Stepwise Post-Modification of Pyridine-Imine COFs for Enhanced Hydrolytic Stability and Proton Conductivity
Hongfei Wang
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorWen-Na Jiao
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorWei-De Zhu
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorSi Huang
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorXiao-Chun Lin
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorTing Chen
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorYanan Fan
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorFangzheng Chen
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Search for more papers by this authorCorresponding Author
Prof. Hai-Sen Xu
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
E-mail: [email protected], [email protected]
Search for more papers by this authorProf. Mei Pan
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Cheng-Yong Su
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
E-mail: [email protected], [email protected]
Search for more papers by this authorHongfei Wang
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorWen-Na Jiao
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorWei-De Zhu
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorSi Huang
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorXiao-Chun Lin
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorTing Chen
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorYanan Fan
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorFangzheng Chen
Department of Chemistry, National University of Singapore, Singapore, 117543 Singapore
Search for more papers by this authorCorresponding Author
Prof. Hai-Sen Xu
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
E-mail: [email protected], [email protected]
Search for more papers by this authorProf. Mei Pan
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Cheng-Yong Su
GBRCE for Functional Molecular Engineering, LIFM, IGCME, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
E-mail: [email protected], [email protected]
Search for more papers by this authorGraphical Abstract
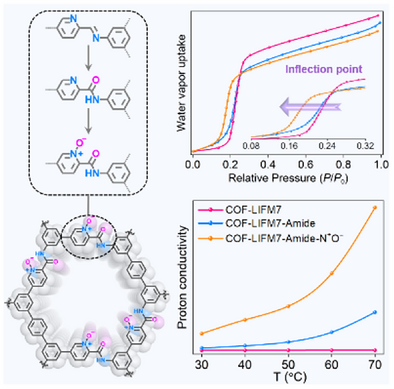
The self-condensation reaction of bifunctional monomers containing both amino and acetal groups yielded COF-LIFM7. Via subsequent amidation and N-oxidation modifications, the pore polarity and hydrophilicity of the post-modified COFs were progressively enhanced, consequently leading to a significant increase in proton conductivity.
Abstract
Emerging as a type of promising material for proton conduction, covalent organic frameworks (COFs) assembled from dynamic imine bonds face a challenge of surmounting hydrolytic instability to achieve long-term performance in humid environments. In this work, we report a post-synthetic strategy to simultaneously enhance the hydrolytic stability and hydrophilicity of a pyridine-imine-based COF, COF-LIFM7, without compromising its crystallinity and porosity. A bifunctional monomer containing amino and acetal groups was employed to construct the primary framework, which was subsequently modified via amide formation and pyridine N-oxidation to yield COF-LIFM7-Amide and COF-LIFM7-Amide-N+O−. These stepwise modifications increased the polarity and hydrogen-binding sites within COF pores to improve water affinity, leading to a three-order-of-magnitude enhancement in the proton conductivity for COF-LIFM7-Amide-N+O−, reaching 1.9 × 10−3 S cm−1 at 95% relative humidity and 70 °C. This study highlights a generalizable post-synthetic approach for tuning the pore chemistry of COFs to achieve high performance in proton-conducting applications under humid conditions.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article. Deposition Number 22450595 (for M2) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via the joint Cambridge Crystallographic Data Centre (CCDC) and Fachinformationszentrum Karlsruhe Access Structures service.
Supporting Information
| Filename | Description |
|---|---|
| anie202511559-sup-0001-SuppMat.pdf8.5 MB | Supporting Information |
| anie202511559-sup-0002-SuppMat.cif183.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. P. Cote, A. I. Benin, N. W. Ockwig, M. OKeeffe, A. J. Matzger, O. M. Yaghi, Science 2005, 310, 1166–1170.
- 2F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klock, M. OKeeffe, O. M. Yaghi, J. Am. Chem. Soc. 2009, 131, 4570–4571.
- 3J. Han, J. Feng, J. Kang, J.-M. Chen, X.-Y. Du, S.-Y. Ding, L. Liang, W. Wang, Science 2024, 383, 1014–1019.
- 4T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L.-H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun, O. M. Yaghi, Science 2018, 361, 48–52.
- 5N. Huang, P. Wang, D. Jiang, Nat. Rev. Mater. 2016, 1, 16068.
- 6B. Yu, R.-B. Lin, G. Xu, Z.-H. Fu, H. Wu, W. Zhou, S. Lu, Q.-W. Li, Y. Jin, J.-H. Li, Z. Zhang, H. Wang, Z. Yan, X. Liu, K. Wang, B. Chen, J. Jiang, Nat. Chem. 2024, 16, 114–121.
- 7P. J. Waller, F. Gándara, O. M. Yaghi, Acc. Chem. Res. 2015, 48, 3053–3063.
- 8S. Kandambeth, K. Dey, R. Banerjee, J. Am. Chem. Soc. 2019, 141, 1807–1822.
- 9K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang, D. Jiang, Chem. Rev. 2020, 120, 8814–8933.
- 10H. Li, Z. Zhou, T. Ma, K. Wang, H. Zhang, A. H. Alawadhi, O. M. Yaghi, J. Am. Chem. Soc. 2024, 146, 35486–35492.
- 11H. Lyu, H. Li, N. Hanikel, K. Wang, O. M. Yaghi, J. Am. Chem. Soc. 2022, 144, 12989–12995.
- 12Z. Zhou, T. Ma, H. Zhang, S. Chheda, H. Li, K. Wang, S. Ehrling, R. Giovine, C. Li, A. H. Alawadhi, M. M. Abduljawad, M. O. Alawad, L. Gagliardi, J. Sauer, O. M. Yaghi, Nature 2024, 635, 96–101.
- 13Y. Yin, Y. Zhang, X. Zhou, B. Gui, W. Wang, W. Jiang, Y.-B. Zhang, J. Sun, C. Wang, Science 2024, 386, 693–696.
- 14Z. Wang, S. Zhang, Y. Chen, Z. Zhang, S. Ma, Chem. Soc. Rev. 2020, 49, 708–735.
- 15S. Yuan, X, L.i, J. Zhu, G. Zhang, P. V. Puyvelde, B. V. d. Bruggen, Chem. Soc. Rev. 2019, 48, 2665–2681.
- 16X. Zhao, P. Pachfule, A. Thomas, Chem. Soc. Rev. 2021, 50, 6871–6913.
- 17X. Yan, B. Wang, J. Ren, X. Long, D. Yang, Angew. Chem. Int. Ed. 2022, 61, e202209583; Angew. Chem. 2022, 134, e202209583.
- 18H.-H. Sun, Z.-B. Zhou, Y. Fu, Q.-Y. Qi, Z.-X. Wang, S. Xu, X. Zhao, Angew. Chem. Int. Ed. 2024, 63, e202409250; Angew. Chem. 2024, 136, e202409250.
- 19W. Lin, F. Lin, J. Lin, Z. Xiao, D. Yuan, Y. Wang, J. Am. Chem. Soc. 2024, 146, 16229–16236.
- 20Q.-J. Wu, D.-H. Si, S. Ye, Y.-L. Dong, R. Cao, Y.-B. Huang, J. Am. Chem. Soc. 2023, 145, 19856–19865.
- 21Y. Yang, P. Zhang, L. Hao, P. Cheng, Y. Chen, Z. Zhang, Angew. Chem. Int. Ed. 2021, 60, 21838–21845; Angew. Chem. 2021, 133, 22009–22016.
- 22Y. Yang, X. He, P. Zhang, Y. H. Andaloussi, H. Zhang, Z. Jiang, Y. Chen, S. Ma, P. Cheng, Z. Zhang, Angew. Chem. Int. Ed. 2020, 59, 3678–3684; Angew. Chem. 2020, 132, 3707–3713.
- 23T. W. Kang, J. H. Lee, J. Lee, J. H. Park, J. H. Shin, J. M. Ju, H. Lee, S. U. Lee, J. H. Kim, Adv. Mater. 2023, 35, 2301308.
- 24Y. Kim, C. Li, J. Huang, Y. Yuan, Y. Tian, W. Zhang, Adv. Mater. 2024, 36, 2407761.
- 25X. Li, H. Wang, Z. Chen, H.-S. Xu, W. Yu, C. Liu, X. Wang, K. Zhang, K. Xie, K. P. Loh, Adv. Mater. 2019, 31, 1905879.
- 26C. Li, D.-D. Wang, G. S. H. Poon Ho, Z. Zhang, J. Huang, K.-T. Bang, C. Y. Lau, S.-Y. Leu, Y. Wang, Y. Kim, J. Am. Chem. Soc. 2023, 145, 24603–24614.
- 27C. Niu, S. Zhao, Y. Xu, J. Am. Chem. Soc. 2024, 146, 3114–3124.
- 28S. Tao, D. Jiang, J. Am. Chem. Soc. 2024, 146, 18151–18160.
- 29Z. Lu, C. Yang, L. He, J. Hong, C. Huang, T. Wu, X. Wang, Z. Wu, X. Liu, Z. Miao, B. Zeng, Y. Xu, C. Yuan, L. Dai, J. Am. Chem. Soc. 2022, 144, 9624–9633.
- 30S. Chandra, T. Kundu, S. Kandambeth, R. BabaRao, Y. Marathe, S. M. Kunjir, R. Banerjee, J. Am. Chem. Soc. 2014, 136, 6570–6573.
- 31L. Hao, S. Jia, X. Qiao, E. Lin, Y. Yang, Y. Chen, P. Cheng, Z. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202217240; Angew. Chem. 2023, 135, e202217240.
- 32J. Li, J. Wang, Z. Wu, S. Tao, D. Jiang, Angew. Chem. Int. Ed. 2021, 60, 12918–12923; Angew. Chem. 2021, 133, 13028–13033.
- 33X. Pang, B. Shi, Y. Liu, H. Wu, J. Shen, J. Guan, X. Wang, C. Fan, L. Cao, T. Zhu, Y. Kong, Z. Jiang, Angew. Chem. Int. Ed. 2025, 64, e202423458; Angew. Chem. 2025, 137, e202423458.
- 34K. T. Tan, S. Tao, N. Huang, D. Jiang, Nat. Commun. 2021, 12, 6747.
- 35H.-S. Xu, Y. Luo, P. Z. See, X. Li, Z. Chen, Y. Zhou, X. Zhao, K. Leng, I. H. Park, R. Li, C. Liu, F. Chen, S. Xi, J. Sun, K. P. Loh, Angew. Chem. Int. Ed. 2020, 59, 11527–11532; Angew. Chem. 2020, 132, 11624–11629.
- 36H.-S. Xu, Y. Luo, X. Li, P. Z. See, Z. Chen, T. Ma, L. Liang, K. Leng, I. Abdelwahab, L. Wang, R. Li, X. Shi, Y. Zhou, X. F. Lu, X. Zhao, C. Liu, J. Sun, K. P. Loh, Nat. Commun. 2020, 11, 1434.
- 37H.-S. Xu, Y. Luo, R. Li, W.-N. Jiao, S. Huang, W.-D. Zhu, H. Wang, T. Chen, M. Nero, F. Chen, Q. Gao, X. Li, M. Pan, T. Willhammar, K. P. Loh, C.-Y. Su, Nat. Synth. 2024, 3, 1498–1506.
- 38L. Grunenberg, G. Savasci, M. W. Terban, V. Duppel, I. Moudrakovski, M. Etter, R. E. Dinnebier, C. Ochsenfeld, B. V. Lotsch, J. Am. Chem. Soc. 2021, 143, 3430–3438.
- 39P. J. Waller, S. J. Lyle, T. M. Osborn Popp, C. S. Diercks, J. A. Reimer, O. M. Yaghi, J. Am. Chem. Soc. 2016, 138, 15519–15522.
- 40X. Guan, Q. Fang, Y. Yan, S. Qiu, Acc. Chem. Res. 2022, 55, 1912–1927.
- 41B. Gui, G. Lin, H. Ding, C. Gao, A. Mal, C. Wang, Acc. Chem. Res. 2020, 53, 2225–2234.
- 42T. Zhang, G. Zhang, L. Chen, Acc. Chem. Res. 2022, 55, 795–808.
- 43R.-R. Liang, S.-Y. Jiang, A. R-H, X. Zhao, Chem. Soc. Rev. 2020, 49, 3920–3951.
- 44M. Xu, W.-Q. Tang, S.-S. Meng, Z.-Y. Gu, Chem. Soc. Rev. 2025, 54, 1613–1633.
- 45Y. Li, L. Guo, Y. Lv, Z. Zhao, Y. Ma, W. Chen, G. Xing, D. Jiang, L. Chen, Angew. Chem. Int. Ed. 2021, 60, 5363–5369; Angew. Chem. 2021, 133, 5423–5429.
- 46Y. Li, Q. Chen, T. Xu, Z. Xie, J. Liu, X. Yu, S. Ma, T. Qin, L. Chen, J. Am. Chem. Soc. 2019, 141, 13822–13828.
- 47 Deposition numbers 2450595 for N-oxide model compound M2 contain the supplementary crystallographic data for this paper. The data is provided free of charge by the joint Cambridge Crystallographic Data Centre Access Structures service (http://www.ccdc.cam.ac.uk/structures).
- 48L. Grunenberg, G. Savasci, S. T. Emmerling, F. Heck, S. Bette, A. Cima Bergesch, C. Ochsenfeld, B. V. Lotsch, J. Am. Chem. Soc. 2023, 145, 13241–13248.
- 49H. Pang, G. Liu, D. Huang, Y. Zhu, X. Zhao, W. Wang, Y. Xiang, Angew. Chem. Int. Ed. 2023, 62, e202313520; Angew. Chem. 2023, 135, e202313520.
- 50F. Kang, X. Wang, C. Chen, C.-S. Lee, Y. Han, Q. Zhang, J. Am. Chem. Soc. 2023, 145, 15465–15472.
- 51A. Volkov, J. Mi, K. Lalit, P. Chatterjee, D. Jing, S. L. Carnahan, Y. Chen, S. Sun, A. J. Rossini, W. Huang, L. M. Stanley, J. Am. Chem. Soc. 2023, 145, 6230–6239.
- 52C. Sun, D. Sheng, B. Wang, X. Feng, Angew. Chem. Int. Ed. 2023, 62, e202303378; Angew. Chem. 2023, 135, e202303378.
- 53H. L. Nguyen, Adv. Mater. 2023, 35, 2300018.
- 54Z. Shi, Y. Guo, X. Zou, J. Zhang, Z. Chen, M. Shan, Z. Zhang, S. Guo, F. Yan, Angew. Chem. Int. Ed. 2025, 64, e202420619; Angew. Chem. 2025, 137, e202420619.
- 55Q. Cheng, S. Liu, Y. He, M. Wang, H. Ji, Y. Huan, T. Qian, C. Yan, J. Lu, Nat. Commun. 2025, 16, 3717.
- 56X. Xu, Y. Feng, H. Chen, N. Huang, J. Am. Chem. Soc. 2025, 147, 16653–16660.
- 57M. Chaplin, “ Water Absorption Spectrum”, can be found under https://water.lsbu.ac.uk/water/water_vibrational_spectrum.html 2000, (accessed: June 2025).