Exceptional Second Harmonic Generation in Ultraviolet Nonlinear Optical Oxyfluoroniobate Crystals via Structural Fingerprint Optimization of Polar Chains
Graphical Abstract
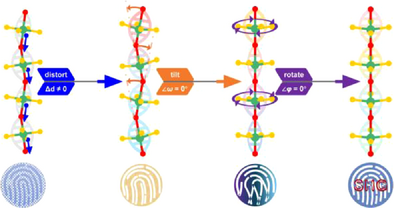
A structural fingerprint optimization strategy is proposed for the 1[TMOF4]∞ chain-based compounds, aimed at modifying the geometric features of functional chains to maximize the optical nonlinearity. In the ultraviolet (UV) nonlinear optical (NLO) crystal (H3O)(Biu)2(NbOF4), the structural fingerprints of the functional chains satisfy the ideal criteria, resulting in a record-breaking second harmonic generation (SHG) response (10.8 × KH2PO4) among all known transition metal (TM) oxyfluorides.
Abstract
Partial fluorination of oxides has positioned oxyfluorides as a promising class of nonlinear optical (NLO) materials owing to their balanced optical properties. However, effectively arranging optical chromophores to achieve strong optical nonlinearity remains challenging. In this study, we explore the structural chemistry of 1[NbOF4]∞ chain-based oxyfluoroniobates and establish a molecular geometric framework to quantify key structural fingerprint factors, namely, the ∠(O─Nb─O′) bond angle, χ[F,Nb,Nb′,F′] torsion angle, chain alignment, and distortion of [NbO2F4] nodes. Theoretical calculations confirm that these factors critically influence second harmonic generation (SHG) activity. By integrating π-conjugated biuret (C2H5N3O2) molecules with optimally aligned 1[NbOF4]∞ chains, we synthesized (H3O)(Biu)2(NbOF4) (Biu = biuret), a crystal exhibiting a record-breaking SHG response, reaching 10.8 times that of KH2PO4, among transition metal (TM) oxyfluorides. Its moderate birefringence (Δn = 0.062 @1064 nm) and wide band gap (Eg = 4.50 eV) further support its potential as a high-performance ultraviolet (UV) NLO material. These results highlight the power of structural fingerprint optimization in fully activating polar chains and offer a new strategy for designing next-generation UV NLO crystals with enhanced SHG performance.
Introduction
Advanced laser applications, such as quantum communications,[1, 2] beam projection and steering,[3, 4] and materials processing,[5] greatly benefit from second-order nonlinear optical (NLO) crystals.[6-8] These materials achieve frequency conversion of lasers across spectral regions from deep-ultraviolet (deep-UV) to terahertz (THz) wavelengths via second-order oscillations of electric dipoles within the bulk, driven by a strong optical electric field.[9-11] Early transition metal ([ETM], d0 transition metals (TMs) such as Ti, V, Mo, W, Nb, Ta, etc.) oxides are among the most widely used NLO crystalline materials.[12, 13] Examples include commercialized KTiOPO4 (KTP),[14] KTiOAsO4 (KTA),[15] and LiNbO3 (LN),[16] which exhibit large second harmonic generation (SHG) effects, and other advantages in the visible to mid-infrared (mid-IR) spectral range. However, their broader technological applicability in shorter-wavelength regions is limited by their narrow band gaps, which arise from electronic structures where covalent bonding involving empty d orbitals and crystal field effects act to lower the conduction band minimum (CBM).[17]
To address this limitation, fluorine-functionalized derivatives known as d0 TM oxyfluorides have been developed, in which some of the oxygen atoms coordinated to the d0 metal are partially replaced by fluorine.[18-22] These materials exhibit wider transparency windows in the UV region, making them promising candidates for UV NLO applications. Oxyfluorides incorporating fluorooxo-functional modules benefit from enlarged bandgaps and enhanced birefringence, thus satisfying the stringent requirements for phase matching in shorter-wavelength regions.[18-23] At the microstructural level, fluorine-functionalized heteroanionic [TMOxFy] units possess enhanced polarizability anisotropy and larger highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) energy gaps, compensating for slightly reduced hyperpolarizability. Consequently, bulk crystalline materials constructed from these fluorooxo-functional units achieve shorter absorption edges and enhanced birefringence, critical for phase matching.[24-26] The d0 TM oxyfluorides inherit the advantages of their fully oxidized counterparts, while their wider bandgaps provide a rich source for exploring UV NLO crystals. However, discovery of UV NLO crystals with large SHG effects (≥5 × KH2PO4@1064 nm) remains sporadic due to unfavorable arrangements, such as cross or reversed configurations of functional modules.[27, 28] The NLO-unfriendly arrangement of highly distorted polyhedra often arises from excessive dimensionality reduction owing to terminal fluorine atoms.[29] The arrangement of dispersed [TMOxFy] anions is influenced by various interactions, including Coulomb forces (from cations), hydrogen bonds, van der Waals forces, and the shapes and sizes of structure-directing agents.[30, 31] These interactions create a delicate and sensitive system, making precise control over the alignment of NLO-functional modules challenging.
Oligomerized or polymerized anions tend to adopt more aligned configurations within the lattice due to steric effects.[32-39] For example, in borates, more than 90% of [B3O6] clusters or 1[BO2]∞ chains exhibit a minimum dihedral angle of zero, ensuring alignment.[40, 41] Similarly, in d0 TM oxides, polyanions exhibit a spontaneous tendency for aligned arrangements. For instance, in K3Nb3Ge2O13, the 1[Nb3O12]∞ tubular chains, constructed from circular [Nb3O15] trimers, are uniformly aligned, resulting in a giant SHG response (17.5 × KH2PO4@1064 nm) and significant birefringence (0.196@546 nm).[42]
While extended secondary structures offer clear advantages, achieving precise modifications to the localized arrangement of NLO-functional modules remains a critical challenge. For example, in the [V2O4F5] dimer, the face-to-face arrangement of two [VO2F4] octahedra completely cancels their net dipole moment, severely limiting their contribution to the SHG effect even within a noncentrosymmetric (NCS) structure.[43] The 1[TMOF4]∞ (TM = Ti, Nb, Ta) chains are particularly promising for the following reasons: (1) compounds containing 1[TMOF4]∞ chains can be readily synthesized via hydro/solvothermal methods by precisely controlling the ratio of metal oxides to fluorine sources; (2) the incorporated octahedra exhibit C4 distortion, with their distortion direction aligning with the chain's polar extension, resulting in additive dipole moments along the chain direction; and (3) compounds with moderately (Δd = 0.4–0.8) or weakly (Δd < 0.4) distorted [TMO2F4] octahedra possess broader band gaps compared to those with highly distorted octahedra (Δd > 0.8).[44] However, most 1[TMOF4]∞ chain-containing compounds, except those with Pb5Cr3F19 structure type, exhibit reversed and antiparallel sequences of chains attributed to dipole–dipole interactions, leading to a center-symmetry-trap.[45] The key challenge is to reverse half of the polar chains while maintaining their ideal geometric configuration and avoiding redshifted absorption edges in the target compound.
Herein, by combining 1[NbOF4]∞ chains with various organic structural guiding agents, we successfully synthesized a series of oxyfluoroniobates. Among them, the hybrid oxyfluoroniobate (H3O)(Biu)2(NbOF4) (Biu = biuret, C2H5N3O2) was identified as a high-performance UV NLO crystal. It exhibits exceptional optical properties, including a giant SHG effect (10.8 × KH2PO4@1064 nm, the largest value reported among NCS TM oxyfluorides), a wide band gap (Eg = 4.50 eV), and sufficient birefringence (0.062@1064 nm) for phase matching. Experimental and theoretical analyses revealed the origins of its excellent NLO properties. Moreover, the geometric system established here highlights the extraordinary SHG efficiency of the completely activated 1[NbOF4]∞ polar chains.
Results and Discussion
The (H3O)(Biu)2(NbOF4) crystals were grown using a two-step method (see details in the Supporting Information). It should be noted that both the preparation of the Nb2O5/HF solution via hydrothermal synthesis and the subsequent evaporation were carried out under mild conditions with low energy consumption. Single-crystal X-ray diffraction analysis reveals that (H3O)(Biu)2(NbOF4) crystallizes in the polar space group, Cm (no. 8) of the monoclinic crystal system (Table S1–S3). Its structure comprises [NbO2F4] anions, neutral biuret molecules, and hydronium ions (Figure 1a). The overall structure features a hydrogen-bond-maintained 3D porous network woven by [H3O] and biuret molecules, with polymerized 1[NbOF4]∞ anionic chains occupying channels along the c-axis. According to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature, the standard chemical name of (H3O)(Biu)2(NbOF4) is hydronium bis(biuret) poly[[tetrafluoridodioxoniobate(V)]-μ-oxo]. The asymmetric unit contains crystallographically independent one niobium, three fluorine, two oxygen, two hydrogen atoms, and one biuret molecular entity (Figure S1). Each niobium atom is coordinated with four equatorial F atoms and two polar O atoms (Figure 1b). The resulting distorted [NbO2F4] octahedron exhibits C4 out-of-center distortion, with significant deviations of the Nb─O bond length (R) from the average bond length (Ra) (Figure 1c). Polymerization of the [NbO2F4] octahedra along the [001] direction produces the 1[NbOF4]∞ polar chain. The extension direction of this polar chain aligns with the distortion direction of the octahedra. Specially, the linear geometry of the Nb─O─Nb configuration (∠Nb─O─Nb = 180°) and the torsion angle χ[F(1),Nb(1),Nb(1′),F(1′)] = 0° demonstrate that the [NbO2F4] octahedron is repeated solely through translation to form the 1[NbOF4]∞ polar chain, with μ2-O acting as bridging linkages (Figure 1b). Table S4 indicates the presence of strong hydrogen-bonding interactions among the hydronium ions, the 1[NbOF4]∞ chains, and the biuret molecules. The alignment of the inorganic components is illustrated in Figure S2, where each hydronium ion forms a hydrogen bond with a neighboring [NbO2F4] node. The electron localization function (ELF) diagrams (Figure S3) reveal no overlap among the electron clouds of the cationic, neutral, and anionic species. Nevertheless, the close proximity of electron clouds between nucleophilic and electrophilic sites suggests that both hydrogen bonding and Coulombic interactions are crucial for stabilizing the overall hybrid structure. The N(1,2,3) atoms of the amino and imino fragments in the biuret molecules, along with the O(1w) atom of the hydronium ion, act as hydrogen bond donors, while the O(2,3) atoms and the branched terminal F(1,2,3) atoms serve as hydrogen bond acceptors. Attributable to the inter-molecular hydrogen bonds (e.g., N(1)−H(1b)…O(3)), the neutral biuret molecule adopts a strictly coplanar geometric configuration without any unfavorable torsion angles, thereby providing the biuret molecule with maximum polarizability anisotropy and hyperpolarizability. The biuret molecules are arranged in a cross-like manner, with a dihedral angle of 64.183° (Figure S4), forming a corrugated 2[Biu]∞ layer, as shown in Figure 1d. The interlayer hydronium ions connect adjacent layers to establish the 3D [(H3O)(Biu)2]∞ cationic framework, which features channels along the c-axis (Figure 1f). The polar 1[NbOF4]∞ chains are located within these channels (Figure 1f), with the dipole moment of each [NbO2F4] octahedron and the net dipole moment of the entire chain oriented in the same direction (Figure 1e). The aligned arrangement of these encapsulated 1[NbOF4]∞ chains, combined with the complex organic-hydronium network, results in a highly anisotropic structure.
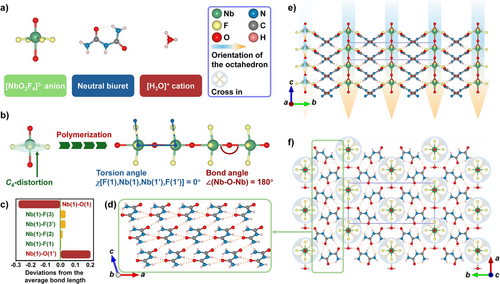
The single crystals of (H3O)(Biu)2(NbOF4) were manually separated from the bulk batch, and the purity of the as-ground samples was confirmed by powder X-ray diffraction analysis (Figure S5). The hydronium ion (H3O)+ forms under strongly acidic conditions. The validity of the hydrogen atom positions was verified using the built-in residual electron density map function in OLEX2.0, which highlights discrepancies between the experimental electron density and the atomic model.[46-48] As shown in Figure S6, the residual electron density maps calculated for the (H3O)(Biu)2(NbOF4) model without explicitly assigning hydrogens on the hydronium ions reveal three probable hydrogen atom positions (indicated by distinct green clouds near the O(1w) atom). Strong hydrogen bonding interactions are also observed (Table S4). These results provide compelling evidence supporting the structural accuracy of the model.
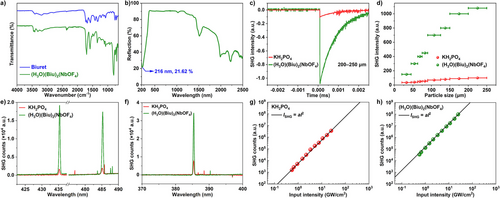
A comparison between the IR spectra of (H3O)(Biu)2(NbOF4) and its organic raw reagent, biuret, reveals a high degree of similarity (Figure 2a). Medium-strong double peaks (3448 and 3410 cm−1 for biuret; 3451 and 3413 cm−1 for (H3O)(Biu)2(NbOF4)) correspond to the asymmetric and symmetric stretching vibrations of ─NH2.[49] The absorption bands near 3190 cm−1 in both spectra are attributed to ─NH stretching vibrations. In the IR spectrum of biuret, strong absorption bands occur at 1724, 1698, and 1679 cm−1, corresponding to C═O stretching vibrations.[50] In contrast, the IR spectrum of (H3O)(Biu)2(NbOF4) displays a single peak at 1699 cm−1, attributed to overlapping of these bands owing to the presence of hydronium ions.[51] The presence of hydronium is further supported by a broad ─OH stretching absorption band at 3309 cm−1. In addition, characteristic absorption peaks at 803 and 745 cm−1 correspond to the asymmetric and symmetric stretching modes of the “─O─Nb─O─” bridge.[28]
The thermal stability of (H3O)(Biu)2(NbOF4) was investigated using thermogravimetric-differential scanning calorimetry (TG-DSC) (see Figure S7). The TG curve shows an initial weight loss beginning around 105 °C, which is attributed to the release of H2O and HF. The corresponding DSC curve displays an endothermic peak at 132 °C, indicating that no phase transition occurs prior to decomposition. This conclusion is further supported by in situ PXRD measurements (Figure S8), which show no changes in diffraction patterns upon heating from 40° to 105 °C.
A wafer with natural growth faces was used for birefringence measurements (Figure S9). The experimental birefringence, Δn = [email protected] nm, was determined using a polarized light microscope with a perpendicular light source. This value reflects the refractive index difference between two crystallographic directions and confirms that the material possesses sufficient phase matching capability. However, it may underestimate the true birefringence, which is defined as the maximum refractive index difference in an optically anisotropic medium.[52]
The diffuse reflectance spectrum (Figure 2b) indicates that the absorption edge of (H3O)(Biu)2(NbOF4) extends down to 216 nm, which is close to the theoretical “perfect” Imm2-LiNbOF4 edge (209 nm).[23] The Tauc plot derived from the Kubelka–Munk function indicates that (H3O)(Biu)2(NbOF4) has an indirect band gap of 4.50 eV (Figure S10).
Since (H3O)(Biu)2(NbOF4) crystallizes in an NCS space group, its NLO properties were experimentally investigated. The results of powder SHG measurement, based on the Kurtz–Perry method, reveal a giant phase-matching SHG response for (H3O)(Biu)2(NbOF4), which is 10.8 times that of KH2PO4 (Figure 2c,d). Notably, this exceptional SHG effect is the largest reported among all known TM oxyfluorides (see details in Table S6 and Figure 5). The SHG capabilities of (H3O)(Biu)2(NbOF4) in the shorter-wavelength region were further investigated under incident radiations of 970, 870, and 770 nm (Figure 2e,f). The SHG intensities of (H3O)(Biu)2(NbOF4) were measured to be 10.2 and 11.2 times that of KH2PO4 under 970 and 870 nm radiation, respectively—values comparable to those obtained under 1064 nm excitation. In addition, the compound exhibits phase-matching behavior under 770 nm radiation, confirming its classification as an experimentally verified UV NLO crystal (Figure S11). Taken together, the excellent optical properties of (H3O)(Biu)2(NbOF4) position it as a high-performance UV NLO crystal. Given that (H3O)(Biu)2(NbOF4) is a hybrid compound, its LIDT was evaluated to assess its stability under laser irradiation. As shown in Figure 2g,h, both the KH2PO4 reference and (H3O)(Biu)2(NbOF4) exhibit SHG responses that fit well with the equation ISHG = a(Iinput)2, where Iinput is the fundamental input intensity, ISHG is the SHG intensity, and a is a constant proportional to |χ(2)|2. This indicates that multiphoton absorption—one of the primary mechanisms of optical breakdown—was not observed within our experimental range (LIDT > 24.7 GW cm−2). The LIDT of (H3O)(Biu)2(NbOF4) is at least 100 times higher than that of AgGaSe2, whose LIDT is reported as 0.24 GW cm−2 under the same measurement conditions.[53] The exceptional SHG response at 532 nm is clearly observed in Figure S12. Although (H3O)(Biu)2(NbOF4) is a hybrid NLO crystal, its exceptionally high LIDT enables it to remain stable under high-power fundamental laser radiation.
A theoretical study based on the GGA-PBE (generalized gradient approximation and Perdew–Burke–Ernzerhof) hybrid functional was conducted to investigate the structure-property relationship in (H3O)(Biu)2(NbOF4).[54, 55] The HSE-06 band structure demonstrates that (H3O)(Biu)2(NbOF4) is an indirect bandgap compound with a wide band gap of 4.80 eV (Figure 3a),[56] closely matching the experimental value. The total and partial densities of states (DOS and PDOS) of (H3O)(Biu)2(NbOF4) are shown in Figure 3c. The valence band maximum (VBM) is dominated by N 2p and O 2p states, while the CBM near the Fermi level is primarily composed of C 2p, N 2p, and Nb 4d5s states. These results indicate that both the organic biuret molecules and the inorganic 1[NbOF4]∞ polar chains contribute to the optical properties.
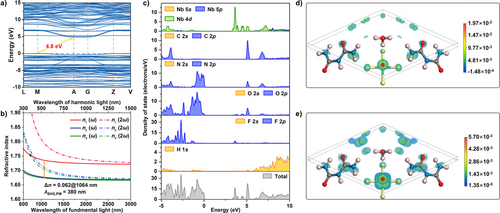
The ELF diagrams (Figure S3) reveal strong d–p hybridization between Nb and nucleophilic atoms (F and O). The lone pair electrons of the protonated water molecule and the delocalized π electrons of the biuret molecules are also clearly observed. The calculated refractive indices of (H3O)(Biu)2(NbOF4) follow the trend nz − ny > ny − nx, characterizing it as a positive biaxial crystal. The three refractive indices (nx < ny < nz) are aligned with the three dielectric principal axes, which correspond to the directions [−1.081, 0, −1.496], [0, , 0], and [−0.306, 0, 2.201], where , , and represent the basis vectors of the conventional lattice. It should be noted that single crystals with such non-integral crystal faces cannot naturally form; the calculated birefringence reaches as high as 0.062@1064 nm, representing the theoretical maximum index difference. This discrepancy arises because the birefringence measured via polarizing microscopy represents the refractive index difference along a specific wafer orientation. Nonetheless, the close numerical agreement between calculated and experimental values supports the validity of the theoretical calculations. Based on the refractive index dispersion curves (Figure 3b), the shortest phase-matching wavelength is below 400 nm (λSHG,PM = 380 nm), indicating that (H3O)(Biu)2(NbOF4) is capable of achieving phase matching in the UV region—an observation that is also experimentally verified. According to the Kleinman approximation,[57] (H3O)(Biu)2(NbOF4), with point symmetry Cs-m, possesses ten valid and non-zero SHG tensors. These are calculated as follows: d11 = −0.304 pm V−1, d12 = d26 = 0.859 pm V−1, d13 = d35 = −0.528 pm V−1, d15 = d31 = 0.561 pm V−1, d24 = d32 = −2.740 pm V−1, and d33 = −1.856 pm V−1. Notably, the largest SHG tensor, d24, is seven times larger than d36(KH2PO4) = 0.39 pm V−1, which aligns well with the experimental results. The experimental effective NLO coefficient (deff,exp.) of (H3O)(Biu)2(NbOF4) is 1.28 pm V−1, calculated using the formula, deff,exp. = deff(KH2PO4) × [I2ω/I2ω(KH2PO4)]1/2. The calculated effective NLO coefficient deff. cal. is 2.14 pm V−1, which is of the same order of magnitude as the experimental value.[58] Theoretically, the relative values of NLO coefficient and powder SHG intensity are not strictly consistent, as the latter is proportional to the square of deff. Conventionally, deff, exp. is influenced by practical measurement conditions such as the incident wavelength, phase-matching angle, and refractive index. Using the visualized SHG-weighted density maps, the SHG origin of the (H3O)(Biu)2(NbOF4) was investigated from the perspective of orbital analysis. The virtual-electron (VE) process dominates the SHG coefficient (more than 82.4%), whereas the virtual-hole (VH) and two-band processes contribute less. Only the contribution of the VE process to the largest SHG tensor (d24) was analyzed, and the findings are shown in Figure 3d,e. The SHG density in the VE occupied state for d24 mainly originates from the O 2p and F 2p orbitals in the 1[NbOF4]∞ chains, the N 2p and O 2p orbitals in biuret molecules, and a minor contribution from the 2p orbitals of O in the [H3O] cations. In the VE unoccupied state, the SHG density also arises from the 5d orbitals of Nb, the 2p orbitals of C, and the lone pair of [H3O] cations. The synergistic effects of the 1[NbOF4]∞ chains and biuret molecules are responsible for the unusual SHG effect of (H3O)(Biu)2(NbOF4), while the contribution of the lone pairs of pronated waters to the SHG response is limited. Further quantum chemical calculations were performed to evaluate the individual contributions of the 1[NbOF4]∞ chains and biuret molecules to the overall SHG effect. The [NbO2F4] and biuret fragments were extracted from the complete structure and analyzed via quantum chemical computation. In addition, structurally similar species—ditriangular [C2N4H7O]+ (guanylurea) and [C(NH2)2NHNO2]+ (nitroguanidium), derived from the recently reported compounds [C2N4H7O][NH2SO3] and [C(NH2)2NHNO2][C(NH2)3](NO3)2─as well as the [CO(NH2)2] (urea) and [C(NH2)3] (guanidium) monomers, were also investigated through quantum chemical calculations.[59, 60] As shown in Figure S13, the hyperpolarizability of [NbO2F4] (163 a.u.) is significantly higher than that of biuret (108 a.u.). However, biuret exhibits a hyperpolarizability comparable to that of the [C2N4H7O]+ cation, which is slightly greater than that of [C(NH2)2NHNO2]+ and substantially higher than those of [CO(NH2)2] and [C(NH2)3]. These results suggest that, disregarding molecular arrangement, biuret and [C2N4H7O]+ may contribute similarly to the SHG effect in NCS structures. Although the density of ditriangular modules in (H3O)(Biu)2(NbOF4) (ρbiuret = 5.96 × 10−3 Å−3) is slightly larger than that in [C2N4H7O][NH2SO3] (ρguanylurea = 5.20 × 10−3 Å−3), the cross-like arrangement of biuret molecules—with a large dihedral angle of 64.183° (Figures 1e and S4)—significantly reduces their SHG contribution. In contrast, the ideal alignment of fully activated 1[NbOF4]∞ chains is primarily responsible for the pronounced SHG effect observed in this compound.
Although NCS ETM oxyfluorides, such as [NH3(CH2)4NH3](TiOF4),[61] (Hdpa)(NbOF4),[28] and compounds with the Pb5Cr3F19 structure type, have previously been reported to include 1[d0-TMOF4]∞ chains, significant SHG effects exceeding five times that of KH2PO4 had never been observed. In contrast, the SHG effect of (H3O)(Biu)2(NbOF4) exceeds ten times that of KH2PO4. To analyze the unusually large SHG effect in (H3O)(Biu)2(NbOF4), a comprehensive system of structural fingerprint factors has been established.
According to anionic group theory, the macroscopic SHG coefficient of a crystal can be regarded as the geometric superposition of the microscopic SHG coefficients of its anionic groups.[62] To further analyze these one-dimensional anionic chains, we introduce a set of descriptive parameters, termed structural fingerprint factors, to visually characterize the geometric features of these functional modules. Specific parameters with defined physical meanings are introduced: the bond angle ∠(Nb─O─Nb) is denoted as α (ranging from 0° to 180°), with its supplementary angle ω (ranging from 0° to 180°); the torsion angle χ[F, Nb, Nb′, F′] is defined as φ (ranging from 0° to 90°); the angle between the net dipole moments is defined as τ (ranging from 0° to 180°); and the distortion of the octahedron is denoted as Δ. The distortion, lacking a natural boundary, is simplified as follows: Δ = 1 if the octahedron is distorted (Δd ≠ 0), and Δ = 0 otherwise.
To expand the research dataset, new oxyfluoroniobates containing one-dimensional 1[NbOF4]∞ chains were synthesized.[63] To investigate how geometric fingerprints—specifically Δ, ω, and (180°−φ)—affect SHG coefficients, a point charge model was used to study the dipole moments of 1[NbOF4]∞ chains extracted from (H-2-methyl-5-hydroxypyridine)(NbOF4) (chain-a), [HCS(NH2)2](NbOF4) (chain-b), and (H3O)(Biu)2(NbOF4) (chain-c).[64] Figure 4a–c illustrate the geometric characteristics of these chains.
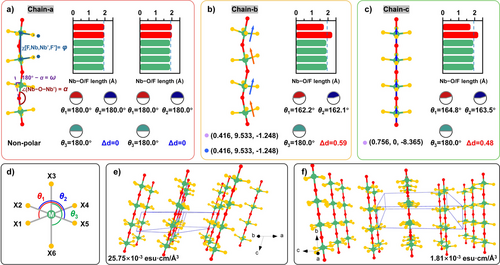
In chain-a, the Nb─F and Nb─O bond lengths show slight deviations from the average, and the ∠(X─Nb─X′) angles (θ1, θ2, and θ3) are all 180°. The octahedra in chain-a are non-polar, with a computed Δd = 0, indicating that chain-a is not activated as an NLO-functional chain. Conversely, the distortions in [NbO2F4] octahedra in chain-b and chain-c are Δd = 0.59 and Δd = 0.48, respectively. These moderately distorted octahedra, according to Halasyamani's definition,[44] exhibit broken symmetry and are considered NLO-active, with Δ = 1.
The directions of dipole moments in [NbO2F4] octahedra are marked as colored arrows in Figure 4b,c. In chain-b, the dipole moments partially cancel due to unfavorable ω = 24.783° and φ = 1.83°, resulting in a zigzag chain. In chain-c, ω and φ are omitted, aligning all octahedra in a linear configuration, with polarization along the [0.756, 0, −8.365] orientation. This comparison shows that large ω and φ hinder the optimization of NLO efficiency in polar chains.
The spatial arrangement of chains is another critical factor influencing SHG effects. Although chain-b has non-zero dipole moments, compound-b crystallizes in a centrosymmetric (CS) space group (P21/c), where antiparallel chain-b′ cancels the dipole moments of chain-b, resulting in no NLO activity. Even in the NCS compound (Hdpa)(NbOF4) (dpa = 2,2′-dipyridylamine), with geometric parameters φ = 4.116° and ω = 0, the antiparallel sequence of the polar chains (Figure 4f) reduces its SHG response to 30 times that of α-SiO2, approximately 0.75 times that of KH2PO4.
Statistics in Table S6 provide information about the structural fingerprints of all available 1[TMOF4)]∞ chain-containing compounds, and (H3O)(Biu)2(NbOF4) is the only compound with all structural fingerprints completely optimized (Δ = 1, φ = 0, ω = 0, and τ = 0), ultimately leading to f(A) = 1.
The exceptional SHG response of (H3O)(Biu)2(NbOF4) arises from its structural uniqueness. Why can the chains within this structure achieve optimal structural fingerprints? The cations of the compounds in Table S6 belong to two categories: π-conjugated and non-π-conjugated cations. In compounds such as [NH3(CH2)2NH3](TiOF4) and A5(d0-TMF7)2(d0-TMOF4) (A = K, Rb, NH4; d0-TM = Nb, Ta), the non-π-conjugated A-site cations, along with [d0-TMF7] fillers featuring isotropic geometric configurations, match well with chains having ω = 0 and large φ values. This chain-like or linear configuration with φ ≠ 0 is thermodynamically stable. This can be understood by the fact that the non-zero internal rotation (φ ≠ 0) of polyhalogen ethane reduces the contribution to the thermal correction of Gibbs free energy (ΔG).[65] The occurrence of planar π-conjugated components may introduce hydrogen bonding interactions perpendicular to the organic plane, making the shapes and sizes of π-conjugated components crucial for affecting the ω fingerprint. In these hybrid oxyfluorides, however, the φ angles are reduced to small values or even approach zero. This indicates the presence of an anisotropy-transferring mechanism, where the planar organic components lead to a well-aligned arrangement of equatorial fluorine atoms, thereby eliminating the φ angle.
For CS compounds, the adjacent chains near inversion centers display opposite sequences with τ = 180°. Even in the NCS (Hdpa)(NbOF4), the 1[NbOF4]∞ chains contribute little to the SHG response owing to the cancelled microscopic NLO SHG effect caused by the large τ value. Considering the possible dipole–dipole interactions between neighboring 1[TMOF4]∞ chains, the spatial distance between adjacent 1[NbOF4]∞ chains determines whether they are arranged in the same direction (parallel) or in opposite directions (antiparallel). In the A5(d0-TMF7)2(d0-TMOF4) series (A = K, Rb, NH4; d0-TM = Nb, Ta), the chains separated by [d0-TMF7] fillers are well-aligned with τ = 0. In the structure of (H3O)(Biu)2(NbOF4), the perfect alignment of [NbO2F4] octahedra within the chains can be attributed to synergistic effects: (1) Intramolecular hydrogen bonds restrict the biuret molecules to adopt a planar configuration, enabling φ = 0; (2) the non-π-conjugated [H3O] cations, as well as the tilt of biuret molecules, result in a minimal ω; (3) the “biuret…biuret…[H3O]…” hydrogen bonding framework effectively separates the 1[NbOF4]∞ chains from each other, allowing them to extend along the same [001] direction.
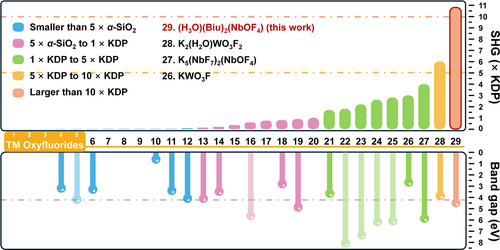
The SHG effects and experimental band gaps of all available TM oxyfluorides are summarized in Figure 5 and Table S7. To the best of our knowledge, the giant SHG response of (H3O)(Biu)2(NbOF4) (10.8 × KH2PO4@1064 nm) is the largest value reported among all known TM oxyfluoride compounds. K2(H2O)WO3F2 also exhibits a large SHG effect (6.0 × KH2PO4@1064 nm), originating from its aligned [WO4F2] functional modules.[24] However, tungsten oxyfluorides with highly distorted octahedra typically exhibit narrower bandgaps compared to niobium oxyfluorides (e.g., the absorption edge of K2(H2O)WO3F2 is 350 nm). Other compounds with blue-shifted absorption edges do not exhibit similarly large SHG responses and provide no advantages over commercially available borate crystals such as β-BaB2O4 (5.8 × KH2PO4@1064 nm, 189 nm)[66] and LiB3O5 (3 × KH2PO4@1064 nm, 145 nm).[67] To assess the SHG performance of (H3O)(Biu)2(NbOF4), a comparative analysis was conducted based on recently reported NLO crystals with similar band gaps (4.0 eV < Eg < 5 eV). As presented in Table S8, the SHG response of (H3O)(Biu)2(NbOF4) exceeds that of over 93% of reported NLO crystals within this band gap range, to the best of our knowledge. Moreover, its SHG response and band gap (10.8 × KH2PO4, 4.50 eV) are comparable to those of leading hybrid NLO crystals such as Ba(H2C6N7O3)2·8 H2O (12 × KH2PO4, 4.10 eV).[68] Therefore, (H3O)(Biu)2(NbOF4) can be regarded as a high-performance NLO crystal.
Conclusions
In conclusion, we have successfully developed a high-performance UV NLO crystal, (H3O)(Biu)2(NbOF4), through structural fingerprint optimization. By fully activating the 1[NbOF4]∞ infinite linear chains within the oxyfluoroniobate structure, we successfully distorted the [NbO2F4] octahedra and eliminated the complementary angles of ∠(Nb─O─Nb) (ω = 0) and the torsion angle χ[F,Nb,Nb′,F′] (φ = 0). In the structure of (H3O)(Biu)2(NbOF4), all 1[NbOF4]∞ chains are well-aligned along the [001] direction, resulting in the largest SHG effect (10.8 × KH2PO4@1064 nm) observed among all TM oxyfluorides. Combined with its low-cost growth, wide band gap (Eg = 4.50 eV), and sufficient birefringence (Δn = 0.067@1064 nm), (H3O)(Biu)2(NbOF4) collectively sets a new performance benchmark as a highly promising candidate for UV NLO applications. This work provides a successful example of precisely tailoring structural fingerprint factors to maximize the efficiency of NLO-functional chains in a polar structure. Moreover, other compounds functionalized by linear NLO-active chain-like structures can have their structure-property relationship investigated with the aid of the structural fingerprint analysis methodology developed here.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Grant Nos. RS-2024-00442105, RS-2021-NR059548, and RS-2025-00512822). This work was also supported by the Natural Science Foundation of Hebei Province (Grant No. B2023201068).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.