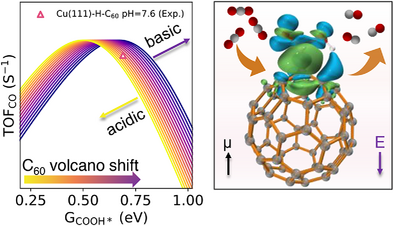
C60 Fullerene as the Active Site for CO2 Electroreduction
Graphical Abstract
We reveal a striking finding: Fullerene (C60), beyond its classical role as an electron buffer, can directly serve as a molecular active site for CO₂ reduction. Through data mining, ab initio simulations, and pH–field coupled microkinetics, we uncover that C60’s unique curvature induces strong dipole responses, stabilizing COOH* intermediates and enabling a pH-dependent volcano. This work redefines the catalytic nature of curved carbon materials.
Abstract
Fullerene (C60) was considered as a catalyst promoter in various electrochemical reactions, yet its catalytic role in enhancing catalytic performance beyond electron transfer remains a puzzle to chemists. Traditional simulations imply C60’s inertness in CO2 reduction reaction (CO2RR) due to weak interaction with COOH* intermediates. Here, according to a pH-field coupled microkinetic model at reversible hydrogen electrode (RHE) scale, we demonstrate that C60 acts as molecular active sites to facilitate the CO2RR toward CO through a strong binding to COOH* in the electrochemical conditions. This binding is mainly due to the unique structure of C60 that induces large dipole moment changes to stabilize COOH* intermediates across different pH conditions. By detailed comparison of experimental CO2RR observations and quantitative pH-dependent modeling, this work provides new insights on C60-based catalysts, highlighting the large dipole moment change upon adsorption at curved surfaces should not be dismissed when analyzing the pH-dependent binding strength and electrocatalytic activity.
Introduction
Fullerene (C60) materials have been widely recognized for its role as an electronic modulator, altering the electron structures of associated metal catalysts in energy storage and conversion processes.[1-6] Its unique cage-like structure and delocalized π-electron system render its excellent properties of electron buffer,[5] enabling efficient charge transfer processes in applications such as hydrogen evolution,[7] oxygen reduction,[3] and carbon dioxide (CO2) reduction reactions.[5, 8] By integrating fullerene into catalytic systems, performances including reaction rates, selectivity, and durability can be principally improved, opening possibilities for making fullerenes as advanced materials in green technologies.[5, 9]
Electrochemical conversion of CO2 to CO holds great promise, as CO is a crucial renewable feedstock for the widely-applied Fischer–Tropsch process and other industrial applications.[10, 11] The use of carbon-based electrocatalysts, such as traditional graphene-based catalysts and carbon-coated catalysts, primarily leverages their physical properties to facilitate rapid electron conduction,[12] prevent corrosion of metal active sites, and shield against electrochemical oxidation and physical agglomeration.[13] However, increasing the applied bias to boost current density at metal active sites will simultaneously promote the competitive hydrogen evolution reaction (HER), which suppresses CO selectivity due to the scaling relationships between COOH* and H* intermediates.[14]
C60, with contributions mainly from s-/p-orbital, may effectively bypass the constraints imposed by conventional d-band characteristics of metal catalysts and scaling relationships between COOH* and H* intermediates.[14, 15] While “electron buffering effects”[5, 6, 16] have recently been exploited in catalysis, C60’s role remains to be explored. Through analysis of extensive experimental data via a large-scale data mining from CO2RR literatures published in the past decade (Figure 1), we identified that C60 can actively participate in CO2RR, directly influencing catalytic performance: even using Cu as the substrate (which conventionally favors multi-carbon products in CO2RR), the inclusion of C60 leads to a high Faradaic efficiency (FE) of CO (−90%). This discovery challenges catalytic assumptions about limitation of C60 on a promoter and exemplifies the need to reassess its intrinsic role. Despite the simplicity of the two-electron transfer process in the CO2-to-CO conversion, the actual reaction mechanisms are complex,[17, 18] including factors like interfacial charge, solvent effects, applied potential at the reversible hydrogen electrode (RHE) scale, as well as the pH conditions. Accurately capturing these components is challenged to traditional ab initio models.[19]
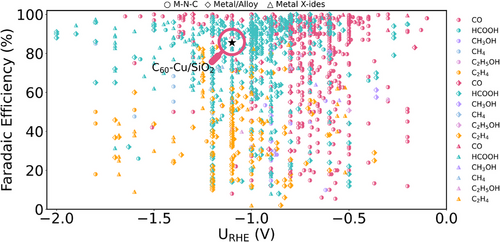
The computational hydrogen electrode (CHE) method, widely used in density functional theory (DFT) calculations,[20] is a standard approach for modeling electrocatalysis. While sometimes effective in capturing general trends, this method might overlook real the surface states and fails to accurately predict experimental observations, such as current densities (j) and Tafel slopes. Moreover, it struggles with different pH conditions, as the traditional H+ activity correction term to the reaction energy (i.e., 0.059 * pH) shifts in the same way as the potential shifts at the standard hydrogen electrode (SHE) scale, which fails to analyze the pH-dependent behavior at the RHE scale,[21] leading to discrepancies between theoretical and experimental observations. Recent studies have shown that activity in metal-nitrogen-carbon (M─N─C) catalysts for CO2-to-CO conversion depends on key descriptors: CO2* and COOH* binding strengths, influenced by narrow d-states and CO2* dipoles.[22] Although new models incorporating electric field effects and microkinetic analysis have improved understanding of CO2RR,[23] they are still difficult to achieve accurate predictions of activity and selectivity under complex conditions, for example, considering the electrode acidity,[24] consequentially causing deviations between real working conditions and models for CO2RR. To address these challenges, it is crucial to understand the atomic-scale behavior of C60 in CO2RR, particularly its interactions with key intermediates, rate-determining steps, and RHE scale's pH-dependent activity under applied potential.
In this work, we present a comprehensive mechanistic study of CO2RR on C60-based catalysts, introducing a robust model that can potentially be extended to other electrocatalytic processes. We reveal that the unique dipole moment and polarizability of C60’s curved surface under electric fields plays a crucial role in tuning the binding energies of reaction intermediates, distinctly differing from planar graphene and metal catalysts. On basis of the analysis of the reaction kinetics of CO2 activation, COOH* reduction to CO*, and CO* desorption, a pH-dependent microkinetic activity volcano at the RHE scale was derived using COOH* binding strength as the descriptor (i.e., the independent variable). This model enables a direct comparison of competing reaction steps across potential and pH conditions. Furthermore, our finding demonstrate that C60-based catalysts exhibit enhanced activity in alkaline media due to the stabilization of COOH* under electric fields, a direct consequence of the C60 molecule's intrinsic curvature-induced dipole. Such a comprehensive analysis offers new insights into the design of C60-based and carbon-based electrocatalysts, highlighting the role of surface curvature geometrics in optimizing COOH* binding strength and strategically tuning of reaction conditions to enhance efficiency and selectivity.
Results and Discussion
The Role of C60
An initial experiment of CO2RR revealed that the Cu/SiO2 catalyst, operating at −1.1 VRHE, displayed conventional Cu surface activity, producing both CO and C2H4, but with CO2RR competing unfavorably against the HER. However, when C60 was integrated with Cu/SiO2, the FE of CO rose dramatically to 85.5%, with negligible C2H4 formation (Figure 2a), signaling a shift in the catalytic active sites. Furthermore, the C60-Cu/SiO2 catalyst demonstrated robust stability, with no deactivation or poisoning of the active sites under experimental conditions (Figures 2b and S1). Details of the experimental methods can be found in the Supporting Information.
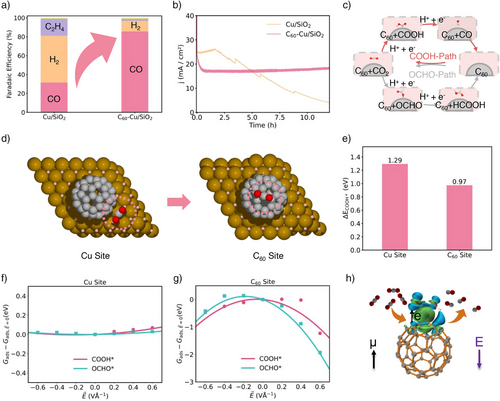
To understand the observed catalytic performances, Figure 2c illustrates the mechanisms leading to CO and HCOOH production on the electrocatalysts. CO is formed via a two-electron pathway: COOH* → CO* → CO, while HCOOH is produced through an alternative pathway: OCHO* → HCOOH* → HCOOH. Specifically, the COOH-pathway can be detailed as: CO2 + (H+ + e−) → COOH*, followed by COOH* + (H+ + e−) → CO* + H2O. In contrast, the OCHO-pathway proceeds as: CO2 + (H+ + e−) → OCHO*, followed by OCHO* + (H+ + e−) → HCOOH*. Product analysis confirms that COOH* is the dominant intermediate formed after the first proton-electron transfer, leading to CO formation.
Figure 2d further illustrates the two distinct active sites within the C60-Cu/SiO2 catalyst: the Cu site and the C60 site. Simulations of the relative electronic energy of the COOH* intermediate at both sites reveal that C60 exhibited a more negative adsorption energy, indicating stronger bonding, which make it more effective at activating CO2 (Figure 2e). Further analysis showed that, unlike the Cu site, which remained almost unresponsive to electric fields (Figure 2f), the C60 site exhibited a pronounced energy decrease under an applied field, stabilizing COOH* intermediate and significantly enhancing CO2 activation (Figure 2g). Differential charge density calculations (Figure 2h) revealed a notable electron flow due to C60’s unique spherical structure, effectively activating the asymmetric COOH* intermediate. This characteristic can be a key factor in C60’s role as an active site for CO2RR. These insights led us to further design a series of model catalysts featuring C60 as an active site, systematically exploring changes in intermediate adsorption energies under various electric field to uncover the origin of C60’s catalytic activity. This is consistent with the recent study uncovering that C60 can combine ammonia intermediates to form C60-NH* and C60-NH2*,[9] resulting in promoted nitrogen reduction activity.
The Effect of C60 Curved Surfaces and Electric Field
Following the catalytic performance analysis of C60-Cu/SiO2 catalyst in CO2RR, as summarized in Figures 2 and S2, we delved into the underlying mechanisms that drive the enhanced activity observed in C60-based catalysts. The distinct curvature of C60, setting it apart from traditional flat catalysts such as graphene, is hypothesized to play a pivotal role in modulating the adsorption energies of CO2RR intermediates under the external electric fields.[25] While C60 itself possesses a wide bandgap, its integration with graphene—especially defected graphene such as Ox-Graphene-C60, significantly reduces the bandgap, potentially even conferring metallic properties (Figure S9). This unique combination of materials renders C60-based systems promising as electrocatalytic materials, offering an approach for enhancing catalytic performance. These electronic modifications likely contribute to the improved interactions with intermediates under external electric fields.
Figure 3a–d demonstrates how an external electric field impacts the adsorption energies of CO2RR intermediates across various C60-based catalysts,[26] including C60, Ox-Graphene-C60, Cu(111)-H-C60, and Graphene-C60. The electric field interacts with intermediates with a substantial dipole moment (μ) and polarizability (α). When comparing the active site on C60 to those on H* saturated Cu(111) surfaces,[27] the unique curvature and electronic properties of C60-based catalysts under electric fields lead to more negative adsorption energies for COOH* intermediates (Figure S3). Conversely, Cu(111) surfaces and planar graphene show only slight changes in adsorption energies under electric fields (Figures 2f, S4, and S5), with negligible values for μ and α, whereas the C60-based catalysts display substantial improvements.
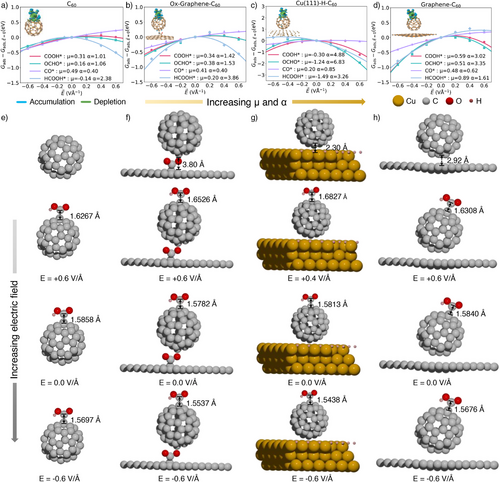
Negative electric fields increase the μ and α values of adsorbed C60-based catalysts, indicating the adsorption energies significantly benefit from the electric field.[21] This substantial polarizability is attributed to the curvature of C60,[28] which boosts its ability to polarize and stabilize adsorbed intermediates (Figure S6). The curved C70 surface has a similar effect (Figure S7). The corresponding enhancement in adsorption energies indicates a strengthened interaction between C60 and COOH* intermediate under the electric field. Specifically, in the case of C60, the bond length between the carbon atom of C60 and the carbon atom of COOH* intermediate shortens from 1.63 Å under a positive electric field to 1.59 Å with no electric field, and further to 1.57 Å under a negative electric field (Figure 3e–h). Under identical electric fields, the more negative ΔGCOOH* on Cu(111)-H-C60 than pristine C60 is primarily attributed to enhanced charge transfer at the interface (Figure S8). The isosurfaces provided further detail, illustrating regions of electron accumulation and depletion, emphasizing how the electric field facilitates stronger binding of COOH* by redistributing the charge on catalyst surfaces.[17] This charge redistribution lowers the activation energy required for CO2RR, thereby enhancing the overall catalytic efficiency of C60-based catalysts.
The Effects of Potentials of Zero Charge (PZCs) and pH
While the electric field has been proven to impose a notable impact on both COOH* and OCHO* intermediates, the differences in selectivity and activity among C60-based catalysts remains to be fully explained. Additional factors such as potentials of zero charge (PZCs), solvation effects, pH (at the RHE scale), as well as reaction thermodynamics, must be considered for comprehensive evaluation of catalytic performances.[29]
Figure 4a illustrates that pure C60 exhibits a highly positive PZC (+0.77 V), whereas C60 catalysts modified with metal or non-metal components display negative PZC values (−0.10 V for Ox-Graphene-C60, −0.30 V for Cu(111)-H-C60, and −0.34 V for Graphene-C60). Explicit model further shows deviations up to 1.0 V/SHE across all C60-based catalysts (Figure 4c), with values of +0.99 V for C60, +0.86 V for Ox-Graphene -C60, +0.48 V for Cu(111)-H-C60, and +0.05 V for Graphene-C60. These deviations indicate inherent limitations of implicit model in accurately capturing the PZC when water molecules interact with active sites or surfaces, as shown in Figure S10 and Table S2. The orientation and arrangement of water molecules create a more realistic electrostatic environment, influencing surface charge distribution and thus PZC values.[30] To establish a more unified model, we employed an average of PZCs derived from the ab initio molecular dynamics (AIMD) simulations to inform our microkinetic activity analysis.
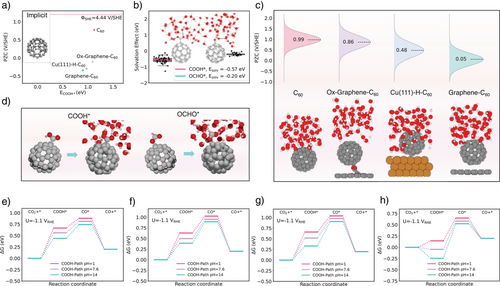
Solvent effects play a critical role, especially when COOH* and OCHO* intermediates are involved.[31] Under the implicit solvent model, the adsorption energy and configuration changes will not be greatly affected by the addition of a water layer, while the explicit model we use can take into account the competitive adsorption of water in AIMD, which is important for the adsorption energy of COOH* and OCHO*. An explicit water layer (thickness: 10 Å, density: ρ = 1 g cm−3) was constructed around the adsorbed COOH* or OCHO* intermediates. Figure 4b shows data through a pool of over 2000 AIMD simulations steps, which sampled more than 50 fully optimized distinct adsorbate-substrate configurations to estimate solvent effect for each intermediate. COOH* forms hydrogen bonds with two neighboring water molecules, whereas OCHO* is bonded only one. These hydrogen bonding interactions significantly lower the energy of the COOH* intermediate and lead to a shortened bond between COOH* and C60, as illustrated in Figure 4d. The mean value of -0.57 and −0.20 eV for COOH* and OCHO*, respectively, were incorporated into our model to adjust the adsorption energies accurately.
Thermodynamic analysis revealed that the first hydrogenation step leading to COOH* is energetically more favorable than the corresponding step leading to OCHO* across all C60-based catalysts (Figure S11), with a strong pH correlation (Figure 4e–h). Higher pH environments generally lower the Gibbs free energy (ΔG) for COOH* formation, making CO production more efficient (Figure S12). Furthermore, when a specific potential is applied, the pH's influence on thermodynamic trends remains consistent. At pH = 14, the ΔG for COOH* formation decreases to 0.33 eV, which is lower than the 0.52 eV required at pH = 7.6 with U = −1.1 VRHE, confirming a strong pH-dependent performance of Cu(111)-H-C60 for CO2RR.
pH-Dependent Microkinetic Volcano
Based on the simulations of COOH* adsorption energies, we constructed conventional volcano activity models to compare the catalytic activities of the COOH-pathway (represented by GCOOH* and GCO*) and the OCHO-pathway (GOCHO* and GHCOOH*) (Figure S13). The result indicates that CO production is thermodynamically more favorable than HCOOH under comparable conditions. Notably, C60-based catalysts appear closer to the volcano summit of the COOH-pathway, suggesting that fine-tuning of potential and pH can push C60-based catalysts towards an optimal CO formation performance, especially under neutral and mild conditions. However, traditional volcano model is unable to capture the pH effects on catalytic behavior, as it treats the pH-correction term in the reaction energetics as −0.059 * pH eV, which shifts in the same way as the potential shifts in the SHE scale and hard to explain the pH-dependency in experiments under an RHE scale.[21] To overcome this limitation, we developed a model that integrates both pH and electric field effects, enabling us to directly observe how pH modulates the stability and formation energies of key intermediates, thus offering deeper insights into the intrinsic catalytic properties of C60-based materials. Details of the microkinetic modeling methods can be found in the Supplementary Information.
On basis of the insights gained from Figures 3 and 4, which examined the effects of PZCs, solvation, and electric field, Figure 5 introduces a comprehensive microkinetic model that consolidates these factors to affect the reaction mechanisms of CO2RR on C60-based catalysts. Figure 5a displays the pH-dependent activity volcanos, demonstrating enhanced CO2RR turnover frequency (TOF) in neutral and alkaline media, with a significant drop on activity observed in acidic environments due to the left-shift of the volcano peaks. This model is based on the linear relationship between CO* and COOH* adsorption energies (Figure S14), and aligns well with our experiment observations (Figure 2a). While the double-layer capacitance in this model is treated as constant, we acknowledge its inherent potential dependence in physical systems. Crucially, within the strong electric field applied in our study, the relative variation of capacitance becomes negligible, justifying this simplification for the present analysis. Figure 5b further extends this analysis to the HER (based on the modeling method developed in Ref. [32]), demonstrating the competitive interplay between HER with CO2RR. In acidic conditions, HER can prevail due to higher proton availability, but under alkaline conditions, C60-based catalysts suppress HER more effectively, favoring higher CO2RR selectivity.
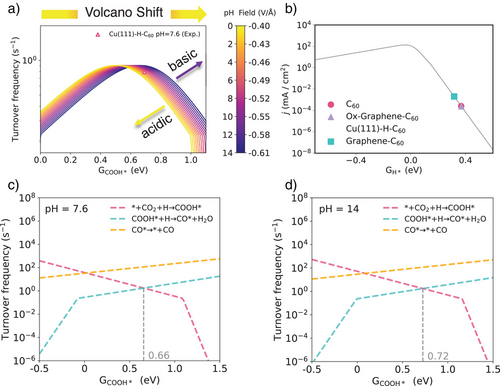
A critical aspect of our analysis lies in identifying the rate-determining step (RDS) under varying pH conditions. As depicted in Figures 5c,d and S15, when the adsorption free energy of COOH* exceeds 0.66 eV, the initial activation of CO2 to form COOH* becomes the RDS in neutral media (pH = 7.6), thereby imposing a significant limitation on CO2RR activity. However, in alkaline media (pH = 14), the RDS shifts toward CO* formation in this potential region, driven by the effective stabilization of COOH* intermediates through reduced adsorption energies. While this transition establishes CO* formation as the kinetic bottleneck, the lowering of the activation energy for COOH* adsorption appropriately accelerates overall reaction, ultimately enhancing the overall CO production. This shift underscores a strong pH dependence, where higher pH values favor the stabilization of COOH* intermediates and enhance overall CO production. These findings align with the pH-sensitive trends in the thermodynamic analysis shown in Figure 4, where the lowering of Gibbs free energy (ΔG) facilitates the conversion of COOH* to CO under alkaline conditions.
The comprehensive pH-field coupled microkinetic analysis enables to study the effect of both pH and precise control over GCOOH* as pivotal factors influencing the catalytic performance of C60-based catalysts. The study reveals that alkaline environments significantly enhance the formation and stabilization of COOH* intermediates, shifting the RDS to a step that promotes more efficient CO production. Therefore, the strategic adjustment of pH and applied potential can effectively lower energy barriers, thereby enhancing both the selectivity and efficiency of CO2RR. These findings make C60-based catalysts highly promising candidates for sustainable CO2 conversion applications.
Conclusion
In summary, C60 is revealed to serve as a molecular active catalytic site rather than just an electronic modulator. Traditional models overlook this complexity and fail to accurately predict the pH-dependent performance of CO2RR under the experimental RHE scale. Alternatively, by integrating pH and electric field effects into a comprehensive microkinetic model, it can be concluded that C60’s unique structure stabilizes COOH* intermediates, particularly under alkaline conditions, enhancing CO2 conversion. The derived pH-field coupled microkinetic volcano model provides a pH-dependent framework under RHE scale, revealing a shift in activity from acidic to alkaline conditions and a transition in the RDS. This work highlights the importance of quantitative pH-dependent modeling and provides new guidance for designing C60-based catalysts. In addition, our study demonstrates that the large dipole moment change upon adsorption at curved surfaces should not be dismissed when analyzing the pH-dependent bonding strength and electrocatalytic activity. The present work clarifies C60’s role in electrocatalytic CO2RR and exemplifies a new tool to study the catalysis under real electrochemical conditions, especially for those catalysts with curved structures.
Author Contributions
All authors have given approval to the final version of the manuscript and declare no competing financial interest.
Acknowledgements
This work was financially supported by the JSPS KAKENHI (Nos. JP25K01737 and JP24K23068), the National Natural Science Foundation of China (NSFC) (92477110, 22372135, and 92361303), the Natural Science Foundation of Xiamen (3502Z202373001), the Fundamental Research Funds for the Central Universities (20720230007), the Science and Technology Cooperation and Communication Project of Shanxi Province (No. 202304041101016), and the Foundation of State Key Laboratory of Coal Conversion (No. J24-25-610). Si-Wei Ying acknowledges the financial support from the China Scholarship Council (No. 202306310120). The authors acknowledge the Center for Computational Materials Science, Institute for Materials Research, Tohoku University for the use of MASAMUNE-IMR (Nos. 202412-SCKXX-0211 and 202412-SCKXX-0209), and the Institute for Solid State Physics (ISSP) at the University of Tokyo for the computational resources.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
All data are available upon reasonable request from the authors. Besides, the key experimental data and computational structures are available in the Digital Catalysis Platform (DigCat: www.digcat.org).