Adsorption of γ-aminopropyltriethoxysilane onto bulk iron from aqueous solutions
Abstract
The adsorption of γ-aminopropyltriethoxysilane (γ-APS) onto mechanically polished iron coupons from 1% aqueous solutions has been investigated using reflection–absorption infrared spectroscopy. Thick films were formed on the coupons during 1-hr exposures to the γ-APS solutions. The outermost portion of these films consisted of highly hydrolyzed polysiloxanes that were weakly bound and easily desorbed by cold water. This fraction of the films was characterized by an infrared band near 1575 cm−1 that was tentatively assigned to the NH2 deformation of acceptor amine groups in strong hydrogen bonds or to coordination of the amino nitrogen atoms to silicon atoms. Removal of the weakly bound outermost portion of the films with water revealed an incompletely hydrolyzed, strongly bound film approximately 60 Å in thickness characterized by a band near 1510 cm−1 that was assigned to NH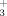 groups. Formation of NH3+ groups indicated that γ-APS may have been adsorbed initially as cyclic, internal zwitterions.
groups. Formation of NH3+ groups indicated that γ-APS may have been adsorbed initially as cyclic, internal zwitterions.




