Dual-Function Tetrabenzylphosphonium Groups as Mitochondria-Targeting Artificial Anion Channels
Dr. Fei Gou
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Both authors contributed equally to this work.
Search for more papers by this authorXinlei Huangfu
College of Chemistry and Molecular Engineering, Peking University, Haidian District, Beijing, 100084 China
Both authors contributed equally to this work.
Search for more papers by this authorQiuting Wang
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorZihong Yang
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorXiyu Yuan
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorDr. Wenju Chang
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jie Shen
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
E-mails: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Wen-Xiong Zhang
College of Chemistry and Molecular Engineering, Peking University, Haidian District, Beijing, 100084 China
E-mails: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Huaqiang Zeng
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
E-mails: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Fei Gou
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Both authors contributed equally to this work.
Search for more papers by this authorXinlei Huangfu
College of Chemistry and Molecular Engineering, Peking University, Haidian District, Beijing, 100084 China
Both authors contributed equally to this work.
Search for more papers by this authorQiuting Wang
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorZihong Yang
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorXiyu Yuan
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorDr. Wenju Chang
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jie Shen
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
E-mails: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Wen-Xiong Zhang
College of Chemistry and Molecular Engineering, Peking University, Haidian District, Beijing, 100084 China
E-mails: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Huaqiang Zeng
College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116 China
E-mails: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
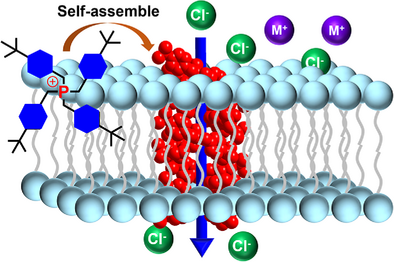
A series of mitochondriotropic artificial anion channels have been constructed from a self-assembled tetrabenzylphosphonium framework with an inherent ability to autonomously target mitochondria. Anion transport is mediated by multiple intermolecular C─H⋯anion H─bonds and electrostatic ion pair interactions. The highly efficient transmembrane anion transport is demonstrated by low micromolar anticancer activity against three cancer cell lines.
Abstract
Artificial ion channels with specific organelle-targeting capabilities have been scarcely investigated. Here, we report the first-in-class mitochondria-targeting anion channels derived from a structurally simple tetrabenzylphosphonium framework, in stark contrast to its phenyl-based counterpart, which lacks anion transport activity. Structural and computational analyses underscore the critical role of the methylene (CH2) linkers in the benzyl groups. These CH2 units reduce positive charge delocalization to enhance σ-hole–anion interactions, while also enabling H-atoms from both the CH2 linkers and aromatic rings to cooperatively form multiple C─H⋯anion H─bonds. In further conjunction with the rigid benzene rings, they help create sufficient spatial voids to accommodate anion translocation, collectively facilitating and energizing the anion transport process. Among the series studied, those bearing methyl and tert-butyl substituents exhibit the highest transport activity via a channel mechanism, with a conductance value as high as 26.5 ± 0.8 pS. Furthermore, leveraging the cationic nature of the quaternary phosphonium center, this family of anion channels readily achieves targeted mitochondrial localization, demonstrating potent anticancer activity, with IC50 values ranging from 1.42 to 3.04 µM across three cancer cell lines.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202511936-sup-0001-SuppMat.pdf1.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. A. Hübner, T. J. Jentsch, Hum. Mol. Genet. 2002, 11, 2435–2445.
- 2X. Wu, E. N. W. Howe, P. A. Gale, Acc. Chem. Res. 2018, 51, 1870–1879.
- 3T. J. Jentsch, K. Steinmeyer, G. Schwarz, Nature 1990, 348, 510–514.
- 4K. Steinmeyer, C. Ortland, T. J. Jentsch, Nature 1991, 354, 301–304.
- 5T. J. Jentsch, M. Pusch, Physiol. Rev. 2018, 98, 1493–1590.
- 6A. Picollo, M. Pusch, Nature 2005, 436, 420–423.
- 7P. A. Gale, C. C. Tong, C. J. E. Haynes, O. Adeosun, D. E. Gross, E. Karnas, E. M. Sedenberg, R. Quesada, J. L. Sessler, J. Am. Chem. Soc. 2010, 132, 3240–3241.
- 8T. Arakawa, T. Kobayashi-Yurugi, Y. Alguel, H. Iwanari, H. Hatae, M. Iwata, Y. Abe, T. Hino, C. Ikeda-Suno, H. Kuma, D. Kang, T. Murata, T. Hamakubo, A. D. Cameron, T. Kobayashi, N. Hamasaki, S. Iwata, Science 2015, 350, 680–684.
- 9H. Valkenier, O. Akrawi, P. Jurček, K. Sleziaková, T. Lízal, K. Bartik, V. Šindelář, Chem 2019, 5, 429–444.
- 10R. Dutzler, FEBS Lett. 2004, 564, 229–233.
- 11E. Park, E. B. Campbell, R. MacKinnon, Nature 2017, 541, 500–505.
- 12J. J. Thomas, J. Physiol 2015, 593, 4091–4109.
- 13Z. Chen, X. Xie, C. Jia, Q. Zhong, Q. Zhang, D. Luo, Y. Cao, Y. Mu, C. Ren, Angew. Chem. Int. Ed. 2024, 63, e202318811.
- 14S. Chattopadhayay, K. V. Banzal, P. Talukdar, Angew. Chem. Int. Ed. 2025, 64, e202414354.
- 15T. Yan, J. Liu, Angew. Chem. Int. Ed. 2025, 64, e202416200.
- 16Y. Lin, B. Wu, Y. Zeng, H. Yuan, C. Ji, Z. Liu, Y. Sui, T. Yin, X. Kong, Y. Zhu, J. Chen, C. Lang, Angew. Chem. Int. Ed. 2024, 63, e202408558.
- 17H. Chen, Y. Liu, X. Cheng, S. Fang, Y. Sun, Z. Yang, W. Zheng, X. Ji, Z. Wu, Angew. Chem. Int. Ed. 2021, 60, 10833–10841.
- 18A. Mondal, S. N. Save, S. Sarkar, D. Mondal, J. Mondal, S. Sharma, P. Talukdar, J. Am. Chem. Soc. 2023, 145, 9737–9745.
- 19S. Deng, Z. Li, L. Yuan, H. Q. Zeng, Molecules 2024, 29, 1118.
- 20T. Saha, M. S. Hossain, D. Saha, M. Lahiri, P. Talukdar, J. Yang, G. Yu, J. L. Sessler, I. Shin, P. A. Gale, F. Huang, J. Am. Chem. 2021, 7, 3256–3291.
- 21L. Yuan, P. Jiang, J. Hu, H. Q. Zeng, Y. Huo, Z. Li, H. Q. Zeng, Chin. Chem. Lett. 2022, 33, 2026–2030.
- 22W.-L. Huang, X.-D. Wang, Y.-F. Ao, Q.-Q. Wang, D.-X. Wang, J. Am. Chem. Soc. 2020, 142, 13273–13277.
- 23R. Cao, R. B. Rossdeutcher, Y. Zhong, Y. Shen, D. P. Miller, T. A. Sobiech, X. Wu, L. S. Buitrago, K. Ramcharan, M. I. Gutay, M. F. Figueira, P. Luthra, E. Zurek, T. Szyperski, B. Button, Z. Shao, B. Gong, Nat. Chem. 2023, 15, 1559–1568.
- 24R. Ye, C. Ren, J. Shen, N. Li, F. Chen, A. Roy, H. Q. Zeng, J. Am. Chem. Soc. 2019, 141, 9788–9792.
- 25X. Wu, J. R. Small, A. Cataldo, A. M. Withecombe, P. Turner, P. A. Gale, Angew. Chem. Int. Ed. 2019, 58, 15142–15147.
- 26X. Li, B. Shen, X.-Q. Yao, D. Yang, J. Am. Chem. Soc. 2007, 129, 7264–7265.
- 27W.-L. Huang, X.-D. Wang, Y.-F. Ao, Q.-Q. Wang, D.-X. Wang, Angew. Chem. Int. Ed. 2023, 62, e202302198.
- 28P. H. Schlesinger, R. Ferdani, J. Liu, J. Pajewska, R. Pajewski, M. Saito, H. Shabany, G. W. Gokel, J. Am. Chem. Soc. 2002, 124, 1848–1849.
- 29N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás, P. A. Gale, J. Am. Chem. Soc. 2011, 133, 14136–14148.
- 30L. Yuan, J. Shen, R. Ye, F. Chen, H. Q. Zeng, Chem. Commun. 2019, 55, 4797–4800.
- 31P. R. Brotherhood, A. P. Davis, Chem. Soc. Rev. 2010, 39, 3633–3647.
- 32A. P. Davis, D. N. Sheppard, B. D. Smith, Chem. Soc. Rev. 2007, 36, 348–357.
- 33J. T. Davis, O. Okunola, R. Quesada, Chem. Soc. Rev. 2010, 39, 3843.
- 34P. A. Gale, J. T. Davis, R. Quesada, Chem. Soc. Rev. 2017, 46, 2497–2519.
- 35G. W. Gokel, S. Negin, Acc. Chem. Res. 2013, 46, 2824–2833.
- 36G. You, Med. Res. Rev. 2004, 24, 762–774.
- 37S. Otto, M. Osifchin, S. L. Regen, J. Am. Chem. Soc. 1999, 121, 7276–7277.
- 38N. Madhavan, E. C. Robert, M. S. Gin, Angew. Chem. Int. Ed. 2005, 44, 7584–7587.
- 39J. Kempf, A. Schmitzer, RSC Adv. 2016, 6, 42713–42719.
- 40C. Zhang, J. Tian, S. Qi, B. Yang, Z. Dong, Nano Lett. 2020, 20, 3627–3632.
- 41N. Akhtar, N. Pradhan, G. K. Barik, S. Chatterjee, S. Ghosh, A. Saha, P. Satpati, A. Bhattacharyya, M. K. Santra, D. Manna, ACS Appl. Mater. Interfaces 2020, 12, 25521–25533.
- 42B. Díaz de Greñu, P. I. Hernández, M. Espona, D. Quiñonero, M. E. Light, T. Torroba, R. Pérez-Tomás, R. Quesada, Chem.-Eur. J. 2011, 17, 14074–14083.
- 43E. Hernando, V. Capurro, C. Cossu, M. Fiore, M. García-Valverde, V. Soto-Cerrato, R. Pérez-Tomás, O. Moran, O. Zegarra-Moran, R. Quesada, Sci. Rep. 2018, 8, 2608.
- 44M. Vidal, A. Schmitzer, Chem.-Eur. J. 2014, 20, 9998–10004.
- 45J. Gravel, R. A. Schmitzer, Supramol. Chem. 2015, 27, 364–371.
- 46S.-P. Zheng, Y.-H. Li, J.-J. Jiang, A. van der Lee, D. Dumitrescu, M. Barboiu, Angew. Chem. Int. Ed. 2019, 58, 12037–12042.
- 47T. Brinck, J. S. Murray, P. Politzer, Int. J. Quantum Chem. 1992, 44, 57–64.
- 48A. V. Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. Metrangolo, G. Resnati, N. Sakai, S. Matile, Nat. Commun. 2012, 3, 905.
- 49A. Vargas Jentzsch, D. Emery, J. Mareda, P. Metrangolo, G. Resnati, S. Matile, Angew. Chem. Int. Ed. 2011, 50, 11675–11678.
- 50L. E. Bickerton, A. J. Sterling, P. D. Beer, F. Duarte, M. J. Langton, Chem. Sci. 2020, 11, 4722–4729.
- 51C. Ren, X. Ding, A. Roy, J. Shen, S. Zhou, F. Chen, S. F. Yau Li, H. Ren, Y. Y. Yang, H. Q. Zeng, Chem. Sci. 2018, 9, 4044–4051.
- 52L. E. Bickerton, A. Docker, A. J. Sterling, H. Kuhn, F. Duarte, P. D. Beer, M. J. Langton, Chem.-Eur. J. 2021, 27, 11738–11745.
- 53S. Benz, M. Macchione, Q. Verolet, J. Mareda, N. Sakai, S. Matile, J. Am. Chem. Soc. 2016, 138, 9093–9096.
- 54H. V. Humeniuk, A. Gini, X. Hao, F. Coelho, N. Sakai, S. Matile, JACS Au. 2021, 1, 1588–1593.
- 55A. Docker, T. G. Johnson, H. Kuhn, Z. Zhang, M. J. Langton, J. Am. Chem. Soc. 2023, 145, 2661–2668.
- 56B. Zhou, F. P. Gabbaï, Chem. Sci. 2020, 11, 7495–7500.
- 57L. M. Lee, M. Tsemperouli, A. I. Poblador-Bahamonde, S. Benz, N. Sakai, K. Sugihara, S. Matile, J. Am. Chem. Soc. 2019, 141, 810–814.
- 58G. Park, D. J. Brock, J.-P. Pellois, F. P. Gabbaï, Chem 2019, 5, 2215–2227.
- 59G. Park, F. P. Gabbaï, Chem. Sci. 2020, 11, 10107–10112.
- 60B. L. Murphy, F. P. Gabbaï, J. Am. Chem. Soc. 2024, 146, 7146–7151.
- 61N. H. Hunter, F. P. Gabbaï, Angew. Chem. Int. Ed. 2025, 64, e202414699.
- 62R. A. J. Smith, C. M. Porteous, A. M. Gane, M. P. Murphy, Proc. Natl. Acad. Sci 2003, 100, 5407–5412.
- 63D.-Y. Kim, H.-J. Kim, K.-H. Yu, J.-J. Min, Bioconjugate Chem. 2012, 23, 431–437.
- 64A. Singh, A. Torres-Huerta, F. Meyer, H. Valkenier, Chem. Sci. 2024, 15, 15006–15022.
- 65W. G. Ryder, A. Levina, M. E. Graziotto, B. A. Hawkins, D. E. Hibbs, E. J. New, P. A. Gale, Chem 2025, 11, 102247.
- 66X. Huangfu, W. Liu, H. Xu, Z. Wang, J. Wei, W.-X. Zhang, Inorg. Chem. 2023, 62, 12009–12017.
- 67 The supplementary crystallographic data for this paper can be found under deposition numbers 2417178 (for 3) and 2417179 (for 5). These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 68CCDC 153818, see: A. L. Spek, CSD Communication 2000, https://doi.org/10.5517/cc551w7.
10.5517/cc551w7 Google Scholar
- 69H. Gill, M. R. Gokel, M. McKeever, S. Negin, M. B. Patel, S. Yin, G. W. Gokel, Coord. Chem. Rev. 2020, 412, 213264.
- 70J. Shen, Y. Gu, L. Ke, Q. Zhang, Y. Cao, Y. Lin, Z. Wu, C. Wu, Y. Mu, Y.-L. Wu, C. Ren, H. Q. Zeng, Nat. Commun. 2022, 13, 5985.