A Bioinspired Diazafluorenone Catalytic System for Aerobic Oxidative Deamination of Primary Amines
Sheng Gong
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
Both authors contributed equally to this work.
Search for more papers by this authorWenhu Zhang
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
Both authors contributed equally to this work.
Search for more papers by this authorYongchang Song
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
Search for more papers by this authorCorresponding Author
Jianfeng Chen
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Baoguo Zhao
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorSheng Gong
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
Both authors contributed equally to this work.
Search for more papers by this authorWenhu Zhang
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
Both authors contributed equally to this work.
Search for more papers by this authorYongchang Song
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
Search for more papers by this authorCorresponding Author
Jianfeng Chen
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Baoguo Zhao
The Education Ministry Key Lab of Resource Chemistry, Shanghai Frontiers Science Center of Biomimetic Catalysis, Shanghai Normal University, Shanghai, 200234 China
State Key Laboratory of Synergistic Chem-Bio Synthesis, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
Abstract
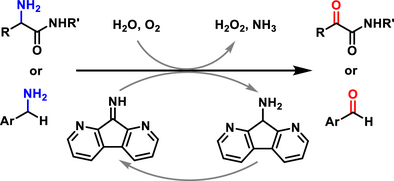
Copper amine oxidases (CAOs) catalyze aerobic oxidation of primary amines into carbonyl compounds, which is an important biological process as well as a useful transformation for organic synthesis. A new bioinspired organocatalyst, 1,8-diazafluoren-9-one (DFO), has been discovered, which exhibits impressive characteristics, including simple structure, high robustness, and strong electrophilicity, enabling it to effectively mimic the function of copper amine oxidases. Oxidative deamination of α-amino amides presents an attractive approach to access α-keto amides; however, it is difficult to achieve due to their electron-withdrawing nature and steric effect. The diazafluorenone displays high catalytic activity in this transformation, producing various biologically significant α-keto amides in moderate to excellent yields. Furthermore, the diazafluorenone catalyst is able to promote direct oxidation of benzylamines into aromatic aldehydes, instead of the generally formed product-reactant imines, which leads to no greater than 50% yields of the desired aldehydes. The current aerobic oxidative deamination proceeds via a pathway similar to the biological process, involving transamination, hydrolysis/aminolysis, and aerobic oxidation.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202511937-sup-0001-SuppMat.pdf21.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. Floris, B. Mondovì, Copper Amine Oxidases: Structures, Catalytic Mechanisms, and Role in Pathophysiology, CRC Press, Boca Raton 2009.
10.1201/9781420076813 Google Scholar
- 2M. Mure, S. A. Mills, J. P. Klinman, Biochemistry 2002, 41, 9269–9278.
- 3M. Mure, Acc. Chem. Res. 2004, 37, 131–139.
- 4M. Mure, J. P. Klinman, J. Am. Chem. Soc. 1995, 117, 8698–8706.
- 5M. Mure, J. P. Klinman, J. Am. Chem. Soc. 1995, 117, 8707–8718.
- 6Y. Lee, L. M. Sayre, J. Am. Chem. Soc. 1995, 117, 3096–3105.
- 7Y. Lee, L. M. Sayre, J. Am. Chem. Soc. 1995, 117, 11823–11828.
- 8Y. Lee, H. Huang, L. M. Sayre, J. Am. Chem. Soc. 1996, 118, 7241–7242.
- 9S. Tang, M. Rauch, M. Montag, Y. Diskin-Posner, Y. Ben-David, D. Milstein, J. Am. Chem. Soc. 2020, 142, 20875–20882.
- 10N. K. Pal, K. Singh, M. Patra, S. Yadav, P. K. Pandey, J. K. Bera, Green Chem. 2023, 25, 6212–6217.
- 11A. Kumar, S. Bhatt, P. P. Samal, S. Krishnamurty, S. L. Jain, ACS Appl. Eng. Mater. 2, 2095–2101.
10.1021/acsaenm.4c00251 Google Scholar
- 12M. Largeron, M.-B. Fleury, Science 2013, 339, 43–44.
- 13M. Largeron, Org. Biomol. Chem. 2017, 15, 4722–4730.
- 14M. Largeron, Pure Appl. Chem. 2020, 92, 233–242.
- 15R. Zhang, S. Luo, Chin. Chem. Lett. 2018, 29, 1193–1200.
- 16M. Largeron, Eur. J. Org. Chem. 2013, 2013, 5225–5235.
- 17A. E. Wendlandt, S. S. Stahl, Angew. Chem. Int. Ed. 2015, 54, 14638–14658.
- 18B. Chen, L. Wang, S. Gao, ACS Catal. 2015, 5, 5851–5876.
- 19M. T. Schümperli, C. Hammond, I. Hermans, ACS Catal. 2012, 2, 1108–1117.
- 20M. Largeron, M.-B. Fleury, J. Org. Chem. 2000, 65, 8874–8881.
- 21M. Largeron, A. Neudorffer, M.-B. Fleury, Angew. Chem. Int. Ed. 2003, 42, 1026–1029.
- 22M. Largeron, M.-B. Fleury, Org. Lett. 2009, 11, 883–886.
- 23M. Largeron, M.-B. Fleury, Angew. Chem. Int. Ed. 2012, 51, 5409–5412.
- 24M. Largeron, M.-B. Fleury, Chem. Eur. J. 2015, 21, 3815–3820.
- 25M. Largeron, M.-B. Fleury, Chem. Eur. J. 2017, 23, 6763–6767.
- 26M. Largeron, P. Deschamps, K. Hammad, M.-B. Fleury, Green Chem. 2020, 22, 1894–1905.
- 27A. E. Wendlandt, S. S. Stahl, Org. Lett. 2012, 14, 2850–2853.
- 28A. E. Wendlandt, S. S. Stahl, J. Am. Chem. Soc. 2014, 136, 506–512.
- 29A. E. Wendlandt, S. S. Stahl, J. Am. Chem. Soc. 2014, 136, 11910–11913.
- 30B. Li, A. E. Wendlandt, S. S. Stahl, Org. Lett. 2019, 21, 1176–1181.
- 31H. Yuan, W.-J. Yoo, H. Miyamura, S. Kobayashi, J. Am. Chem. Soc. 2012, 134, 13970–13973.
- 32Y. Qin, L. Zhang, J. Lv, S. Luo, J.-P. Cheng, Org. Lett. 2015, 17, 1469–1472.
- 33R. Zhang, Y. Qin, L. Zhang, S. Luo, J. Org. Chem. 2019, 84, 2542–2555.
- 34Y. Goriya, H. Y. Kim, K. Oh, Org. Lett. 2016, 18, 5174–5177.
- 35G. Golime, G. Bogonda, H. Y. Kim, K. Oh, ACS Catal. 2018, 8, 4986–4990.
- 36J. Baek, H. Y. Kim, K. Oh, Org. Chem. Front. 2023, 10, 4353–4358.
- 37R. Grigg, T. Mongkolaussavaratana, C. A. Pounds, S. Sivagnanam, Tetrahedron Lett. 1990, 31, 7215–7218.
- 38J. Li, S. Gong, S. Gao, J. Chen, W.-W. Chen, B. Zhao, Nat. Commun. 2024, 15, 939.
- 39J. S. Yadav, B. V. S. Reddy, T. Swamy, K. S. Shankar, Monatsh. Chem. 2008, 139, 1317–1320.
- 40Y. Xie, H. Pan, M. Liu, X. Xiao, Y. Shi, Chem. Soc. Rev. 2015, 44, 1740–1748.
- 41X. Xiao, B. Zhao, Acc. Chem. Res. 2023, 56, 1097–1117.
- 42X. Xiao, K. Xu, Z.-H. Gao, Z.-H. Zhu, C. Ye, B. Zhao, S. Luo, S. Ye, Y.-G. Zhou, S. Xu, S.-F. Zhu, H. Bao, W. Sun, X. Wang, K. Ding, Sci. China Chem. 2023, 66, 1553–1633.
- 43X. Xiao, Y. Xie, C. Su, M. Liu, Y. Shi, J. Am. Chem. Soc. 2011, 133, 12914–12917.
- 44Y. Wu, L. Deng, J. Am. Chem. Soc. 2012, 134, 14334–14337.
- 45Y. E. Liu, Z. Lu, B. Li, J. Tian, F. Liu, J. Zhao, C. Hou, Y. Li, L. Niu, B. Zhao, J. Am. Chem. Soc. 2016, 138, 10730–10733.
- 46Q.-K. Kang, S. Selvakumar, K. Maruoka, Org. Lett. 2019, 21, 2294–2297.
- 47W. Cai, X. Qiao, H. Zhang, B. Li, J. Guo, L. Zhang, W.-W. Chen, B. Zhao, Nat. Commun. 2021, 12, 5174.
- 48W. Cai, D. Cai, H. Liang, X. Ren, B. Zhao, J. Org. Chem. 2023, 88, 7849–7857.
- 49J. Lu, Y. Yu, Z. Li, J. Luo, L. Deng, J. Am. Chem. Soc. 2024, 146, 16706–16713.
- 50M. Robello, E. Barresi, E. Baglini, S. Salerno, S. Taliani, F. D. Settimo, J. Med. Chem. 2021, 64, 3508–3545.
- 51C. De Risi, G. P. Pollini, V. Zanirato, Chem. Rev. 2016, 116, 3241–3305.
- 52D. Kumar, S. R. Vemula, G. R. Cook, ACS Catal. 2016, 6, 4920–4945.
- 53N. A. Meanwell, M. R. Krystal, B. Nowicka-Sans, D. R. Langley, D. A. Conlon, M. D. Eastgate, D. M. Grasela, P. Timmins, T. Wang, J. F. Kadow, J. Med. Chem. 2018, 61, 62–80.
- 54J. Li, G. Chen, J. M. Webster, Can. J. Microbiol. 1997, 43, 770–773.
- 55A. Arasappan, F. Bennett, S. L. Bogen, S. Venkatraman, M. Blackman, K. X. Chen, S. Hendrata, Y. Huang, R. M. Huelgas, L. Nair, A. I. Padilla, W. Pan, R. Pike, P. Pinto, S. Ruan, M. Sannigrahi, F. Velazquez, B. Vibulbhan, W. Wu, W. Yang, A. K. Saksena, V. Girijavallabhan, N.-Y. Shih, J. Kong, T. Meng, Y. Jin, J. Wong, P. McNamara, A. Prongay, V. Madison, et al., ACS Med. Chem. Lett. 2010, 1, 64–69.
- 56T. F. Buckley, H. Rapoport, J. Am. Chem. Soc. 1982, 104, 4446–4450.
- 57L. S. Witus, C. Netirojjanakul, K. S. Palla, E. M. Muehl, C.-H. Weng, A. T. Iavarone, M. B. Francis, J. Am. Chem. Soc. 2013, 135, 17223–17229.
- 58K. S. Palla, L. S. Witus, K. J. Mackenzie, C. Netirojjanakul, M. B. Francis, J. Am. Chem. Soc. 2015, 137, 1123–1129.
- 59J. Dong, H.-J. Jeong, H. Ueda, J. Biosci. Bioeng. 2016, 122, 125–130.
- 60C. Cennamo, B. Carafoli, E. P. Bonetti, J. Am. Chem. Soc. 1956, 78, 3523–3527.
- 61K. Tatsumoto, M. Haruta, A. E. Martell, Inorg. Chim. Acta 1987, 138, 231–239.
- 62J. M. Gilmore, R. A. Scheck, A. P. Esser-Kahn, N. S. Joshi, M. B. Francis, Angew. Chem. Int. Ed. 2006, 45, 5307–5311.
- 63R. A. Scheck, M. T. Dedeo, A. T. Iavarone, M. B. Francis, J. Am. Chem. Soc. 2008, 130, 11762–11770.
- 64M. Zhang, X. Zhang, J. Li, Q. Guo, Q. Xiao, Chin. J. Chem. 2011, 29, 1715–1720.
- 65S. H. Lee, H. Kyung, R. Yokota, T. Goto, T. Oe, Chem. Res. Toxicol. 2014, 27, 637–648.
- 66H. B. F. Dixon, Biochem. J. 1964, 92, 661–666.
- 67A. Papanikos, J. Rademann, M. Meldal, J. Am. Chem. Soc. 2001, 123, 2176–2181.
- 68J. Srogl, S. Voltrova, Org. Lett. 2009, 11, 843–845.
- 69S. Wang, Q. Zhou, X. Chen, R.-H. Luo, Y. Li, X. Liu, L.-M. Yang, Y.-T. Zheng, P. Wang, Nat. Commun. 2021, 12, 2257.
- 70J. Chen, X. Gong, J. Li, Y. Li, J. Ma, C. Hou, G. Zhao, W. Yuan, B. Zhao, Science 2018, 360, 1438–1442.
- 71A. Cheng, L. Zhang, Q. Zhou, T. Liu, J. Cao, G. Zhao, K. Zhang, G. Song, B. Zhao, Angew. Chem. Int. Ed. 2021, 60, 20166–20172.
- 72E. Schön, I. Born, H.-U. Demuth, J. Faust, K. Neubert, T. Steinmetzer, A. Barth, S. Ansorge, Biol. Chem. Hoppe. Seyler. 1991, 372, 305–312.
- 73D. V. Patel, R. D. Gless, Jr., H. K. Webb HSU, S. K. Anandan, B. R. Aavula, PCT Int. Appl. WO 2008073623, 2008.
- 74H. Knust, M. Nettekoven, E. Pinard, O. Roche, M. Rogers-Evans, PCT Int. Appl. WO 2009016087, 2009.
- 75S. Golla, H. P. Kokatla, J. Org. Chem. 2022, 87, 9915–9925.
- 76Z. Zhu, X. Lv, J. E. Anesini, D. Seidel, Org. Lett. 2017, 19, 6424–6427.
- 77A. Muthukumar, G. Sekar, J. Org. Chem. 2018, 83, 8827–8839.
- 78H. Tian, L. Ermolenko, M. Gabant, C. Vergne, C. Moriou, P. Retailleau, A. Al-Mourabit, Adv. Synth. Catal. 2011, 353, 1525–1533.
- 79M. W. Weatherburn, Anal. Chem. 1967, 39, 971–974.
- 80J. Fajardo, Jr., J. C. Peters, J. Am. Chem. Soc. 2017, 139, 16105–16108.
- 81R. Breslow, Acc. Chem. Res. 1995, 28, 146–153.