Some Novel Dinuclear phenylboronates of biologically potent β-enaminoesters: Synthesis, Spectroscopic characterization, antimicrobial activity and their antiandrogenic effect
Vinita Jangir
Department of Chemistry, University of Rajasthan, Jaipur, India
Search for more papers by this authorRajesh Saharan
Department of Chemistry, University of Rajasthan, Jaipur, India
Search for more papers by this authorRamavatar Sharma
Department of Botany, University of Rajasthan, Jaipur, India
Search for more papers by this authorJyoti Sharma
Department of Chemistry, University of Rajasthan, Jaipur, India
Search for more papers by this authorCorresponding Author
Yashpal Singh
Department of Chemistry, University of Rajasthan, Jaipur, India
Correspondence
Yashpal Singh, Department of Chemistry, University of Rajasthan. Jaipur, India.
Email: [email protected]
Search for more papers by this authorVinita Jangir
Department of Chemistry, University of Rajasthan, Jaipur, India
Search for more papers by this authorRajesh Saharan
Department of Chemistry, University of Rajasthan, Jaipur, India
Search for more papers by this authorRamavatar Sharma
Department of Botany, University of Rajasthan, Jaipur, India
Search for more papers by this authorJyoti Sharma
Department of Chemistry, University of Rajasthan, Jaipur, India
Search for more papers by this authorCorresponding Author
Yashpal Singh
Department of Chemistry, University of Rajasthan, Jaipur, India
Correspondence
Yashpal Singh, Department of Chemistry, University of Rajasthan. Jaipur, India.
Email: [email protected]
Search for more papers by this authorAbstract
Organoboron derivatives of biologically potent β-enamino esters of the type 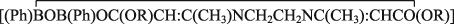 [Where R = CH3(1a), C2H5 (1b), C3H7(1c) and C (CH3)3 (1d)] have been prepared by the reactions of β-enamino esters and Phenyl boronic acid [PhB (OH)2] in 1:2 molar ratio in refluxing tetrahydrofuran (THF). All these derivatives have been characterized by physico-chemical properties, elemental analyses and molecular weight measurements. The structures of these compounds have been proposed on the basis of IR, 1H, 13C, 11B NMR spectral data and GC-mass spectrometry. Phenyl boronic acid, β-enamino esters and their respective phenylboronates derivatives have been screened for the antibmicrobial activities against pathogenic bacteria (B. subtilis and E. coli) and fungi (A. niger and P. peniculosum) to access their growth inhibiting potential. In addition to this, antiandrogenic effect of Ligand, LaH2 and its boron derivative (1a) has also been tested in male albino rats.
[Where R = CH3(1a), C2H5 (1b), C3H7(1c) and C (CH3)3 (1d)] have been prepared by the reactions of β-enamino esters and Phenyl boronic acid [PhB (OH)2] in 1:2 molar ratio in refluxing tetrahydrofuran (THF). All these derivatives have been characterized by physico-chemical properties, elemental analyses and molecular weight measurements. The structures of these compounds have been proposed on the basis of IR, 1H, 13C, 11B NMR spectral data and GC-mass spectrometry. Phenyl boronic acid, β-enamino esters and their respective phenylboronates derivatives have been screened for the antibmicrobial activities against pathogenic bacteria (B. subtilis and E. coli) and fungi (A. niger and P. peniculosum) to access their growth inhibiting potential. In addition to this, antiandrogenic effect of Ligand, LaH2 and its boron derivative (1a) has also been tested in male albino rats.
REFERENCES
- 1P. Sharma, V. Vajpayee, J. Sharma, Y. P. Singh, Appl. Organometal. Chem. 2010, 24, 774.
- 2V. Jangir, J. Sharma, R. A. Sharma, Y. P. Singh, Main Group Met. Chem. 2016, 39,9.
- 3M. Singhal, A. Paul, Int. J. Pharm. Sci. Res. 2011, 2, 2604.
- 4P. Nagpal, R. V. Singh, Appl. Organometal. Chem. 2004, 18, 221.
- 5J. Bhomia, J. Sharma, R. Lakhne, R. Sharma, R. S. Gupta, R. A. Sharma, Y. P. Singh, Appl. Organometal. Chem. 2018, 32. https://doi.org/10.1002/aoc.3983
- 6V. Barba, N. Farfan, Inorg. Chim. Acta 2010, 363, 4112.
- 7Q. Hou, S. Jiang, J. Luminescence 2007, 126, 447.
- 8F. M. Hwang, H. Y. Chen, P. S. Chen, C. S. Liu, Y. Chi, C. F. Shu, F. L. Wu, P. T. Chou, S. M. Peng, G. H. Lee, Inorg. Chem. 2005, 44, 1344.
- 9J. Li, P. I. Djurovuch, B. D. Alleyne, M. Yousufuddin, N. N. Ho, J. C. Thomas, R. Peters, M. E. Thompson, Inorg. Chem. 2005, 44, 1713.
- 10R. C. Evans, P. Douglas, C. J. Winscom, Coord. Chem. Rev. 2006, 250, 2093.
- 11J. W. Faller, A. R. Lavole, Organometallics 2001, 20, 5245.
- 12P. Pelagatti, M. Carcell, F. Calbiani, C. Cassi, L. Elvir, C. Pellzzl, D. Rogollno, Organometallics 2005, 24, 5836.
- 13V. Barba, E. Gallegos, R. Santillan, N. Farf, J. Org. Chem. 2001, 622, 259.
- 14C. Olliver, P. J. Renaud, J. Am. Chem. Soc. 2001, 11, 101.
- 15S. A. Khan, J. H. Morris, M. Haran, M. B. Hursthouse, J. Chem. Soc. Dalton Transactions 1992, 119.
10.1039/dt9920000119 Google Scholar
- 16K. Shelly, C. B. Knobler, M. R. Howthorne, Inorg. Chem. 1992, 31, 2889.
- 17H. P. Singh, C. S. Chauhan, S. N. Pandeya, C. S. Sharma, A. Shrivastava, PharmaLett. 2010, 2, 472.
- 18M. Singhal, A. Paul, Res. J. Pharmacol. 2011, 5, 52.
- 19R. B. Oliveira, E. M. S. Fagundes, R. P. Soares, A. A. Andrade, A. U. Krettli, C. L. Zani, Eur. J. Med. Chem. 2008, 43, 1988.
- 20S. N. Pandeya, P. Yogeeswari, J. P. Stable, Eur. J. Med. Chem. 2000, 35, 886.
- 21N. Murmu, P. Ghosh, A. Games, S. Mitra, M. Das, S. E. Besra, J. Majumdar, S. Bhattacharya, P. Sur, J. R. Vedasiromonl, J. Exp. Clin. Cancer Res. 2002, 21, 351.
- 22R. I. Scorei, R. Popa, Anti-Cancer Agents Med. Chem. 2010, 10, 346.
- 23V. I. Bvegadze, I. B. Sivaeu, S. A. Glazun, Anti-Cancer Agents Med. Chem. 2006, 6, 75.
- 24C. A. Perk, A. J. G. Mill, K. G. Harrison, J. A. Gibson, Brit. J. Radiol 1998, 61, 1115.
- 25H. Nemato, J. Cai, N. Asao, J. Med. Chem. 1995, 38, 1673.
- 26P. Pignol, J. C. Abbe, O. Lefebvre, A. Stampfler, G. Methlin, J. C. R. Sahel, J. Acad. Sci III 1994, 317, 548.
- 27M. Chen, L. Yu, Z. H. Ren, Y. Y. Wang, Z. H. Guan, Chemical Communications 2017, 53, 6245.
- 28G. Bartoli, C. Cimarelli, E. Marcantoni, G. Palmieri, M. Petrini, J. Org. Chem. 1994, 59, 5328.
- 29C. Cimarelli, G. Palmieri, J. Org. Chem. 1996, 61, 5557.
- 30R. S. Bhosale, P. A. Suryawanshi, S. A. Ingle, M. N. Lokhande, S. P. More, S. B. Mane, S. V. Bhosale, R. P. Pawar, Synlett 2006, 933.
- 31Z. H. Zhang, L. Yin, Y. M. Wang, Adv. Synth. Catal. 2006, 348, 184.
- 32Z. H. Zhang, J.-Y. Hu, J. Braz. Chem. Soc. 2006, 17, 1447.
- 33W. L. F. Armarego, D. D. Perrin, Purification of Laboratory Chemicals, 4th ed., Butterworth-Heinemann, London 1997.
- 34N. P. Priya, S. Aranachalam, N. Sathya, C. Jayabalakrishnam, J. Coord. Chem. 2010, 63, 1440.
- 35L. H. Abdel-Rahman, M. S. Adam, A. M. Abu-Dief, H. Moustafa, M. Basha, A. H. Aboria, B. S. Al-Farhan, H. E. I. Sayed Ahmed, Appl. Organometal. Chem. 2018, 32, e4527.
- 36L. H. Abdel-Rahman, A. M. Abu-Dief, H. Moustafa, S. K. Hamdana, Appl. Organometal. Chem. 2017, 31, e3555.
- 37L. H. Abdel- Rahman, N. M. Ismail, M. Ismael, A. M. Abu-Dief, E. A. H. Ahmed, J. Mol. Struct. 2017, 1134, 851.
- 38L. H. Abdel-Rahman, A. M. Abu-Dief, H. Moustafa, A. A. H. Abdel-Mawgoud, Arab. J. Chem. 2017. https://doi.org/10.1016/j.arabjc.2017.07.007
- 39L. H. Abdel-Rahman, A. M. Abu-Dief, A. A. H. Abdel-Mawgoud, J. KingSaud Univ, Science 2017, 31, 52. https://doi.org/10.1016/j.jksus.2017.05.011
- 40L. H. Abdel-Rahman, A. M. Abu-Dief, E. F. Newair, S. K. Hamdan, J. Photochem. Photobiol. B 2016, 162, 298.
- 41R. Montogomery, Arch. Biochem.Biophys. 1957, 67, 378.
- 42B. L. Oser, Hawka's Physiology Chemistry, 14 th ed., McGraw- Hill, New York, NY 1956 246.
- 43T. Mann, Biochemistry of Semen and of the Male Reproductive Tract, Vol. 237, Methuen, London, UK 1964.
- 44M. R. N. Prasad, N. J. Chinoy, K. M. Kadam, Fertil. Steril 1972, 23, 186.
- 45M. Abercrombie, The Anatomical Record. 1946, 94, 239.
- 46O. Kotova, S. Semenov, S. Eliseeva, S. Troyanov, K. Lyssenko, N. Kuzmina, Eur. J. Inorg. Chem. 2009, 2009, 3467.
- 47Y. P. Singh, P. Rupani, A. Singh, A. Rai, R. C. Mehrotra, R. D. Rogers, J. L. Atwood, Inorg.Chem. 1986, 25, 3076.
- 48N. B. Coltthup, L. H. Daly, S. E. Wiberly, Introduction to Infrared and Raman Spectroscopy, 2nd ed., Academic Press, New York 1995.
- 49M. Sanchez, O. Sanchez, H. Hopfl, M. Ochoa, D. Castillo, N. Farfan, S. R. Lima, J. Organomet. Chem. 2004, 689, 811.
- 50R. V. Singh, M. K. Biyala, Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 1477.
- 51W. Kliegel, J. Metge, S. J. Rettig, J. Trotter, Can. J. Chem. 1997, 75, 1203.
- 52L. H. Abdel-Rahman, R. M. EI-Khatib, L. A. E. Nassr, A. M. Abu-Dief, J. Mol. Struct. 2013, 1040, 9.
- 53L. H. Abdel- Rahman, A. M. Abu-Dief, R. M. EI-Khatib, S. M. Abdel- Fatah, J. Bioorg. Chem. 2016, 69, 140.
- 54L. H. Abdel- Rahman, A. M. Abu-Dief, M. Basha, A. A. H. Abdel-Mawgoud, Appl. Organometal Chem. 2017, 31, e3750.
- 55L. H. Abdel–Rahman, A. M. Abu-Dief, R. M. EI-Khatib, S. M. Abdel-Fatah, Int. J. Nano. Chem. 2018, 4, 1.
- 56A. M. Irving, C. M. Vogels, L. G. Nikolcheva, J. P. Edwards, X. F. He, M. G. Hamilton, M. O. Baerlocher, F. J. Baerlocher, A. Decken, S. A. Westcott, New J. Chem. 2003, 27, 1419.
- 57E. Steinberger, A. Steinberger, Handbook of Physiology, Williams and Williams, Maryland, Md 1975.
- 58K. C. Chitra, C. Latchoumycandane, P. P. Mathur, Asian J. Androl. 1999, 1(4), 203.
- 59K. M. Avari, D. A. Bhiwgade, Indian J. Exp. Biol. 1992, 30(12), 1118.
- 60A. Graca, J. S. Ramalho, Asian J. Androl. 2004, 6(3), 237.
- 61M. C. V. Jagot, R. Schoysman, G. Smets, W. Gepts, Int. J. Androl. 1980, 3(1), 15.




