Chloromethoxytrityls: Tuning the SOMO Level of Stable Luminescent Radicals
Graphical Abstract
Abstract
Luminescent radicals are of significant recent interest because of the unique quantum properties of the doublet excited state. Control of the energy level of the singly-occupied molecular orbital (SOMO) is essential for the design and application of open-shell materials, but most triarylmethyl radicals are very electron-poor and exhibit similar SOMO values. In this work, we show how a combination of chlorine and methoxy substituents in triarylmethyl radicals allows tuning of their SOMO level over a wide range of –5.7 to –3.9 eV. We demonstrate that both methoxy and chlorine groups provide sufficient stabilization of the radical against peroxide formation, but one-electron oxidation in air limits the SOMO ≤ -4.5 eV to maintain bench stability. Two new stable radicals are characterized by X-ray crystallography, cyclic voltammetry, and luminescence measurements in solution and doped films. We show that the combination of chlorine and methoxy substituents improves the photostability of triarylmethyl radicals compared to tris(trichlorophenyl)methyl (TTM). The extraordinarily large range of SOMO levels in these radicals provides an opportunity to benchmark computational methods for predicting redox potentials. We find that most common density functional theory functionals and even coupled-cluster (CCSD) calculations overestimate the substituent effects in these open-shell species.
Introduction
Luminescent radicals are a rapidly growing area of research,[1-3] with their unusual doublet excited state bringing about new opportunities for organic light-emitting diodes (OLEDs),[4] quantum materials[5] sensing[6, 7] and bioimaging.[8] While low-temperature photoluminescence of trityl radical has been known since the 1940s,[9] it was of little practical interest due to the compound's instability. The development of air-stable polychlorinated trityl radicals PTM and TTM (Figure 1) since the 1970s has established these molecules as viable materials for various applications.[10-13] Different derivatives of chlorinated trityl, including those with bromine,[14, 15] iodine[16] and nitrile substituents,[17] as well as structurally related alkylsulfanyl-substituted,[18] benzannulated[19] and heterosubstituted (e.g., PyBTM[20]) triarylmethyl radicals have been reported in the following decades. Nonetheless, photoluminescence properties of stable radicals remained obscure to the broad community until the work of Juliá’s group, who showed high quantum yield (>50%) in carbazole-functionalized TTM derivatives.[21, 22] The further development of these materials in Li's and Friend's groups, and demonstrated gain of efficiency in radical-doped OLEDs,[4, 23, 24] have led to the recent surge of research in this field. Over a hundred luminescent trityl radicals have been reported in the last decade, most of them obtained by substituting p-chlorine in TTM with carbazole or another arylamine pendant group.[2, 21, 25-27] This synthetic effort led to further improvement of quantum yield and photostability of radical dyes,[21, 28] uncovered new photophysical phenomena[26, 29] and expanded their applications.[5, 8]
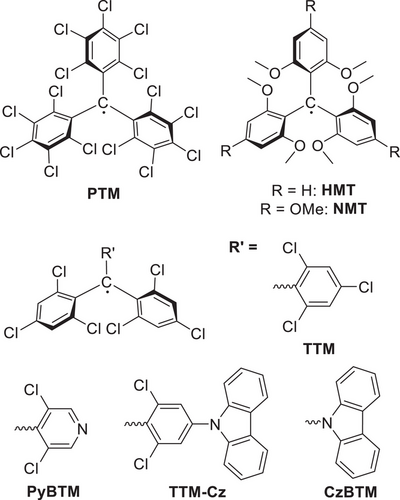
Notably, with very few exceptions (e.g., CzBTM[30] and related structures[31, 32]), all stable luminescent trityl radicals rely on the same molecular design pattern with six halogen atoms blocking the ortho positions of the aryl rings. The steric bulk of these substituents stabilizes the radicals against dimerization and peroxide formation in air. However, this structural feature also limits the tunability of the frontier orbital energies, and most reported trityl radicals exhibit very similar SOMO levels.[25]
Another type of trityl radical, which has received relatively little attention, is hexamethoxytrityl (HMT).[33] Although it has been known since the 1960s,[33, 34] to our knowledge, its luminescence properties have never been documented. HMT is isolable as an orange crystalline solid, persisting in solution for minutes to hours under ambient conditions.[35] Compared to the electron-deficient chlorinated trityl radicals (TTM, PTM, PyBTM and their derivatives), the electron-rich nature of methoxylated radicals makes them prone to oxidation in air via electron transfer to oxygen: for example, nonamethoxyltrityl (NMT) radical immediately oxidizes in air forming a highly stable carbocation.
We envisioned a hybrid class of radicals between HMT and PTM, chloromethoxytrityls (CMTs). Such substitution can provide a critical ability to tune a radical's SOMO level, which is essential for its application in semiconducting devices such as OLEDs. To our knowledge, this new series represents the only example of systematic tuning of the SOMO level while maintaining effective steric protection of the trityl radical core. Two air-stable radicals of this class show high photoluminescence quantum yields (PLQYs) in doped films and improved photostability compared to TTM. Finally, we use an extraordinarily large range of oxidation potentials of substituted trityl radicals to benchmark common computational methods and find that all examined common DFT functionals, HF, MP2 and even coupled clusters (CCSD) calculations overestimate the effect of substituents on the orbital energy levels.
Results and Discussion
Synthesis of the CMTs was achieved in three to four steps by a modular strategy (Scheme 1). Pentachlorobenzaldehyde and decachlorobenzhydrol were used as building blocks in a Friedel-Crafts reaction to give the triarylmethanes (SI-2). Condensation of decachlorobenzhydrol with chlorodimethoxy- and trimethoxybenzene in triflic acid produced CM2T-H and CM3T-H intermediates with 85% and 35% yield, respectively. CM4T-H and CM6T-H were prepared by condensation of pentachlorobenzaldehyde either in triflic acid or in nitromethane using scandium triflate as a Lewis acid. A major side product in the synthesis of CM4T-H, at 20% yield, was its regioisomer CM4T-H’; the structure was confirmed by X-ray crystallography (Figure S22). To generate the CM2T and CM3T radicals, the corresponding triarylmethanes were deprotonated with sodium hydride in DMF and resulting anions were spontaneously oxidized in air. The radicals were subsequently purified by silica column chromatography and characterised by HRMS and single crystal X-ray diffraction. The low acidity of CM6T-H prevented its deprotonation (with BuLi, DBU, NaH, LDA, Bu4NOH), so instead a benzylic oxidation with ceric ammonium nitrate (CAN)[36] was pursued to form the corresponding carbocation. The CM6T radical was formed and studied in situ by reduction with aqueous chromous sulfate (CrSO4).[33] Unfortunately, benzylic oxidation of CM4T-H led to decomposition into multiple side products, while its deprotonation with strong bases (NaH, Bu4NOH) produces a mixture of products.
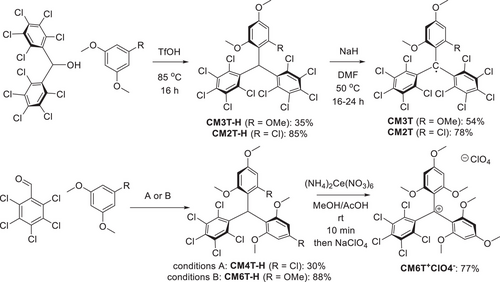
Figure 2 shows X-band EPR spectra of isolated TTM, CM3T and CM2T along with in-situ generated NMT and CM6T radicals. All spectra are represented by a relatively broad peak (∼15 G). No hyperfine coupling (hfc) was observed on hydrogens even at low temperature (Figure S17), a result of vanishing spin density on the meta position. The broader signal of HMT (ΔH = 25 G) compared to other radicals is likely a result of unresolved hfc with its para-hydrogens. The g-values within the CMT series decreased with increasing electron richness, though NMT showed a higher g-value than the CMTs.
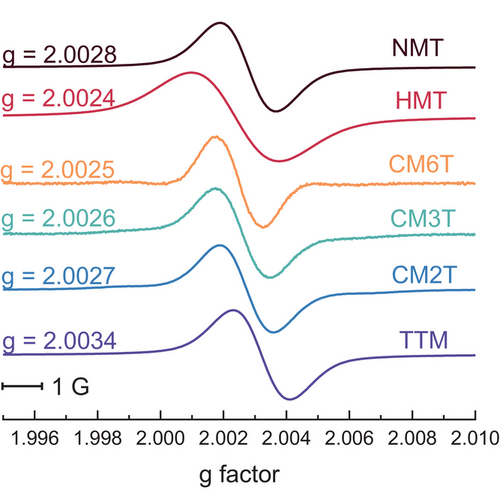
Single crystals of CM3T and CM2T were analyzed via X-ray diffraction and their structures were compared with the known trityl radicals PTM,[37] TTM[11] and HMT[38] to assess the impact of substitution (Figure 3). In all cases, the radical's central carbon is trigonal planar. Across the series, the methoxylated rings show slightly smaller dihedral angles and shorter C*─CAr bonds (between the trigonal radical center and aryl ring) than for the chlorinated rings. The lesser steric bulk of oxygen (van der Waals radius = 1.52 Å) versus chlorine (1.75 Å), as well as the stronger π-conjugation of the radical with the methoxy group, likely both contribute to the shortening of the C*─CAr bond with the methoxylated rings. DFT calculations show that the smaller dihedral angle correlated with an increased spin density in the aryl ring, as expected (Table S1, Figure S4). In HMT and PTM, the crystal packing enforces a low-symmetry conformation (with one ring showing a different dihedral angle than the others), though DFT calculations suggest these geometries are slightly (∼2.5 kcal mol−1) less stable than the symmetric gas-phase geometries.
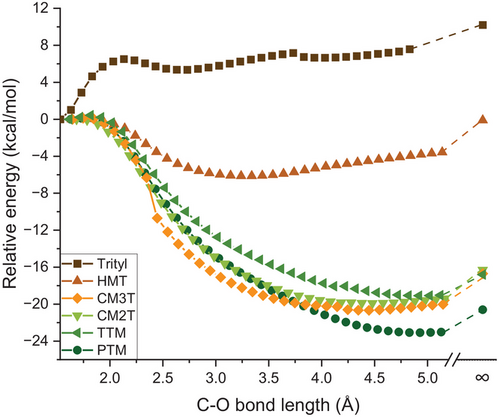
DFT calculations of the radicals’ reactivity towards triplet oxygen were conducted in order to assess the efficiency of methoxy groups in steric protection of the radical center. For all ortho-substituted derivatives, the C-O bond of the corresponding peroxy radical is enthalpically unfavorable (Figure 4). Furthermore, the free energy calculations show that at room temperature the peroxide dimer is thermodynamically stable only for the unsubstituted trityl radical but for none of the other derivatives, confirming that the ortho methoxy groups provide sufficient protection of the radical center (Table S5).
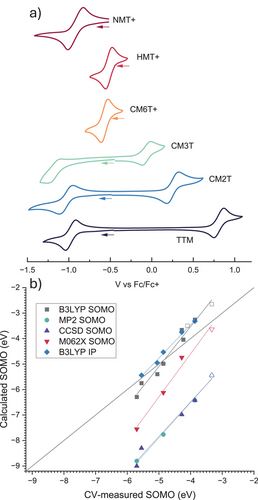
Cyclic voltammetry (CV, Figure 5a) measurements were performed on solutions of radicals (CM3T, CM2T, TTM) or corresponding carbocations (NMT, HMT, CM6T). All of them showed reversible radical-to-cation oxidation waves, which shift negatively upon increasing the number of electron-donating methoxy groups. The reversible radical-to-anion reduction waves were also observed for TTM, PTM, CM3T and CM2T, but could not be detected in the more electron-rich CM6T, HMT and NMT within the accessible electrochemical window.
|
Potential vs Fc/Fc+, Vb) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESOMO eVa) | ESUMO eVa) |
E1/2ox |
E1/2red |
λabs, nm D2/D1 | ε, M−1cm−1 D2 / D1z | λemrt, nmb) | ΦPLrt, %b) | λem77K, nmb) | λemrt, nm matrix | D0-D1a) λabs, nm (f) | D1-D0a) λem, nm (f) | |
| NMT | −3.26 | −1.13 | −0.92c) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 465 (0.020) | 544 (0.022) |
| HMT | −3.66 | −1.44 | −0.52c) | n.d. | 370 / 551 | n.d. | 590 | 1.7 | 597 | n.d. | 466/ (0.008) | 570 (0.014) |
| CM6T | −4.04 | −1.96 | −0.57c) | n.d. | 389 / 564 | n.d. | n.d. | n.d. | n.d. | n.d. | 505 (0.049) | 702 (0.032) |
| CM3T | −4.98 | −2.92 | 0.06 | −1.17 | 395 / 561 | 19 400 / 2310 | 750 | 0.1 | 638 | 659 | 517 (0.062) | 815 (0.037) |
| CM2T | −5.40 | −3.22 | 0.25 | −1.05 | 390 / 568 | 29 800 / 3450 | 707 | 1.7 | 613 | 656 | 520 (0.062) | 734 (0.038) |
| TTM | −5.75 | −3.44 | 0.81 | −0.98 | 373 / 542 | 43 800 / 1030 | 571 | 0.56 | 563 | 563 | 467 (0.022) | 556 (0.027) |
| PTM | −6.29 | −3.92 | 0.9[40] | −0.9[40] | 384 / 565[41] | 30 000 / 910[42],d) | 604[42],d) | 1[42],d) | 501 (0.009) | 631 (0.011) | ||
The ρ-value of the Hammett plots is smaller for oxidation versus reduction (ρox = 0.27; ρred = 0.079, Figure S8). This is likely because stabilization of the carbocation via the resonance effect is larger than the chlorines’ stabilization of the carbanion via the inductive effect of substituents. As a result, the CMTs show a contraction in the electrochemical gap between the oxidation and reduction waves (1.23 V for CM3T vs. 1.80 V for PTM).
CV is commonly used to determine the energies of frontier molecular orbitals of closed-shell molecules by referencing against the vacuum level of electrochemical standards. We have calculated the SOMO/SUMO energies of the radicals from their oxidation/reduction potential versus ferrocene (Fc) standard at –4.8 eV (as is most commonly used in the literature, although other values, from –4.8 to –5.4 eV, have been debated[44]). The air-stable radicals PTM, TTM, CM2T and CM3T show SOMO levels at or below –4.86 eV. The more electron-rich radicals HMT, NMT and CM6T are prone to one-electron oxidation in air to corresponding carbocations. We thus estimate the cutoff for the oxidative stability of triarylmethyl radicals in air between the SOMO levels of CM3T and CM6T, ca. –4.5 eV.
The orbital energies can also be calculated with DFT, and excellent agreements with the electrochemical values are often found for HOMO energies calculated with B3LYP functional (though larger deviations are often observed for LUMO).[45, 46] The validity of such correlations for open-shell molecules (i.e., Eox vs. SOMO), however, is not established. The extraordinarily large range of the Eox values (1.8 V) for the substituted trityls with almost unperturbed orbital localization provides a rare opportunity to examine such relationships. We compared different DFT functionals [B3LYP, M06-2x, ωB87XD), Hartree-Fock (HF), Moller-Plesset (MP2) and Coupled Cluster (CCSD) calculations] for their ability to predict the electrochemically derived SOMO energies (Figures 5b and S1, S2). As expected, B3LYP provided the best absolute match with the electrochemical values, while the ab initio methods (HF, MP2, CCSD) underestimate the energy by >2 eV. However, DFT does not predict well the trend of redox potentials, significantly overestimating the effect of substituents (correlation slope of ∼1.6). An even larger slope (3.6) is observed in correlating the radical reduction potential (Figure S2). This is in contrast to closed-shell systems where a near-unity (∼0.9–1.1) slopes are commonly reported for HOMO–Eox correlations.[47, 48] The deviation is lower for the HF, MP2 and CCSD methods but still far from unity slope (∼1.4) despite the higher computational cost. We suspected that charge localization in closed-shell cations formed by oxidation of neutral radicals may lead to different substituent effects on the vertical ionization in vacuum (represented by SOMO) compared to the adiabatic oxidation in electrolyte. To keep a reasonable computational cost, we used a polarized continuum solvation model (smd: DCM) to represent the effect of electrolyte and calculated adiabatic ionization potentials with the standard B3LYP functional. The results indeed show an improved agreement with the experimental trend (slope ∼1.3).
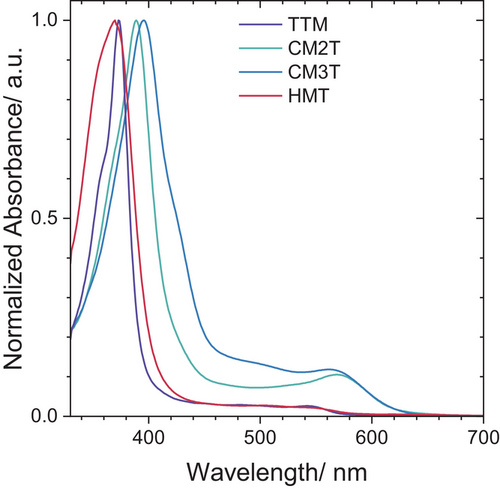
The UV-vis absorptions of all trityl radicals are qualitatively similar, with weak, broad D0→D1 peaks, and more intense and narrower D0→D2 peaks (Figures 6 and S9). Interestingly, the relative intensity of the D0→D1 versus the D0→D2 (and the extinction coefficient for the former band) increases significantly in the donor-acceptor substituted CMTs compared to the symmetric HMT and TTM. TD-DFT calculations [B3LYP/6–31G(d)] show that the lowest energy D1 excitation occurs mostly from the HOMO to SUMO for the radicals (Table S4). The oscillator strength for CM2T and CM3T is several times higher than that of TTM (Table 2), which correlates with the higher D1 absorption coefficients of CMT derivatives. This effect can be rationalized in the context of the symmetry-forbidden D1 transition in symmetric trityl radicals (Table 1).[24, 49] The higher HOMO coefficient on the methoxylated ring breaks the symmetry of this transition endowing it with a weak charge-transfer character, which explains the red-shifted absorption of CMTs, by ∼10–20 nm, compared to both fully methoxylated HMT and fully chlorinated TTM. Natural transition orbital (NTO) analysis of the D1 transition also shows predominant hole localization on the methoxylated ring and electron delocalization between the radical center and chlorinated rings (Figure S5).
| Medium | Radical | λem (nm) | PLQY | τ (ns) | kr (s−1) | knr (s−1) |
|---|---|---|---|---|---|---|
| cyclohexane | TTM | 570 | 0.009 | 5.3 | 1.7 × 106 | 1.9 × 108 |
| CM2T | 646 | 0.013 | 3.9 | 3.4 × 106 | 2.5 × 108 | |
| CM3T | 677 | 0.0037 | 3.7 | 1.0 × 106 | 2.7 × 108 | |
| dioxane | TTM | 571 | 0.007 | 7.3 | 9.6 × 105 | 1.4 × 108 |
| CM2T | 693 | 0.017 | 4.9 | 3.4 × 106 | 2.0 × 108 | |
| CM3T | 719 | 7 × 10−4 | 6.8 | 1.0 × 105 | 1.5 × 108 | |
| DCM (rt) | TTM | 571 | 0.006 | 6.0 | 9.4 × 105 | 1.7 × 108 |
| CM2T | 702 | 0.017 | 4.6 | 3.7 × 106 | 2.2 × 108 | |
| CM3T | 742 | 0.001 | 4.8 | 2.1 × 105 | 2.1 × 108 | |
| DCM (77 K) | TTM | 563 | − | 138 | − | − |
| CM2T | 621 | − | 48 | − | − | |
| CM3T | 646 | − | 30 | − | − | |
| HTTM | 1 w% TTM | 568 | 0.63 | 208 | 3.0 × 106 | 1.8 × 106 |
| CM2T−H | 0.15 w% CM2T | 606 | 0.84 | 48 | 1.8 × 107 | 3.4 × 106 |
| CM3T−H | 1 w% CM3T | 635 | 0.47 | 30 | 1.5 × 107 | 1.8 × 107 |
The emission bands of CM3T and CM2T are significantly red-shifted compared to TTM and HMT, and show larger Stokes shifts of >100 nm (Figure 7, Table 2). Unlike TTM, the CMTs display a large solvatochromic effect (Table 2, Figure S10), with ∼60 nm shift of λem from cyclohexane to dichloromethane. TD-DFT calculations of the relaxed D1 excited state show that for TTM and PTM, the rings’ torsion angles show minimal changes compared to the D0 geometry. However, for CM2T and CM3T, the out-of-plane twist increases for the methoxylated ring (e.g., from 37° to 60° for CM3T) and decreases for the chlorinated rings (e.g., from 53° to 34° for CM3T), explaining the larger Stokes shifts in these derivatives (Table S3).
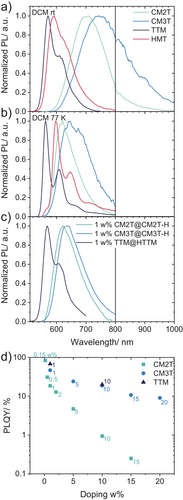
Emission lifetimes were collected by time-correlated single photon counting (TCSPC) measurements. In all solvents tested, TTM has a consistently longer lifetime (τ = 5.3–7.3 ns, Table 2) than the CMTs (τ = 3.7–6.8 ns). Like HMT, PTM and TTM, the CMTs show low PLQYs (PLQY < 2%) in solution (Table 1) due to slower rates of radiative (kr) compared to nonradiative (knr) relaxation (Table 2). The significantly lower PLQY of CM3T (0.07%–0.1%) compared to CM2T (1.3%–1.7%) is likely a result of the energy gap law considering its emission in the NIR region (λem = 742 nm). We note that the intersystem crossing, a common deactivation process for many closed-shell dyes, should not occur in simple trityl radicals because their quartet state Q1 normally has a higher energy than D1.[20, 43] Also, the photocyclization, which occurs with a quantum yield of ∼30% in PTM,[43] is significantly less important in CMTs (see below). A previous detailed TD-DFT analysis of PyBTM suggested that the internal conversion occurs mainly via vibronic coupling involving the C-C stretching modes with the central carbon, and that the internal conversion rates are slightly slower than those in triphenylamine dyes.[50]
To test the role of the vibrational relaxation, we studied the emission of trityl radicals in glassy DCM matrices at 77 K. Compared to room temperature, the emission bands are narrowed, indicative of a conformationally restrictive environment (Figure 7b). Unlike TTM and HMT, the CMTs exhibit large blue shifts (∼80–100 nm) at 77 K compared to room temperature, consistent with the inhibition of their relaxation to the D1 minimum. The substantially longer emission lifetimes at 77 K (τ = 30–138 ns) suggest that the vibrational relaxation is the major contributor to knr, at room temperature.
To assess the luminescence properties of the new trityl radicals in solid films, they were co-sublimed with their parent triarylmethanes (to minimize possible aggregation effects).[16] The so-prepared films show very similar emission maxima and lifetimes to the frozen DCM glasses (Figure 7c). As expected, the PLQY is significantly improved in the solid-state compared to solution (up to 84% for CM2T, 47% for CM3T, Table 2). However, this improvement is not only a result of reduced knr, but also increased kr. The latter can be attributed to greater coupling of the D1 and D0 states around the Franck-Condon geometries compared to the relaxed conformations. This increase of kr is significant for CMT derivatives (ca. an order of magnitude) but is lower for TTM, which is corroborated by DFT calculations showing higher D0-D1 oscillator strength in the D1 geometry compared to the D0 geometry for the CMTs, whereas for TTM they are approximately the same (Table 1).
We examined the effect of the doping concentration on the solid-state emission (Figure 7d, Table S7). As expected,[51] PLQYs decrease with increasing radical doping concentration (Figure S14). The most rapid decrease was observed for CM2T and the least rapid for TTM. It's possible that the higher polarity of donor-acceptor substituted CMTs (Table S3) leads to stronger aggregation, resulting in concentration quenching at lower doping levels. This is similar to what is observed for other trityls: for example, the non-polar I3-PTM[16] retains PLQY of >90% at doping concentration of up to 4% while the highly polar PyBTM[6] shows a sharp decrease in PLQY at doping concentrations <1%. A distinct emission band, attributed to the aggregate, at λ ∼ 650 nm is visible in TTM's spectra with increasing concentration (Figure S13a),[52] which allows TTM to retain moderate quantum yields even at high doping concentrations (Figure 7d). We did not detect any red-shifted aggregate emission in the CMTs, likely due to the expectedly low PLQY in the NIR region.
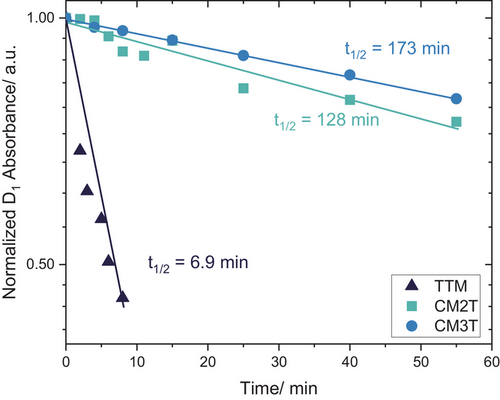
The above-mentioned photocyclization of TTM derivatives is the main reason for their low photostability and the major barrier to their application.[1, 43] To elucidate the effect of methoxy groups on photostability, we compared CM2T, CM3T and TTM's photodecomposition by irradiating with UV light solutions of the radicals in aerated chlorobenzene solutions, under identical conditions (Figures 8, S16). The CMTs showed significantly improved photostability, with ∼20 times longer half-life (t1/2) compared to TTM.
Conclusion
In conclusion, we present new stable CMT radicals with tunable SOMO levels (from -5.7 to -3.9 eV) and examine the interrelationship between electron richness, steric bulk and stability. We show that the methoxy groups in the ortho positions provide sufficient steric protection of the radical center and that the air stability of these radicals is controlled by the electron transfer to oxygen. The asymmetric functionalization of trityl with methoxy and chlorine groups results in the contraction of the SOMO-SUMO gap and red-shifted emission compared to the symmetric trityls TTM and HMT. The new stable CMT radicals show bright luminescence in doped films and improved photostability compared to TTM. Taking advantage of the wide range of SOMO/SUMO levels in these radicals, we show that the common computational methods systematically overpredict the effect of substituents on the redox potentials of radicals.
Acknowledgements
This work was funded by the National Sciences and Engineering Research Council of Canada. H.E.H. acknowledges the NSERC Alexander Graham Bell Doctoral Scholarship as well as the Walter C. Sumner Foundation scholarship. C.R. acknowledges support from Fonds de recherche du Québec, Nature et technologies sector. The authors thank Hatem Titi (McGill University) for the crystallographic analysis and Nadim Saade and Alex Wahba for their assistance with mass spectrometry.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.





