Nylon-6 Precursor Electrosynthesis From Low-Concentration NO via Carbon-Enhanced NO Adsorption and Improved Mass Transfer
Graphical Abstract
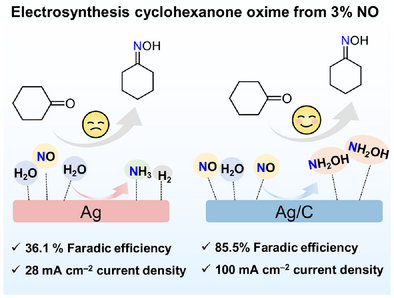
Porous carbon-supported ultrasmall Ag nanoparticles are designed to achieve 85.5% FE at a partial current density of 100 mA cm−2 for cyclohexanone oxime with a 3% NO concentration, outperforming pure Ag nanoparticles. Porous carbon can optimize the NO transport path and H2O molecule distribution on the catalyst surface, accelerating the reaction dynamics for cyclohexanone oxime electrosynthesis and suppressing the formation of ammonia and hydrogen byproducts.
Abstract
Cyclohexanone oxime electrosynthesis from low-concentration NO is important but suffers from low Faraday efficiency and current density because of severe competitive hydrogen and ammonia evolution reactions. Here, porous carbon-supported ultrasmall Ag nanoparticles are designed to achieve 85.5% FE at a partial current density of 100 mA cm−2 for cyclohexanone oxime with a 3% NO concentration, outperforming pure Ag nanoparticles. Mechanistic studies reveal that porous carbon can optimize the NO transport path and H2O molecule distribution on the catalyst surface, accelerating the reaction dynamics for cyclohexanone oxime electrosynthesis and suppressing ammonia and hydrogen formation. This work paves the way for accessing low-concentration gaseous reactants for various catalytic reactions.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.