Cooperative Activation of CO and Pyridine by an Aluminum(I) Complex Ligated with a Silylene–Borane Ligand
Jinghuang Lv
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
Both authors contributed equally to this work.
Search for more papers by this authorXiao Fang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
Both authors contributed equally to this work.
Search for more papers by this authorFanshu Cao
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenbo Mo
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]
Search for more papers by this authorJinghuang Lv
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
Both authors contributed equally to this work.
Search for more papers by this authorXiao Fang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
Both authors contributed equally to this work.
Search for more papers by this authorFanshu Cao
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenbo Mo
State Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Academy for Advanced Interdisciplinary Studies, College of Chemistry, Nankai University, Tianjin, 300071 China
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
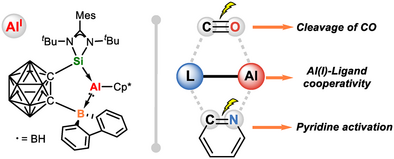
A silylene–borane-ligated aluminum(I) complex featuring Si(II)→Al(I) and Al(I)→B(III) donor–acceptor interactions were synthesized and structurally characterized. The ambiphilic ligand- aluminum cooperation enables C≡O bond cleavage and pyridine dearomatization, uncovering new avenues in low-valent aluminum chemistry.
Abstract
Cooperative main-group systems based on alumylenes are highly attractive due to their potential for activating and transforming inert chemical bonds and small molecules. However, their development has been hindered by the scarcity of suitable supporting ligands. Herein, we report the synthesis of an amphiphilic carboranyl silylene–borane ligand (1) and demonstrate its effectiveness in stabilizing an aluminum(I) complex 2. Complex 2 has been unambiguously characterized by spectroscopic analysis, X-ray diffraction analysis, and DFT calculations, which reveals a unique structure featuring both silicon(II)→aluminum(I) and aluminum(I)→boron(III) donor–acceptor bonds. The synergistic interplay between the silylene–borane ligand and the aluminum(I) center in 2 drives its unusual reactivity toward CO and pyridine activation, facilitating cleavage of the C≡O bond and dearomatization of pyridine.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202512084-sup-0001-SuppMat.pdf2.4 MB | Supporting Information |
| anie202512084-sup-0002-SuppMat.pdf392.5 KB | Supporting Information |
| anie202512084-sup-0003-SuppMat.cif11.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. W. Roesky, S. S. Kumar, Chem. Commun. 2005, 32, 4027–4038.
- 2S. Nagendran, H. W. Roesky, Organometallics 2008, 27, 457–492.
- 3M. Asay, C. Jones, M. Driess, Chem. Rev. 2011, 111, 354–396.
- 4Y. Liu, J. Li, X. Ma, Z. Yang, H. W. Roesky, Coord. Chem. Rev. 2018, 374, 387–415.
- 5K. Hobson, C. J. Carmalt, C. Bakewell, Chem. Sci. 2020, 11, 6942–6956.
- 6M. Zhong, S. Sinhababu, H. W. Roesky, Dalton Trans. 2020, 49, 1351–1364.
- 7X. Zhang, Y. Mei, L. L. Liu, Chem. - Eur. J. 2022, 28, e202202102.
- 8C. Dohmeier, C. Robl, M. Tacke, H. Schnöckel, Angew. Chem. Int. Ed. 1991, 30, 564–565.
- 9C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao, F. Cimpoesu, Angew. Chem. Int. Ed. 2000, 39, 4274–4276.
10.1002/1521-3773(20001201)39:23<4274::AID-ANIE4274>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 10G. Tan, T. Szilvási, S. Inoue, B. Blom, M. Driess, J. Am. Chem. Soc. 2014, 136, 9732–9742.
- 11A. Hofmann, T. Tröster, T. Kupfer, H. Braunschweig, Chem. Sci. 2019, 10, 3421–3428.
- 12S. K. Mellerup, Y. Cui, F. Fantuzzi, P. Schmid, J. T. Goettel, G. Bélanger-Chabot, M. Arrowsmith, I. Krummenacher, Q. Ye, V. Engel, B. Engels, H. Braunschweig, J. Am. Chem. Soc. 2019, 141, 16954–16960.
- 13J. D. Queen, A. Lehmann, J. C. Fettinger, H. M. Tuononen, P. P. Power, J. Am. Chem. Soc. 2020, 142, 20554–20559.
- 14X. Zhang, L. L. Liu, Angew. Chem. Int. Ed. 2021, 60, 27062–27069.
- 15J. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, Nature 2018, 557, 92–95.
- 16J. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, Angew. Chem. Int. Ed. 2021, 60, 1702–1713.
- 17M. D. Anker, C. L. McMullin, N. A. Rajabi, M. P. Coles, Angew. Chem. Int. Ed. 2020, 59, 12806–12810.
- 18S. Kurumada, S. Takamori, M. Yamashita, Nat. Chem. 2020, 12, 36–39.
- 19K. Koshino, R. Kinjo, J. Am. Chem. Soc. 2020, 142, 9057–9062.
- 20R. A. Jackson, A. J. R. Matthews, P. Vasko, M. F. Mahon, J. Hicks, D. J. Liptrot, Chem. Commun. 2023, 59, 5277–5280.
- 21M. P. Coles, M. J. Evans, Chem. Commun. 2023, 59, 503–519.
- 22D. Sarkar, P. Vasko, A. F. Roper, A. E. Crumpton, M. M. D. Roy, L. P. Griffin, C. Bogle, S. Aldridge, J. Am. Chem. Soc. 2024, 146, 11792–11800.
- 23T. Agou, K. Nagata, N. Tokitoh, Angew. Chem. Int. Ed. 2013, 52, 10818–10821.
- 24P. Bag, A. Porzelt, P. J. Altmann, S. Inoue, J. Am. Chem. Soc. 2017, 139, 14384–14387.
- 25C. Weetman, A. Porzelt, P. Bag, F. Hanusch, S. Inoue, Chem. Sci. 2020, 11, 4817–4827.
- 26X. Liu, S. Dong, J. Zhu, S. Inoue, J. Am. Chem. Soc. 2024, 146, 23591–23597.
- 27T. Chu, I. Korobkov, G. I. Nikonov, J. Am. Chem. Soc. 2014, 136, 9195‒9202.
- 28R. J. Schwamm, M. D. Anker, M. Lein, M. P. Coles, Angew. Chem. Int. Ed. 2019, 58, 1489–1493.
- 29J. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, J. Am. Chem. Soc. 2019, 141, 11000–11003.
- 30J. Hicks, P. Vasko, A. Heilmann, J. M. Goicoechea, S. Aldridge, Angew. Chem. Int. Ed. 2020, 59, 20376–20380.
- 31S. Grams, J. Eyselein, J. Langer, C. Färber, S. Harder, Angew. Chem. Int. Ed. 2020, 59, 15982–15986.
- 32R. J. Schwamm, M. P. Coles, M. S. Hill, M. F. Mahon, C. L. McMullin, N. A. Rajabi, A. S. S. Wilson, Angew. Chem. Int. Ed. 2020, 59, 3928–3932.
- 33J. T. Boronski, L. R. Thomas-Hargreaves, M. A. Ellwanger, A. E. Crumpton, J. Hicks, D. F. Bekiş, S. Aldridge, M. R. Buchner, J. Am. Chem. Soc. 2023, 145, 4408–4413.
- 34D. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 46–76.
- 35D. W. Stephan, Acc. Chem. Res. 2015, 48, 306–316.
- 36D. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441.
- 37D. W. Stephan, Science 2016, 354, aaf7229.
- 38J. Paradies, Coord. Chem. Rev. 2019, 380, 170–183.
- 39J. Lam, K. M. Szkop, E. Mosaferi, D. W. Stephan, Chem. Soc. Rev. 2019, 48, 3592–3612.
- 40D. W. Stephan, Chem 2020, 6, 1520–1526.
- 41D. W. Stephan, J. Am. Chem. Soc. 2021, 143, 20002–20014.
- 42J. R. Khusnutdinova, D. Milstein, Angew. Chem. Int. Ed. 2015, 54, 12236–12273.
- 43G. Bouhadir, D. Bourissou, Chem. Soc. Rev. 2016, 45, 1065–1079.
- 44F.-G. Fontaine, É. Rochette, Acc. Chem. Res. 2018, 51, 454–464.
- 45A. A. Omaña, R. K. Green, R. Kobayashi, Y. He, E. R. Antoniuk, M. J. Ferguson, Y. Zhou, J. G. C. Veinot, T. Iwamoto, A. Brown, E. Rivard, Angew. Chem. Int. Ed. 2021, 60, 228–231.
- 46B. L. Frenette, J. Trach, M. J. Ferguson, E. Rivard, Angew. Chem. Int. Ed. 2023, 62, e202218587.
- 47A. A. Omaña, B. L. Frenette, E. Dornsiepen, R. Kobayashi, M. J. Ferguson, T. Iwamoto, E. Rivard, Dalton Trans. 2023, 52, 774–786.
- 48V. O. Smirnov, A. D. Volodin, A. A. Korlyukov, A. D. Dilman, Angew. Chem. Int. Ed. 2020, 59, 12428–12431.
- 49B. L. Frenette, E. Rivard, Chem. - Eur. J. 2023, 29, e202302332.
- 50F. Krämer, J. Paradies, I. Fernández, F. Breher, Nat. Chem. 2024, 16, 63–69.
- 51M. J. Krahfuss, U. Radius, Dalton Trans. 2021, 50, 6752–6765.
- 52L. Wang, Y. Li, Z. Li, M. Kira, Coord. Chem. Rev. 2022, 457, 214413.
- 53S. Yao, A. Saddington, Y. Xiong, M. Driess, Acc. Chem. Res. 2023, 56, 475–488.
- 54A. Saddington, S. Dong, S. Yao, J. Zhu, M. Driess, Angew. Chem. Int. Ed. 2024, 63, e202410790.
- 55X. Chen, D. Yang, F. Cao, Z. Mo, J. Am. Chem. Soc. 2024, 146, 29278–29284.
- 56X. Su, T. A. Bartholome, J. R. Tidwell, A. Pujol, S. Yruegas, J. J. Martinez, C. D. Martin, Chem. Rev. 2021, 121, 4147–4192.
- 57J. Krebs, A. Häfner, S. Fuchs, X. Guo, F. Rauch, A. Eichhorn, I. Krummenacher, A. Friedrich, L. Ji, M. Finze, Z. Lin, H. Braunschweig, T. B. Marder, Chem. Sci. 2022, 13, 14165–14178.
- 58H. Wang, L. Wu, Z. Lin, Z. Xie, J. Am. Chem. Soc. 2017, 139, 13680–13683.
- 59H. Wang, L. Wu, Z. Lin, Z. Xie, Angew. Chem. Int. Ed. 2018, 57, 8708–8713.
- 60H. Wang, J. Zhang, H. K. Lee, Z. Xie, J. Am. Chem. Soc. 2018, 140, 3888–3891.
- 61Y. Quan, Z. Xie, Chem. Soc. Rev. 2019, 48, 3660–3673.
- 62S. Yao, A. Kostenko, Y. Xiong, A. Ruzicka, M. Driess, J. Am. Chem. Soc. 2020, 142, 12608–12612.
- 63S. Yao, T. Szilvási, Y. Xiong, C. Lorent, A. Ruzicka, M. Driess, Angew. Chem. Int. Ed. 2020, 59, 22043–22047.
- 64Z. Qiu, Z. Xie, Acc. Chem. Res. 2021, 54, 4065–4079.
- 65Y. Xiong, D. Chen, S. Yao, J. Zhu, A. Ruzicka, M. Driess, J. Am. Chem. Soc. 2021, 143, 6229–6237.
- 66S. Yao, A. Kostenko, Y. Xiong, C. Lorent, A. Ruzicka, M. Driess, Angew. Chem. Int. Ed. 2021, 60, 14864–14868.
- 67Z. Qiu, Z. Xie, Chem. Soc. Rev. 2022, 51, 3164–3180.
- 68L. Wu, X. Zhang, M. Moos, I. Krummenacher, M. Dietz, A. Jayaraman, R. Bertermann, Q. Ye, M. Finze, M. Wenzel, R. Mitric, C. Lambert, H. Braunschweig, L. Ji, J. Am. Chem. Soc. 2024, 146, 17956–17963.
- 69S. Yruegas, J. C. Axtell, K. O. Kirlikovali, A. M. Spokoyny, C. D. Martin, Chem. Commun. 2019, 55, 2892–2895.
- 70A. Benton, J. D. Watson, S. M. Mansell, G. M. Rosair, A. J. Welch, J. Organomet. Chem. 2020, 907, 121057.
- 71C. Zhang, J. Wang, W. Su, Z. Lin, Q. Ye, J. Am. Chem. Soc. 2021, 143, 8552–8558.
- 72M. O. Akram, J. R. Tidwell, J. L. Dutton, C. D. Martin, Angew. Chem. Int. Ed. 2022, 61, e202212073.
- 73M. O. Akram, C. D. Martin, J. L. Dutton, Inorg. Chem. 2023, 62, 13495–13504.
- 74L. Xiang, J. Wang, A. Matler, Q. Ye, Chem. Sci. 2024, 15, 17944–17949.
- 75L. Xiang, A. Matler, L. Tan, Q. Ye, Dalton Trans. 2024, 53, 11655–11658.
- 76C. E. Radzewich, M. P. Coles, R. F. Jordan, J. Am. Chem. Soc. 1998, 120, 9384–9385.
- 77I. L. Fedushkin, M. V. Moskalev, A. N. Lukoyanov, A. N. Tishkina, E. V. Baranov, G. A. Abakumov, Chem. - Eur. J. 2012, 18, 11264–11276.
- 78M.-A. Courtemanche, J. Larouche, M.-A. Légaré, W. Bi, L. Maron, F.-G. Fontaine, Organometallics 2013, 32, 6804–6811.
- 79I. L. Fedushkin, M. V. Moskalev, E. V. Baranov, G. A. Abakumov, J. Organomet. Chem. 2013, 747, 235–240.
- 80Y. Zhao, Y. Liu, Y. Lei, B. Wu, X.-J. Yang, Chem. Commun. 2013, 49, 4546–4548.
- 81T. W. Myers, L. A. Berben, Chem. Sci. 2014, 5, 2771–2777.
- 82T. J. Sherbow, C. R. Carr, T. Saisu, J. C. Fettinger, L. A. Berben, Organometallics 2016, 35, 9–14.
- 83S. P. Sarish, P. P. Samuel, H. W. Roesky, C. Schulzke, K. Nijesh, S. De, P. Parameswaran, Chem. - Eur. J. 2015, 21, 19041–19047.
- 84E. J. Thompson, L. A. Berben, Angew. Chem. Int. Ed. 2015, 54, 11642–11646.
- 85M. V. Moskalev, A. N. Lukoyanov, E. V. Baranov, I. L. Fedushkin, Dalton Trans. 2016, 45, 15872–15878.
- 86T. E. Stennett, J. Pahl, H. S. Zijlstra, F. W. Seidel, S. Harder, Organometallics 2016, 35, 207–217.
- 87S. Chen, B. Li, X. Wang, Y. Huang, J. Li, H. Zhu, L. Zhao, G. Frenking, H. W. Roesky, Chem. - Eur. J. 2017, 23, 13633–13637.
- 88T. J. Sherbow, E. J. Thompson, A. Arnold, R. I. Sayler, R. D. Britt, L. A. Berben, Chem. - Eur. J. 2019, 25, 454–458.
- 89J. Campos, Nat. Rev. Chem. 2020, 4, 696–702.
- 90C. R. Carr, J. I. Vesto, X. Xing, J. C. Fettinger, L. A. Berben, ChemCatChem 2022, 14, e202101869.
- 91C. L. Shaves, N. Villegas-Escobar, E. R. Clark, I. M. Riddlestone, Chem. - Eur. J. 2023, 29, e202203806.
- 92J. S. Scott, M. L. Maenaga, A. J. Woodside, V. W. Guo, A. R. Cheriel, M. R. Gau, P. R. Rablen, C. R. Graves, Inorg. Chem. 2024, 63, 4028–4038.
- 93T. W. Myers, L. A. Berben, J. Am. Chem. Soc. 2013, 135, 9988–9990.
- 94A. Heilmann, J. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, Angew. Chem. Int. Ed. 2020, 59, 4897–4901.
- 95F. Ebner, L. M. Sigmund, L. Greb, Angew. Chem. Int. Ed. 2020, 59, 17118–17124.
- 96L. M. Sigmund, L. Greb, Chem. Sci. 2020, 11, 9611–9616.
- 97H. Ruppert, L. M. Sigmund, L. Greb, Chem. Commun. 2021, 57, 11751–11763.
- 98L. M. Sigmund, C. Ehlert, M. Enders, J. Graf, G. Gryn'ova, L. Greb, Angew. Chem. Int. Ed. 2021, 60, 15632–15640.
- 99S. J. Kohl, L. M. Sigmund, M. Schmitt, L. Greb, Chem. Sci. 2024, 15, 10803–10809.
- 100B. M. Hoffman, D. Lukoyanov, Z.-Y. Yang, D. R. Dean, L. C. Seefeldt, Chem. Rev. 2014, 114, 4041–4062.
- 101J. Yano, V. Yachandra, Chem. Rev. 2014, 114, 4175–4205.
- 102G. Knör, Coord. Chem. Rev. 2015, 304–305, 102–108.
- 103F. A. Schultz, R. L. Lord, M.-H. Baik, Inorg. Chim. Acta. 2020, 510, 119746.
- 104Y. Wang, M. Karni, S. Yao, A. Kaushansky, Y. Apeloig, M. Driess, J. Am. Chem. Soc. 2019, 141, 12916–12927.
- 105L. Greb, F. Ebner, Y. Ginzburg, L. M. Sigmund, Eur. J. Inorg. Chem. 2020, 2020, 3030–3047.
- 106X. Chen, Y. Yang, H. Wang, Z. Mo, J. Am. Chem. Soc. 2023, 145, 7011–7020.
- 107B. Lei, F. Cao, M. Chen, X. Wang, Z. Mo, J. Am. Chem. Soc. 2024, 146, 17817–17826.
- 108S. G. Kim, D. Kim, J. Oh, Y. J. Son, S. Jeong, J. Kim, S. J. Hwang, J. Am. Chem. Soc. 2024, 146, 11440–11449.
- 109A. Fukazawa, J. L. Dutton, C. Fan, L. G. Mercier, A. Y. Houghton, Q. Wu, W. E. Piers, M. Parvez, Chem. Sci. 2012, 3, 1814–1818.
- 110H. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas, Q. Ye, Nature 2015, 522, 327–330.
- 111C. Ganesamoorthy, J. Schoening, C. Wölper, L. Song, P. R. Schreiner, S. Schulz, Nat. Chem. 2020, 12, 608–614.
- 112D. Reiter, R. Holzner, A. Porzelt, P. Frisch, S. Inoue, Nat. Chem. 2020, 12, 1131–1135.
- 113Y. Ding, J. Zhang, Y. Li, C. Cui, J. Am. Chem. Soc. 2022, 144, 20566–20570.
- 114V. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Angew. Chem. Int. Ed. 2006, 45, 3488–3491.
- 115X. Wang, Z. Zhu, Y. Peng, H. Lei, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2009, 131, 6912–6913.
- 116M. A. Dureen, D. W. Stephan, J. Am. Chem. Soc. 2010, 132, 13559–13568.
- 117H. Braunschweig, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer, I. Krummenacher, J. Mies, A. K. Phukan, A. Vargas, Nat. Chem. 2013, 5, 1025–1028.
- 118M. Sajid, L. M. Elmer, C. Rosorius, C. G. Daniliuc, S. Grimme, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2013, 52, 2243–2246.
- 119M. J. Cowley, Y. Ohmori, V. Huch, M. Ichinohe, A. Sekiguchi, D. Scheschkewitz, Angew. Chem. Int. Ed. 2013, 52, 13247–13250.
- 120M. Sajid, A. Lawzer, W. Dong, C. Rosorius, W. Sander, B. Schirmer, S. Grimme, C. G. Daniliuc, G. Kehr, G. Erker, J. Am. Chem. Soc. 2013, 135, 18567–18574.
- 121M. J. Cowley, V. Huch, D. Scheschkewitz, Chem. - Eur. J. 2014, 20, 9221–9224.
- 122R. Y. Kong, M. R. Crimmin, J. Am. Chem. Soc. 2018, 140, 13614–13617.
- 123Y. Kratish, D. Pinchuk, A. Kaushansky, V. Molev, B. Tumanskii, D. Bravo-Zhivotovskii, Y. Apeloig, Angew. Chem. Int. Ed. 2019, 58, 18849–18853.
- 124A. V. Protchenko, P. Vasko, D. C. H. Do, J. Hicks, M. Á. Fuentes, C. Jones, S. Aldridge, Angew. Chem. Int. Ed. 2019, 58, 1808–1812.
- 125M. Xu, A. R. Jupp, D. W. Stephan, Angew. Chem. Int. Ed. 2019, 58, 3548–3552.
- 126Y. Xiong, S. Yao, T. Szilvási, A. Ruzicka, M. Driess, Chem. Commun. 2020, 56, 747–750.
- 127M. Xu, Z.-w. Qu, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2021, 143, 634–638.
- 128R. Y. Kong, M. Batuecas, M. R. Crimmin, Chem. Sci. 2021, 12, 14845–14854.
- 129A. Heilmann, M. M. D. Roy, A. E. Crumpton, L. P. Griffin, J. Hicks, J. M. Goicoechea, S. Aldridge, J. Am. Chem. Soc. 2022, 144, 12942–12953.
- 130S. Fujimori, S. Inoue, J. Am. Chem. Soc. 2022, 144, 2034–2050.
- 131M. Jörges, F. Krischer, V. H. Gessner, Science 2022, 378, 1331–1336.
- 132P. Garg, A. Carpentier, I. Douair, D. Dange, Y. Jiang, K. Yuvaraj, L. Maron, C. Jones, Angew. Chem. Int. Ed. 2022, 61, e202201705.
- 133R. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 4974–4977.
- 134M. Sajid, G. Kehr, C. G. Daniliuc, G. Erker, Angew. Chem. Int. Ed. 2014, 53, 1118–1121.
- 135M. Majumdar, I. Omlor, C. B. Yildiz, A. Azizoglu, V. Huch, D. Scheschkewitz, Angew. Chem. Int. Ed. 2015, 54, 8746–8750.
- 136Y. Katsuma, N. Tsukahara, L. Wu, Z. Lin, M. Yamashita, Angew. Chem. Int. Ed. 2018, 57, 6109–6114.
- 137Y. Wang, A. Kostenko, T. J. Hadlington, M. P. Luecke, S. Yao, M. Driess, J. Am. Chem. Soc. 2019, 141, 626–634.
- 138S. Yao, M. S. Budde, X. Yang, Y. Xiong, L. Zhao, M. Driess, Angew. Chem. Int. Ed. 2025, 64, e202414696.
- 139Y. P. Zhou, S. Raoufmoghaddam, T. Szilvási, M. Driess, Angew. Chem. Int. Ed. 2016, 55, 12868–12872.
- 140Y. Wang, A. Kostenko, S. Yao, M. Driess, J. Am. Chem. Soc. 2017, 139, 13499–13506.
- 141M. P. Luecke, L. Giarrana, A. Kostenko, T. Gensch, S. Yao, M. Driess, Angew. Chem. Int. Ed. 2022, 61, e202110398.
- 142Y. Xiong, S. Dong, S. Yao, C. Dai, J. Zhu, S. Kemper, M. Driess, Angew. Chem. Int. Ed. 2022, 61, e202209250.
- 143X. Wang, B. Lei, Z. Zhang, M. Chen, H. Rong, H. Song, L. Zhao, Z. Mo, Nat. Commun. 2023, 14, 2968.
- 144 Deposition Numbers 2449069 (for 1), 2449070 (for 2), 2449071 (for 3), 2449072 (for 4) and 2449073 (for 5) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 145J. Li, M. Zhong, H. Keil, H. Zhu, R. Herbst-Irmer, D. Stalke, S. De, D. Koley, H. W. Roesky, Chem. Commun. 2019, 55, 2360–2363.
- 146Y. Ding, M. Nazish, P. N. Ruth, R. Herbst-Irmer, D. Stalke, H. W. Roesky, Dalton Trans. 2023, 52, 6175–6179.
- 147F. M. Mück, J. A. Baus, R. Bertermann, C. Burschka, R. Tacke, Organometallics 2016, 35, 2583–2588.
- 148J. D. Gorden, A. Voigt, C. L. B. Macdonald, J. S. Silverman, A. H. Cowley, J. Am. Chem. Soc. 2000, 122, 950–951.
- 149P. E. Romero, W. E. Piers, S. A. Decker, D. Chau, T. K. Woo, M. Parvez, Organometallics 2003, 22, 1266–1274.
- 150Z. Yang, X. Ma, R. B. Oswald, H. W. Roesky, H. Zhu, C. Schulzke, K. Starke, M. Baldus, H. G. Schmidt, M. Noltemeyer, Angew. Chem. Int. Ed. 2005, 44, 7072–7074.
- 151N. Dettenrieder, H. M. Dietrich, C. Schädle, C. Maichle-Mössmer, K. W. Törnroos, R. Anwander, Angew. Chem. Int. Ed. 2012, 51, 4461–4465.
- 152W. Lu, H. Hu, Y. Li, R. Ganguly, R. Kinjo, J. Am. Chem. Soc. 2016, 138, 6650–6661.
- 153D. Dange, C. P. Sindlinger, S. Aldridge, C. Jones, Chem. Commun. 2017, 53, 149–152.
- 154A. Hofmann, M. A. Légaré, L. Wüst, H. Braunschweig, Angew. Chem. Int. Ed. 2019, 58, 9776–9781.
- 155A. Hofmann, C. Pranckevicius, T. Tröster, H. Braunschweig, Angew. Chem. Int. Ed. 2019, 58, 3625–3629.
- 156Z. Güven, L. Denker, D. Wullschläger, J. Pablo Martínez, B. Trzaskowski, R. Frank, Angew. Chem. Int. Ed. 2022, 61, e202209502.
- 157S. Kurumada, M. Yamashita, J. Am. Chem. Soc. 2022, 144, 4327–4332.
- 158H.-Y. Liu, M. F. Mahon, M. S. Hill, Inorg. Chem. 2023, 62, 15310–15319.
- 159M. R. Mason, B. Song, K. Kirschbaum, J. Am. Chem. Soc. 2004, 126, 11812–11813.
- 160X. Li, C. Ni, H. Song, C. Cui, Chem. Commun. 2006, 16, 1763–1765.
- 161R. Y. Kong, M. R. Crimmin, Chem. Commun. 2019, 55, 6181–6184.
- 162R. Y. Kong, M. R. Crimmin, Dalton Trans. 2020, 49, 16587–16597.
- 163M. J. Evans, M. G. Gardiner, M. D. Anker, M. P. Coles, Chem. Commun. 2022, 58, 5833–5836.
- 164M. Batuecas, R. Kong, A. White, M. Crimmin, Angew. Chem. Int. Ed. 2022, 61, e202202241.
- 165M. J. Evans, S. E. Neale, M. D. Anker, C. L. McMullin, M. P. Coles, Angew. Chem. Int. Ed. 2022, 61, e202117396.
- 166J. H. Barnard, S. Yruegas, K. Huang, C. D. Martin, Chem. Commun. 2016, 52, 9985–9991.
- 167K. R. Bluer, L. E. Laperriere, A. Pujol, S. Yruegas, V. A. K. Adiraju, C. D. Martin, Organometallics 2018, 37, 2917–2927.
- 168S. Yruegas, J. H. Barnard, K. Al-Furaiji, J. L. Dutton, D. J. D. Wilson, C. D. Martin, Organometallics 2018, 37, 1515–1518.
- 169S. Yruegas, J. J. Martinez, C. D. Martin, Chem. Commun. 2018, 54, 6808–6811.
- 170W. Yang, K. E. Krantz, D. A. Dickie, A. Molino, D. J. D. Wilson, R. J. Gilliard, Angew. Chem. Int. Ed. 2020, 59, 3971–3975.
- 171T. Bischof, X. Guo, I. Krummenacher, L. Beßler, Z. Lin, M. Finze, H. Braunschweig, Chem. Sci. 2022, 13, 7492–7497.
- 172T. Bischof, L. Beßler, I. Krummenacher, L. Erhard, H. Braunschweig, M. Finze, Chem. - Eur. J. 2023, 29, e202300210.
- 173N. Tokitoh, Y. Mizuhata, T. Sato, Heterocycles 2012, 84, 413–418.
- 174R. Holzner, D. Reiter, P. Frisch, S. Inoue, RSC Adv. 2020, 10, 3402–3406.
- 175H. Zhu, A. Kostenko, D. Franz, F. Hanusch, S. Inoue, J. Am. Chem. Soc. 2023, 145, 1011–1021.