL.18 Because HAp could be detected in the composite NRL/PLA films, it was possible to evaluate the presence of intermolecular interactions between the HAp filler and the proteins from the NRL component through evaluation of the amide-I region (1620–1700 cm−1) of the AS and this spectral region is displayed in Figure 4d. It should be noted, that the OH-bend of water appears within the amide I region (at 1640 cm−1),31 however, inspection of the full spectra (Figure S6) reveals only minor differences of the relative intensity of the OH-stretch (at 3360 cm−1) making it valid to evaluate the amide I region in the current study. From the data presented in Figure 4d it appears that the main band in the NRL/PLA film is due to the secondary protein structures α-helix and/or random coil (at 1665 cm−1)31 and this band is present in all films incorporating HAp. For the NRL/PLA-D-DO-HAp and NRL/PLA-DO-HAp composite films (prepared using a DO dispersion of HAp nanoparticles), an additional band is present at approximately 1625 cm−1. This position corresponds to β-sheet which would imply enrichment of specific proteins and/or change of secondary structure, however, may also be related to for example, hydrogen-bonding between the protein and the HAp nanoparticles.31 This data thus gives evidence for intermolecular interactions between the proteins inherently present in NRL and the HAp nanoparticles.
The morphology of the NRL/PLA composite films was observed by SEM (Figure 5). The NRL/PLA film (without nanoparticles, Figure 5a, e, i) displays a rough AS while the GS is much smoother. The surface of pure NRL films has previously been reported as smooth and continuous, with the presence of streaks resulting from stretching since the polymer is an elastomer.12, 32 The surfaces of pure PLA films are influenced by solvent evaporation leading to formation of cavities on the surface exposed to air during the drying process. Thus, the morphological features of the NRL/PLA films are similar to those previously observed for pure polymer films. Large clusters of HAp are observed on the GS of the film prepared by addition of dry HAp (NRL/PLA-D-HAp) and to a lesser extent on the AS. The clusters are several microns on the AS and more than 20 μm on the GS. The larger proportion of HAp on the GS can be attributed to nanoparticle sedimentation under gravity during film formation. When preparing the films by incorporating HAp using DO as dispersant of the dry nanoparticles (NRL/PLA-D-DO-HAp) the resulting dispersion of the filler in the film appears to be improved. Much smaller HAp clusters are found on the AS, less than a micron in size. On the GS, large clusters of HAp nanoparticles are again observed, but these somewhat smaller in size (~10 μm) compared to that observed on the GS for the NRL/PLA-D-HAp sample. In contrast, for the NRL/PLA-DO-HAp sample, only very few and very small HAp clusters are visible, both on the GS and the AS. For this sample it thus appears that the nanoparticles are well distributed throughout the polymer matrix.
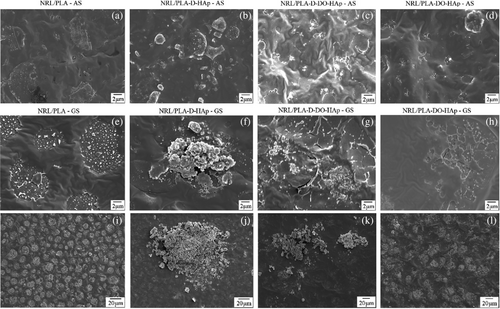
In order to obtain a semi-quantitative measure of the extent to which HAp clusters were present on the AS and GS for each of these films, the HAp percent coverage on the surface of the films was analyzed with details provided in the Supporting Information (Figure S7 and Table S1). Because the NRL/PLA films display features as a result of phase separation, the pure films were also analyzed to evaluate the threshold above which the HAp percent coverage determined by image analysis was considered significant. It can be seen from the data in Table S1 that depending on the method of HAp addition the percent HAp coverage changes. The sample NRL/PLA-D-HAp had the largest percent coverage on the AS, while the value for GS is considered similar to that found for the NRL/PLA-D-DO-HAp sample. A large reduction in HAp coverage is, however, found for the GS of the NRL/PLA-D-DO-HAp sample compared to NRL/PLA-D-HAp. For the NRL/PLA-DO-HAp sample, the values obtained were not significantly different to that of the control NRL/PLA film indicating that no HAp could confidently be identified on these surfaces. These values corroborate the general observations made above.
These results support the evaluation presented in Section 3.3 and indicate that the films fabricated with the suspension prepared through solvent exchange are more homogeneous than the films fabricated using the dispersion prepared from dry nanoparticles. A similar approach of solvent exchange has previously been used to fabricate HAp-decorated carbon nanotube composite PLLA materials where uniform dispersion of the particles were reported.8 It can thus be concluded that avoiding the drying process of the HAp nanoparticles improve their dispersion. This is furthermore supported by the TEM data where individual nanoparticles <200 nm were observed when imaging a DO suspension prepared using solvent exchange (Figure 1a, b) whereas the images of dried HAp nanoparticles appeared as agglomerates with sizes >500 nm indicating poor redispersion after drying. Previous studies have likewise observed that drying of HAp nanoparticles cause formation of nanoparticle agglomerates that are difficult to redisperse23 impacting the properties of the polymeric biomaterials, such as mechanical properties and degradation behavior.5, 19, 20, 23 Overcoming this issue can be achieved using water soluble polymers to in-situ template HAp nanoparticle precipitation.30, 33 However, this approach is not applicable to hydrophobic polymers such as NRL and PLA of the current study. For such polymers a common reported strategy is the use of surface modification of HAp,3 however, the simple solvent exchange process used in the current study has the advantage of simplicity and low cost.
The SEM images in Figure 5 give evidence of the level of phase separation and/or de-wetting of the two polymers in the pure polymer film compared to the different composite films specifically regarding the lateral distribution of domains and the domain size. Phase separation has been reported previously for the 75/25 composition of NRL/PLA.16 In the current study, we observed that the domains that form due to this process are more easily recognized on the GS where they were spherical and 5–10 μm in diameter. On the AS they had more varied size and shape. The AS of all the composite films had less pronounced de-wetting compared to the NRL/PLA polymer film. The sample NRL/PLA-D-HAp appeared to have some phase separation on the GS, where the domains were of similar size but less pronounced. On the GS of the NRL/PLA-D-DO-HAp sample, less regular domains appeared to be present, this was especially clear from the low magnification image. For the NRL/PLA-DO-HAp sample, the GS showed very irregular phase separation, however, based on the low magnification images the size of the domains was larger than for the pure polymer film. It thus appeared that, in general and to a large extent for the NRL/PLA-D-DO-HAp sample, the incorporation of HAp reduced the level of phase separation/de-wetting. Similar observations of improving the compatibility between polymers in blends have previously been made for other blend-nanocomposites, for example, PLA/PCL with various fillers including carbon nanotubes,34 organoclays,35 micro-talc,36 cellulose nanowhiskers,37 and nanosilica.38
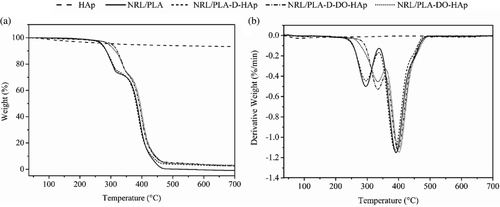
The thermal behavior of NRL/PLA based matrices with and without HAp nanoparticles was analyzed through TGA and the data are shown in Figure 6. The NRL/PLA film underwent a two-step degradation process with the first mass loss displaying a peak in the DTG curve (Figure 6b) at 296°C, which accounted for 25% of the total mass and was attributed to PLA degradation.39 The second mass loss of 75% displaying a peak at 390°C and is attributed to NRL degradation.40 Such two-step degradation indicates poor or complete lack of polymer interactions resulting in an immiscible blend.41 The HAp nanoparticles showed a mass loss of 6.4% associate with bound water in agreement with literature.42 The composite films displayed different thermal stability depending on the method of fabrication. The behavior of the NRL/PLA-D-HAp film mimicked that of the pure polymer while for the NRL/PLA-D-DO-HAp and NRL/PLA-DO-HAp films, a shift in the first peak to higher temperature (334 °C) was observed while there was no major change for the second peak (shifted to 400 °C for NRL/PLA-DO-HAp). In general, the addition of ceramics into a polymer matrix can provide a thermal barrier and therefore enhance the thermal stability and this has been observed in composite materials using lower filler loadings than in the current study.43, 44 However, when nanoparticle agglomerates were present in the polymer matrix, no such improvement was observed.39 Furthermore, when adding fillers into polymer blends, the changes in thermal properties can be used to evaluate in which component of the blend the nanoparticles are localized.45 In the current study, when HAp was added directly into the PLA solution (NRL/PLA-D-HAp), HAp agglomerates were observed and no change in thermal stability resulted when compared to the pure polymer. This can thus be attributed to limited interaction of the HAp agglomerates with the polymer matrix. However, when using a DO suspension, the particle dispersion in the polymer matrix was improved (from SEM) and intermolecular interactions with the proteins were evident (from FTIR). The improvement in dispersion of the HAp nanoparticles possibly resulted from improved interaction of the nanoparticles with the polymer matrix leading to an improvement in the thermal stability of the blend. Furthermore, considering that only the mass loss associated with PLA was affected by the presence of HAp, it would appear that the nanoparticles interacted more strongly with PLA than NRL, and this was potentially mediated by the proteins from the NRL.
3.5 Properties of NRL/PLA composite films
In order to further investigate the influence of the presence of HAp on the wettability of the composite NRL/PLA films, the swelling ratio as a measure of the water absorption capability was evaluated. This was done over a period of 168 h by immersion in water (Figure 7) to gain longer term evaluation and complement the results obtained by the contact angle measurements. The NRL/PLA film displayed a swelling ratio of more than 10% within the first 1.5 h and continued to take up water over the entire period with a plateau being reached at the 120 and 168 h time points (S(%) = 58% at 168 h). The water absorption of NRL/PLA films has previously been evaluated in phosphate buffered saline at 37°C, resulting in a swelling ratio of 25% after 48 h.16 In comparison, the current study found a swelling ratio of 42% in water after 48 h at room temperature. Both temperature and ionic strength are known to affect the water uptake, and the larger swelling ratio observed in water compared to phosphate buffered saline is in agreement with previous studies.46
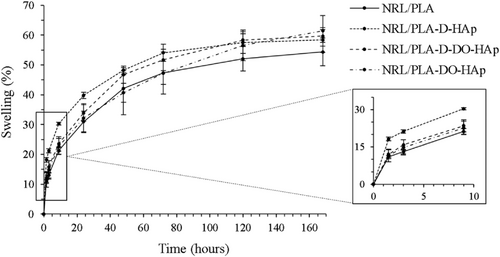
The composite NRL/PLA films generally displayed a higher S(%) and as such, the water absorption capacity was improved with the addition of the HAp nanoparticles in agreement with the water contact angle results discussed above and in agreement with literature.47 Specifically, the S(%) at 168 h was approximately 10% higher for the composite films compared to the NRL/PLA film. In the first 10 h, the NRL/PLA-D-HAp sample showed a significantly higher S(%) compared to the other composite films, while after 168 h, all composite films had reached similar values. Both NRL/PLA-D-HAp and NRL/PLA-D-DO-HAp appeared to have reached an equilibrium value at 168 h while NRL/PLA-DO-HAp appeared to still increase the S(%). Considering that all the composite films displayed similar water contact angle, the higher initial swelling (at 10 h) of NRL/PLA-D-HAp is attributed to the presence of large HAp agglomerates that are poorly integrated into the polymer film. The increased homogeneity observed for the composite films produced using nanoparticles dispersed in DO (NRL/PLA-D-DO-HAp and NRL/PLA-DO-HAp) resulted in an initial lower S(%) and a longer time to reach equilibrium swelling. This correlates with the better nanoparticle dispersion and stronger interactions between the nanoparticles and the polymer matrix (evident from SEM, FTIR and TGA). Furthermore, the NRL/PLA-DO-HAp film display the slowest swelling which correlates with the analysis by SEM where the nanoparticles were seen to be most effectively dispersed.
The samples were subjected to a puncture test to evaluate the impact of the incorporation of the HAp nanoparticles on the mechanical properties. The samples displayed yielding behavior with higher strength at the yield point compared to the breakpoint. We therefore report both the ultimate puncture strength and the puncture strength at rupture together with the elongation at break for all samples in Table 3. The value obtained for the puncture strength at rupture and elongation at break are similar to those previously reported for the NRL/PLA film.16 Incorporation of 5 wt% of HAp nanoparticles did not affect the puncture strength of the composite films, however, it did significantly reduce the elongation at break. Previous studies in the literature have observed various effects on mechanical properties with incorporation of HAp nanoparticles into polymeric materials.3 While for some materials an improvement in mechanical properties is observed,5, 26 for other materials, no effect is seen5, 23 and still other studies find that the properties decline.39 For polyester-based materials, even when dispersion of the nanoparticles is successful, no change in mechanical properties may be found and this has previously been associated with the nanoparticles being present in the amorphous phase of the polymer in composites of relatively low filler content. At higher filler content, however, the nanoparticles can cause an increase in the crystallinity of the material thereby causing enhanced mechanical properties.3, 23 Considering the various degrees of dispersion of the HAp nanoparticle filler in the composite films of the current study, it is likely that the well-dispersed nanoparticles are mainly in the amorphous region. Furthermore, since we found evidence for interactions of the HAp nanoparticles with the PLA component of the blend, potentially mediated by the proteins from NRL, future studies should evaluate higher amounts of HAp incorporation as previous studies have shown improvements in mechanical properties of PLA with high filler content.21, 48
| Sample | Parameters | ||
|---|---|---|---|
| Ultimate Ps (MPa) | Ps at rupture (MPa) | Elongation at break (%) | |
| NRL/PLA | 2.3 ± 0.3a | 2.1 ± 0.3b | 430 ± 40c |
| NRL/PLA-D-HAp | 2.7 ± 0.6a | 2.1 ± 0.3b | 370 ± 20d |
| NRL/PLA-D-DO-HAp | 2.4 ± 0.4a | 2.1 ± 0.3b | 340 ± 30d |
| NRL/PLA-DO-HAp | 2.6 ± 0.5a | 2.2 ± 0.3b | 330 ± 30d |
- Note: Measurements were done with five or six repeats and the SD is given. Mean followed by the same letter within a series indicates no significant differences (p > 0.05).
4 CONCLUSIONS
The first aim of this study was to evaluate the incorporation of HAp nanoparticles into NRL/PLA blend films by different methods and compare to that of the composite PLA and NRL films. Three different methods were used to incorporate 5 wt% HAp into films of these materials, and all displayed an improvement of material hydrophilicity, indicating that incorporation of HAp acts as a surface modifier which could be used for improvement of material properties, making it more suitable for biological application such as bone regeneration. The different methods of adding HAp yielded visibly very different levels of particle dispersion within the polymers and FTIR was observed to give valuable information regarding relative proportion of nanoparticles present in the outmost one micron leading to the conclusion that the use of a nanoparticle solvent dispersion improves dispersion in the polymer matrix. The second aim was to evaluate in detail the HAp composite of the blend film. SEM confirmed the improved dispersion in the polymer when using a DO suspension of HAp nanoparticles, especially for nanoparticles that had not been dried. This was attributed to enhanced interactions between the nanoparticles and PLA mediated by proteins from NRL based on data obtained from FTIR and TGA. The water uptake by the films were affected by the presence of HAp and by the method of HAp incorporation. However, the mechanical properties did not discriminate between the different composite materials with all displaying a reduced elongation while the puncture strength was not changed compared to that of the pure polymer blend and future work will assess higher loadings of HAp nanoparticles to further explore how the mechanical properties may be altered. Overall, the method presented in the current study for improving nanoparticle dispersion is relatively simple and cheap compared to other approaches such as chemical modification of the HAp nanoparticles.
ACKNOWLEDGMENTS
The authors wish to thank Alexandra Louise Mutch, Bruna Cambraia Garms and Imanda Jayawardena for their assistance in laboratory activities, including obtaining SEM images as well as Professor Maria Palmira Daflon Gremião for assistance in obtaining mechanical performance of the materials. The authors acknowledge the facilities, and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis (CMM), UQ. Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
AUTHOR CONTRIBUTIONS
Mariana Biondi Cesar: Conceptualization (equal); data curation (lead); writing – original draft (lead). Hamish Poli: Data curation (supporting); formal analysis (supporting); methodology (supporting); writing – review and editing (equal). Rodolfo Debone Piazza: Data curation (supporting); funding acquisition (supporting); resources (supporting). Rodrigo Fernando Costa Marques: Data curation (supporting); funding acquisition (supporting); resources (supporting). Rondinelli Donizetti Herculano: Conceptualization (equal); funding acquisition (lead); resources (equal); supervision (equal); writing – original draft (supporting). Lisbeth Grondahl: Conceptualization (supporting); formal analysis (lead); project administration (lead); resources (supporting); supervision (equal); writing – review and editing (lead).
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




