Site-selective multi-emitter gold-silver metallopolymers: A novel class of self-assembled materials
Funding information: POCTEFA FEDER, Grant/Award Number: EFA 356/19; Ministerio de Ciencia e Innovación, Grant/Award Number: PID2019-104379RB-C22
Abstract
New luminescent heterometallopolymers [(Au-C6F5)m(AgOSO2CF3)n (PVP)] with different Au:Ag molar ratios were synthesized. The luminescent emissions of these polymers are strongly dependent on the temperature, excitation wavelengths, and the Au(I):Ag(I) molar ratio. The incorporation of a precise amount of Ag(I) atoms in the backbone of [(Au-C6F5)m(PVP)] metallopolymer allows a controlled tuning of the emission energy in the green-blue range.
1 INTRODUCTION
Luminescence in gold and gold-heterometal molecular systems is a fascinating property that these types of materials display. One of the most active areas of research is the study of these photophysical properties associated to self-assembled gold-based molecular systems through the interplay of non-covalent interactions such as aurophilicity, metallophilicity, hydrogen bonding, π-π stacking, halogen bonding, and other types of weak interactions.[1, 2] The rational choice of the metal centers, ligands, and the aforementioned interactions allow the control of the structure–property relationships in these self-assembled systems, leading to the observation of amazing stimuli-responsive properties such as mechano-, thermo-, solvato-, or chemo-chromism.[3] In most cases, these properties are observed in self-assembled systems in solution or in the crystalline solid state; however, other types of supramolecular organizations provide alternative forms of self-assembly through non-covalent interactions whose characteristics allow to deepen into the properties of these systems from alternative points of view. These supramolecular organizations include soft-materials such as metallogelators,[4-8] which produce the immobilization of organic solvents or water in the supramolecular assembly and may lead to particular emissive properties, or liquid crystals, whose mesomorphic behavior can also provide additional and interesting photoluminescent properties, not affordable in solution or in crystalline states.[9, 10]
On the other hand, metallopolymeric materials constitute a promising class of non-crystalline self-assembled materials with potential applications[11-13]; nevertheless, gold-based metallopolymer materials are scarcely represented in the literature.[14-16] Some of us previously reported the study of metallopolymers of the type [(Au-C6X5)m(PVP)] (X = F and Cl; PVP = poly[4-vinylpyridine])[15] and, recently, Hobbollahi et al.[16] reported a study on [(Au-Cl)m(PVP)]. Interestingly, although there are many examples of supramolecular coordination or organometallic crystal structures displaying metallophilic interactions,[1] as far as we are aware, there is no report on the formation of metallophilic Au(I)···M (M = closed shell metal) interactions within an organic polymeric matrix.
In these systems, there is no periodic assembly of molecular units, since the polymerization process takes place in the crystalline solid state. This allows the appearance of metal atoms with different environments within the same polymeric matrix, what provides a fine-tuning of the emissive properties of the metallopolymers thanks to site selective excitations, which lead to different emissions within the same material. In the present study, we envisage the possibility of including Ag(I) ions in the gold-based metallopolymer [(Au-C6F5)m(PVP)], what introduces additional chemical tools to tune the emission energy, the site-selective emission, and the thermochromism of the materials thanks to the delicate control of the Ag(I):Au(I) ratio.
2 SYNTHESIS AND CHARACTERIZATION
The homometallic polymer [(Au-C6F5)m(PVP)] was prepared by mixing a dichloromethane solution of [Au(C6F5)(tht)] (tht = tetrahydrothiophene) with the PVP (poly[4-vinylpyridine]) polymer in a 1:1 (Au:Py-units) molar ratio. After a gravimetric analysis, it was determined that the most of the pyridine positions (84%) were occupied leading to a final stoichiometry of [(Au-C6F5)0.84(PVP)] (1).[15] On the other hand, two different strategies were carried out to incorporate increasing amounts of Ag(I) cations in the backbone of the metallopolymer (see Scheme 1). The first one consists of a two step-process, in which the metallopolymer [(Au-C6F5)m(PVP)] with different gold(I) amounts is first prepared, and, in a second step, the addition of the corresponding diethyl ether solution of [AgOSO2CF3] is carried out. Based on this methodology, two different initial Au:Ag:Py-units molar ratios were tried: 3:1:4 and 2:2:4, leading to metallopolymers 2 and 3, respectively, matching the number of metal centers with the number of coordination sites, in order to try to complete all the available pyridine positions. Attempts to increase the Ag(I) content following this synthetic procedure were unsuccessful due to solubility problems. Thus, a second strategy was used. A higher Ag(I) content can be achieved by the direct mixing of the metal precursors in solution in a 1:3 Au(I):Ag(I) molar ratio, and the further addition of PVP, using a 1:3:4 Au:Ag:Py-units molar ratio, what led to the synthesis of the metallopolymer 4. Despite the use of stoichiometric metal:pyridine ratios, the metal content is lower to that of pyridine units, since the chemical reduction of the resulting materials with hydrazine followed by calcination of the organic residue at 700°C led to products with 32% (2), 26% (3) and 27% (4) metal weight content.
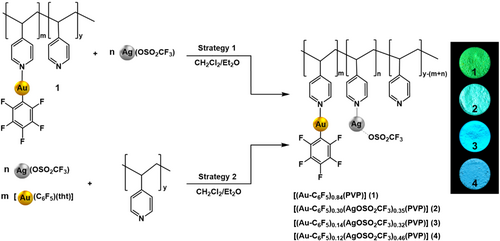
The new solids obtained in these reactions were insoluble in all the solvents tested, unlike the gold polymer [(Au-C6F5)0.84(PVP)] (1), which is slightly soluble in polar solvents such as dichloromethane or methanol.[15] This is likely to be related to an increasing degree of entanglement of the polymeric chains as the number of possible metal–metal interactions increases.
As a direct consequence of the insolubility of the new materials, it was not possible to characterize them by solution techniques such as NMR. However, solid state techniques such as infrared spectroscopy (FT-IR) or X-ray photoelectron spectroscopy (XPS) provide interesting conclusions. Thus, the coordination of the metal centers to the nitrogen atoms of the PVP was confirmed in the IR spectra of metallopolymers 2–4 (see the supporting information). All of them show, among others, three absorption bands due to the presence of the [Au-C6F5] fragments at ca. 807, 960, and 1505 cm−1, as well as bands related to the stretching vibrations υ(C=N) arising from the pyridine rings, which appear around 1615 cm−1. Finally, the presence of the silver salt in the materials was detected by the stretching bands at 1250 and 1160 cm−1, assigned to the trifluoromethylsulfonate anion. We also performed X-ray photoelectron spectroscopy (XPS) analysis of all the samples to gain insight into the atomic composition of the metallopolymers. The wide spectra of the samples confirm the atomic composition of the metallopolymers with signals corresponding to Au, Ag, C, N F, O, and S (see supporting information). The integration of the signals also provides information on the silver:gold ratio within these materials. The Ag(I)/Au(I) ratio present in the final products according to this analysis is 1.15 (2), 2.3 (3), and 3.8 (4) in agreement with the increasing amounts of Ag(I) salt added. In view of the above, and considering the results obtained by the thermogravimetric analysis that indicates the metal content in the polymers, the final formula of the heterometallopolymers can be described as [(Au-C6F5)0.3(AgOSO2CF3)0.35(PVP)] (2) [(Au-C6F5)0.14(AgOSO2CF3)0.32(PVP)] (3) and [(Au-C6F5)0.12(AgOSO2CF3)0.46(PVP)] (4). These results indicate that the addition of the silver salt produces both the coordination of the Ag(I) centers to uncoordinated nitrogen atoms and the displacement of some of the [Au-C6F5] units from the metallopolymer initially formed as [Au(C6F5)(tht)], considering that tetrahydrothiophene molecules are present in the reaction mixture. In addition, according to the broad variety of coordination numbers of Ag(I) ions, we cannot currently discard the possibility of that the Ag(I) centers could be coordinated to more than one pyridine unit. This situation would provoke an increasing degree of entanglement in the polymer, increasing insolubility and displacing [Au-C6F5] units to some extent. In any case, these experiments provide a series of Au(I)-Ag(I) metallopolymers with an increasing silver(I) content if compared to the Au(I) metallopolymer 1 (see supporting information).
3 PHOTOPHYSICAL PROPERTIES AT RT AND 77 K
The metallopolymers described in this study display very intense luminescent emissions in the solid state at room temperature and at 77 K when they are irradiated with UV-light (see Scheme 1 and the supporting information).
Considering that PVP does not display luminescence at room temperature, and it is only very weakly emissive at 77 K (λemis 430 nm),[16] the emissions observed for the metallopolymers are likely to be related to the presence of the metal centers coordinated to the pendant pyridine fragments in the polymer.
The absorption spectra of the heterometallopolymers and the free polymer PVP in solid state display a very intense absorption band at 255 nm. When both Au(I) and Ag(I) metal centers are coordinated to the polymer, a red-shift of the absorption band-edge is observed (see supporting information). This result could be interpreted as a participation of the metal fragments in the lower energy absorptions, probably through charge transfer transitions.
On our previous reported results,[15] the luminescent properties of the homometallopolymers [(Au-C6X5)(PVP)] (X = F, Cl) were assigned to excited states related to aurophilic interactions, which can be both intra- and interchain along the polymer. This fact leads to the existence of multiple metal–metal interactions along the polymeric chains that differ in their Au···Au distances, number of next-neighbor gold(I) atoms, geometries, and so forth. Consequently, there is a large number of possible excitation sites and, subsequently, of emission energies, which are associated to the different Au···Au structural arrangements. A similar assignment has been carried out by Hobbollahi et al.[16] in the case of the [(Au-Cl)0.4(PVP)] polymer ruling out the possibility of emissions associated with the molecular entity [AuClPy], since this complex does not display any luminescent emission. Nevertheless, in our case, apart from a similar origin, we cannot discard charge transfer transitions involving the [Au-C6F5] groups and the pyridine rings as an additional origin of the luminescent properties of this kind of metallopolymers, since it has been shown that complex [Au(C6Cl5)(Me-Py)] is emissive in solid state at room temperature and 77 K, even in the absence of aurophilic interactions.[17]
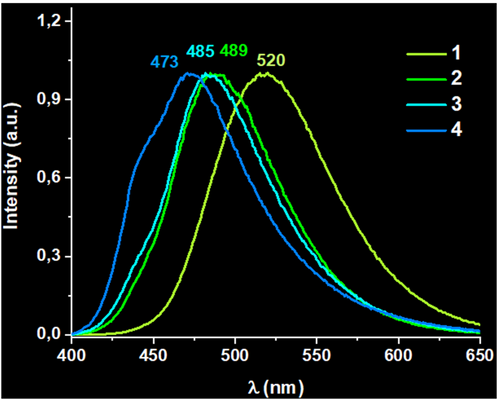
In the case of the Au-Ag heterometallopolymers, interesting luminescent properties are observed since their emission wavelengths are strongly dependent on and can be easily tuned by (i) the Ag:Au molar ratio, (ii) the excitation energy, and (iii) the temperature. Regarding the Ag:Au ratio, in Figure 1, we can observe that, at room temperature and with a fixed λexc = 375 nm, an increase of the silver content in the metallopolymer leads to a blue-shift of the emission energies from 520 nm (τ = 1.80 μs, ϕ = 0.51, 1)[15] to 489 nm (τ1 = 0.55 μs, τ2 = 1.83 μs, ϕ = 0.41, 2), 485 nm (τ1 = 0.83 μs, τ2 = 2.17 μs, ϕ = 0.41, 3) or 473 nm (τ1 = 0.44 μs, τ2 = 1.64 μs, ϕ = 0.05, 4). A similar emission blue-shift upon Ag(I) addition has been previously described for heterometallic Cu(I)-Ag(I) coordination polymers.[18] All the emissions display lifetimes related to forbidden transitions of triplet parentage. It is worth mentioning that when the only metal center present is silver, no emissive properties are observed. In addition, the lifetime decays display several components for 2–4 metallopolymers that would arise from different emissive pathways, consequence of the possibility of several metal environments within the polymer and/or different number of metal···metal interactions.
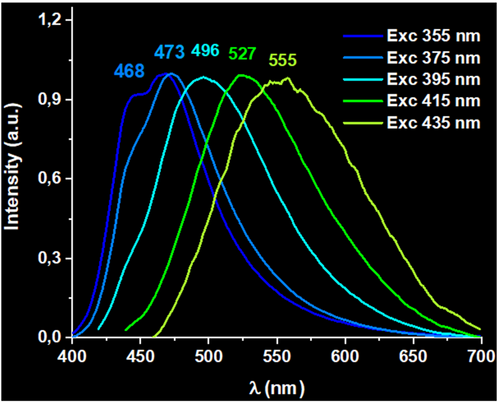
On the other hand, the emissive behavior of polymers 2–4 can be tuned by a site-selective excitation. The site-selective emission spectra of these metallopolymers range from 480 to 523 nm (2); 479 to 535 nm (3) and 468 to 555 nm (4) with λexc between 355–435 nm. In addition, the emissions collected are broader and blue-shifted when they are compared with those recorded for the homometallopolymer 1 when the same excitation energies are used (see supporting information). As an example, Figure 2 depicts the site-selective excitation emission spectra for 4, which displays the broadest emission range. In a similar way, site-selective excitation spectra can be collected upon changing the emission maximum (see supporting information). Unexpectedly, under 435 nm excitation at RT, the emission wavelength shifted to shorter wavelengths in the following order, 4 > 3 > 2. A plausible reason would be related to the possible shorter inter-chain interactions between gold(I) centers of different polymeric chains.
Finally, the third factor that allows a tuning of the emissive properties of these metallopolymers is the temperature. We have obtained similar site-selective excitation emission spectra for all the metallopolymers in solid state at 77 K, obtaining emissions between 474 and 560 nm (2); 470 and 560 nm (3), and 466 and 565 nm (4) with λexc between 355 and 435 nm (see Figure 3 and supporting information), in all cases displaying larger lifetimes. In this case, the effect of the temperature is more pronounced for metallopolymer 2, for which a larger red-shift of the lower energy site-selective emissions is observed. This could be probably due to a larger number of aurophilic interactions in 2, which are more sensitive to thermal contraction effects if compared to 3 or 4, leading to the observed red-shift. In contrast, when a higher Ag(I) content is present in 3 or 4, the luminescent emissions are likely to be influenced by the presence of an increasing number of Au(I)···Ag(I) short interactions, which would preclude the formation of the Au(I)···Au(I) ones (see computational studies below), avoiding the observation of large red-shifts of the lower energy site-selective emissions.
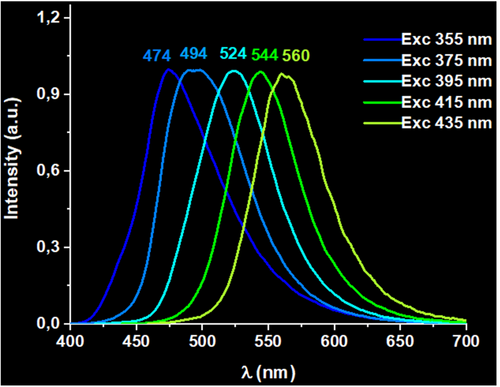
4 COMPUTATIONAL STUDIES
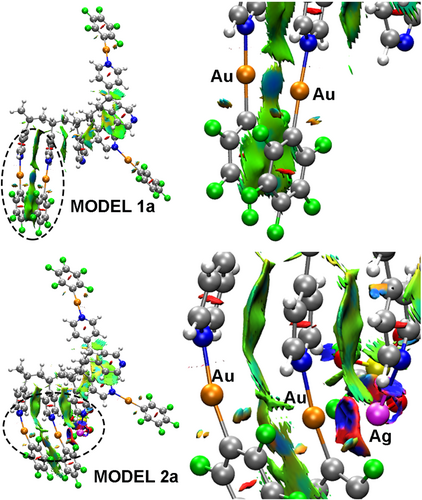
To deepen on the origin of the emissive properties of the metallopolymers, we performed DFT and TD-DFT calculations on the fully optimized fragment model systems [(Au-C6F5)4(CH3-Py-(CH-[Py]-CH2)4-CH2Py)] (1a) and [(Au-C6F5)4(AgOSO2CF3)(CH3-Py-(CH-[Py]-CH2)4-CH2Py)] (2a) (See supporting information). Model system 1a represents a simplified distribution of [Au-C6F5] units within the polymer leading to isolated [(Au-C6F5)(Py)] moieties and [(Au-C6F5)(Py)]···[(Au-C6F5)(Py)] weakly interacting aurophilic ones. Model 2a represents an heterometallic Au-Ag metallopolymer, in which the influence of a Ag(I) center on the Au(I) coordination environment is considered.
Since the presence or absence of metallophilic or other types of weak interactions can influence the resulting photophysical properties, we first carried out the topological analysis of the computed density by the calculation of spatial distribution of the non-covalent interactions (NCI). Figure 4 depicts the density spatial distribution of the non-covalent interactions (NCI) in the real space for model systems 1a and 2a[19] (see supporting information for details). Red colored isosurfaces represent steric and repulsive contacts; green isosurfaces are assigned to weak van der Waals interactions; blue or green-blue isosurfaces represent attractive non-covalent contacts, where the blue ones are the strongest. In the case of model 1a, a green-blue isosurface appears between interacting Au(I)···Au(I) centers, in agreement with the presence of weak aurophilic interactions in the metallopolymer. Moreover, broad green isosurfaces, representing weak π-π stacking interactions, appear along the metallopolymer between pyridine and pentafluorophenyl ligands. The analysis of the NCI isosurfaces for model 2a shows up interesting changes that take place upon coordination of Ag(I) ions to pyridine. The first observation is that, although the π-π stacking interactions between pyridine or C6F5− rings are still present, the Au(I)···Au(I) interaction represented by the green-blue isosurface is almost lost. Instead, a blue NCI isosurface, representing a new and strong metallophilic Au(I)···Ag(I) interaction, appears. This interaction is also supported by a Ag(I)···Cipso one as it is shown in the detail image on Figure 4.
The study of the electronic structures found for models 1a and 2a through the analysis of the frontier MOs and the TD-DFT computed singlet-singlet excitations, provide information about the electronic transitions that describe the theoretical absorption spectra. We have represented the computed absorption spectra (the most intense among the first 90 singlet-singlet excitations) for models 1a and 2a with the experimental absorption ones in solid state (see Figure S20 and Tables S2 and S3 in the supporting information). These results show that the most intense computed excitations consist of charge transfer transitions from the electron rich C6F5− ligands or the [Au(C6F5)] fragments, to π* orbitals of the pyridine groups in the polymer. In addition, the lowest energies of the singlet-singlet excitations representing the experimental low energy shoulders observed in the solid-state absorption spectra for Au-Ag metallopolymers, consist of charge transfer transitions from the C6F5− ligands to π* orbitals of the pyridine groups in the polymer.
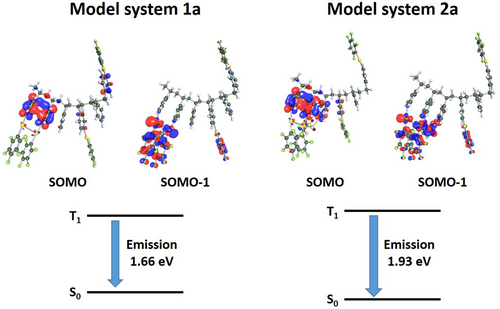
To gain insight into the emissive behavior of the metallopolymers we also computed the optimization of model systems 1a and 2a in the lowest triplet excited state (T1). These calculations allow the analysis of the structural distortions when the metallopolymers are excited to this electronic state as well as the character of the singly occupied MOs (SOMO and SOMO-1) directly involved in the phosphorescent emissions. In addition, the computed emission energies can be compared with the experimental ones. The structural changes of 1a and 2a in the T1 state are collected in Table S1. Figure 5 depicts the frontier SOMO and SOMO-1 for both model systems and the computed emission energies. The most important structural changes observed for both models when going from the S0 to the T1 excited state are the shortening of the intermetallic Au···Au and Au···Ag interactions as well as the Au-N distances, in agreement with the involvement of these atoms in the emissive properties. The character of SOMO and SOMO-1 orbitals confirms this idea, showing that the excited electron in SOMO is located in the pyridine units bonded to Au(I) centres, whereas the SOMO-1 is placed in the interacting metals and the pentafluorophenyl rings. Therefore, the emission for both models is ascribed to a Ligand to Metal–Ligand Charge Transfer transition 3(LMLCT). Finally, the calculation of the energy of the T1 computed structures in the S0 ground state permits to estimate the energy of the emissive vertical transition T1-S0. As it is shown in Figure 5, the T1-S0 vertical emission for the Au-Ag metallopolymer appears at higher energy than the Au one, in agreement with the observed experimental results.
5 CONCLUSIONS
We have designed a novel strategy for the preparation of very intense luminescent heterometallopolymer systems [(Au-C6F5)m(AgOSO2CF3)n (PVP)] displaying site-selective emissions in the green-blue range. Experimental and computational results confirm that the presence of perhalophenylgold(I) groups coordinated to pyridine rings are a required condition to obtain luminescent emissions. Moreover, as concluded from the computational studies, the inclusion of silver(I) centers in the polymeric arrangement promotes an important modification in the environment of the gold(I) centers. This modification of the Au-based metallopolymer gives rise to the formation of short Au(I)···Ag(I) interactions and, therefore, to the breakup of several aurophilic interactions or to the increase of the Au···Au distances, making the silver(I) centers aurophillic-disturbing agents. Consequently, a blue shift is observed for the luminescent emissions, which are much more noticeable as the silver content increases. In addition, these Au-Ag metallopolymers also display site-selective emissions and very interesting thermochromic properties.
6 EXPERIMENTAL SECTION
6.1 General
The compound [Au(C6F5)(tht)][20] was prepared by literature methods. The starting materials PVP (poly[4-vinylpyridine], MW = 60,000) and Ag(CF3SO3) were purchased from Sigma-Aldrich and used as received.
6.2 Instrumentation
Infrared spectra were recorded in the 4000–500 cm−1 range on a PerkinElmer FT-IR Spectrum Two with an ATR accessory. Diffuse reflectance UV–vis spectra of pressed powder samples diluted with KBr were recorded on a Shimadzu (UV-3600 spectrophotometer with a Harrick Praying Mantis accessory) and recalculated following the Kubelka−Munk function. Excitation and emission spectra in the solid state as well as lifetime measurements were recorded with an Edinburgh FLS 1000 fluorescence spectrometer. Quantum yields were measured in the solid state using a Hamamatsu Quantaurus-QY C11347–11 integrating sphere with excitation at 375 nm. XPS experiments were performed on an AXIS Supra spectrometer (Kratos) using a monochromatized Al Kα source (1486.6 eV) operating at 12 kV and 10 mA. Wide scans were acquired at analyzer pass energy of 160 eV, whereas high resolution narrow scans were performed at constant pass energy of 20 eV and steps of 0.1 eV. The photoelectrons were detected at a take-off angle of F = 0° with respect to the surface normal. Basal pressure in the analysis chamber was less than 5 × 10−9 Torr. The spectra were obtained at room temperature. The binding energy (BE) scale was internally referenced to the C 1 s peak (BE for C − C = 284.9 eV).
6.3 Synthesis
- Synthesis of metallopolymers [(Au-C6F5)0.3(AgOSO2CF3)0.35(PVP)] (2) and [(Au-C6F5)0.14(AgOSO2CF3)0.32(PVP)] (3): To a solution of [Au(C6F5)(tht)] (145 mg, 0.33 mmol (2); 97 mg, 0.22 mmol (3)) in 10 ml of dichloromethane was added poly(4-vinylpyridine) (46 mg, 0.44 mmol) appearing a solid. The reaction was stirred for 30 minutes at RT. After this time, a solution of Ag(OSO2CF3) (28 mg, 0.11 mmol (2); 57 mg, 0.22 mmol (3)) in 5 ml of diethyl ether was added to the reaction. The resulting mixture was stirred for 2 hours and the solid was isolated by filtration. The metallopolymers 2 and 3 were isolated as pale yellow or white solids.
Experimental data for 2: ATR-IR: ν 807, 957, 1504 cm−1 (Au-C6F5); 1615 cm−1 (C=N, Py); 1161, 1255 cm−1 (Ag(OSO2CF3)).
- Synthesis of metallopolymer [(Au-C6F5)0.12(AgOSO2CF3)0.46(PVP)] (4): To a solution of [Au(C6F5)(tht)] (48 mg, 0.11 mmol) in 5 ml of dichloromethane was added a solution of Ag(OSO2CF3) (28 mg, 0.11 mmol) in 7 ml of diethyl ether. The resulting suspension was added to a solution of poly(4-vinylpyridine) (46 mg, 0.44 mmol) in 5 ml of dichloromethane, leading to a white precipitate. The reaction was stirred for 2 hours and the solid was isolated by filtration. Metallopolymer 4 was isolated as a white solid.
Experimental data for 4: ATR-IR: ν 807, 960, 1506 cm−1 (Au-C6F5); 1613 cm−1 (C=N, Py); 1164, 1245 cm−1 (Ag(OSO2CF3)).
6.4 Computational details
The model systems 1a and 2a used in the theoretical studies were built using Gauss View 6.1[21] and fully optimized at DFT level of theory using the B3LYP functional[22] as implemented in TURBOMOLE 6.4.[23] TD-DFT calculations[24] were performed to compute the lowest singlet-triplet excitation for the model systems. In all calculations, the Karlsruhe split-valence quality basis sets SV(P) were used for C, N, S, O and H,[25] whereas def2-TZVP basis sets were used for Au and Ag.[26] The NCI gradient isosurface that displays the non-covalent interactions in the real space for model systems 1a and 2a were computed with Multiwfn 3.6[27] and graphically represented with VMD.[28] Red isosurfaces represent steric and repulsive contacts; green isosurfaces are assigned to van der Waals weak interactions; blue or green-blue isosurfaces represent attractive non-covalent contacts.
ACKNOWLEDGMENTS
We acknowledge Grant PID2019-104379RB-C22 funded by (Ministerio de Ciencia e Innovación) MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe” and the EC through POCTEFA FEDER project NUTRIA (EFA 356/19) for financial support. I. Soldevilla also acknowledges Universidad de La Rioja for a FPI grant.
AUTHOR CONTRIBUTIONS
Inés Soldevilla: Data curation; investigation. Montserrat Santamaría-Paganini: Data curation; investigation. M. Elena Olmos: Funding acquisition; investigation; project administration; supervision. Miguel Monge: Funding acquisition; investigation; project administration. José M. López-de-Luzuriaga: Funding acquisition; investigation; project administration. María Rodríguez-Castillo: Conceptualization; data curation; investigation.
Open Research
DATA AVAILABILITY STATEMENT
Supplementary material