Ordered Copper Triangular Atomic Sites for Industrial-Grade Electromethanation of CO2 via Self-Regulated Adsorption of Reactants
Dr. Fanglei Yao
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Yuntong Sun
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 62 Nanyang Drive, Singapore, 637459 Singapore
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorLong Nie
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Search for more papers by this authorDr. Cheng Zhang
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
Search for more papers by this authorDr. Hongwei Shou
College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Search for more papers by this authorZhiming Li
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Xiaoping Gao
School of New Energy, Ningbo University of Technology, Ningbo, 315336 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jin Wang
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Fanglei Yao
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Yuntong Sun
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 62 Nanyang Drive, Singapore, 637459 Singapore
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorLong Nie
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Search for more papers by this authorDr. Cheng Zhang
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
Search for more papers by this authorDr. Hongwei Shou
College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060 P.R. China
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Search for more papers by this authorZhiming Li
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Xiaoping Gao
School of New Energy, Ningbo University of Technology, Ningbo, 315336 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jin Wang
College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518071 P.R. China
Key Laboratory of Functional Molecular Solids Ministry of Education, College of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241002 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
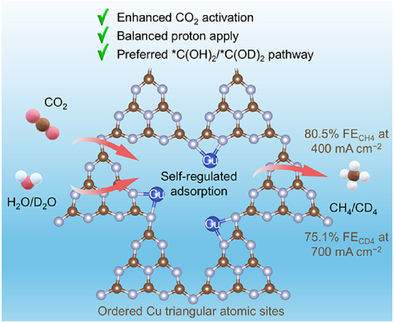
Ordered copper triangular atomic site motifs loaded on poly(heptazine imide) (Cu TAS/PHI) were synthesized via a precise ion exchange strategy. Cu TAS/PHI enables enhanced CO2 coverage and activation with balanced proton supply through self-regulated adsorption of reactants, as well as unlocks an energetically favorable *C(OH)2 pathway, achieving an FECH4 of 80.5% at 400 mA cm−2 and an FECD4 of 75.1% at 700 mA cm−2.
Abstract
Copper single-atom catalysts have shown considerable potential for electrocatalytic CO2 reduction reaction (CO2RR) to methane but face constraints of low selectivity at industrial-grade current densities (>400 mA cm−2) and limited economic viability. Herein, we report an ion exchange strategy to precisely construct ordered Cu triangular atomic sites loaded on poly(heptazine imide) (Cu TAS/PHI), achieving a methane Faradaic efficiency (FE) of 80.5% at 400 mA cm−2 and >60% across 100–800 mA cm−2. Remarkably, it enables CO2 deuteration to high-value methane-d4 with an FE of 75.1% at 700 mA cm−2 and an estimated annual return on investment of 425.35%. In situ spectroscopy and theoretical calculations demonstrate that Cu triangular atomic sites enable strengthened adsorption and activation of CO2, as well as balanced proton supply via self-regulated adsorption of reactants, thus favoring CO2 deep hydrogenation over hydrogen evolution. Moreover, Cu TAS/PHI unlocks an energetically favorable *C(OH)2 pathway, circumventing the conventional *CO pathway that typically yields diverse CO2RR products. This work demonstrates a strategy to construct ordered multiatomic sites for highly selective CO2RR at industrial-grade current density and highlights the extraordinary financial potential of electrocatalytic CO2RR to produce high-value deuterated chemicals.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202511459-sup-0001-SuppMat.pdf5.3 MB | Supporting information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Fang, W. Guo, R. Lu, Y. Yan, X. Liu, D. Wu, F. M. Li, Y. Zhou, C. He, C. Xia, H. Niu, S. Wang, Y. Liu, Y. Mao, C. Zhang, B. You, Y. Pang, L. Duan, X. Yang, F. Song, T. Zhai, G. Wang, X. Guo, B. Tan, T. Yao, Z. Wang, B. Y. Xia, Nature 2024, 626, 86–91.
- 2X. She, L. Zhai, Y. Wang, P. Xiong, M. M.-J. Li, T.-S. Wu, M. C. Wong, X. Guo, Z. Xu, H. Li, H. Xu, Y. Zhu, S. C. E. Tsang, S. P. Lau, Nat. Energy 2024, 9, 81–91.
- 3F. P. García de Arquer, C.-T. Dinh, A. Ozden, J. Wicks, C. McCallum, A. R. Kirmani, D.-H. Nam, C. Gabardo, A. Seifitokaldani, X. Wang, Y. C. Li, F. Li, J. Edwards, L. J. Richter, S. J. Thorpe, D. Sinton, E. H. Sargent, Science 2020, 367, 661–666.
- 4C. Zhan, F. Dattila, C. Rettenmaier, A. Herzog, M. Herran, T. Wagner, F. Scholten, A. Bergmann, N. López, B. R. Cuenya, Nat. Energy 2024, 9, 1485–1496.
- 5L. Fan, F. Li, T. Liu, J. E. Huang, R. K. Miao, Y. Yan, S. Feng, C.-W. Tai, S.-F. Hung, H.-J. Tsai, M.-C. Chen, Y. Bai, D. Kim, S. Park, P. Papangelakis, C. Wu, A. Shayesteh Zeraati, R. Dorakhan, L. Sun, D. Sinton, E. Sargent, Nat. Synth. 2025, 4, 262–270.
- 6M. McKee, M. Kutter, Y. Wu, H. Williams, M.-A. Vaudreuil, M. Carta, A. K. Yadav, H. Singh, J.-F. Masson, D. Lentz, M. F. Kühnel, N. Kornienko, Nat. Chem. 2025, 17, 92–100.
- 7Z. Xu, R. Lu, Z.-Y. Lin, W. Wu, H.-J. Tsai, Q. Lu, Y. C. Li, S.-F. Hung, C. Song, J. C. Yu, Z. Wang, Y. Wang, Nat. Energy 2024, 9, 1397–1406.
- 8R. Cai, H. Zhu, F. Yang, M. Ju, X. Huang, J. Wang, M. D. Gu, J. Gao, S. Yang, Angew. Chem. Int. Ed. 2025, 64, e202424098.
- 9J. Zhu, Y. Zhang, Z. Chen, Z. Zhang, X. Tian, M. Huang, X. Bai, X. Wang, Y. Zhu, H. Jiang, Nat. Commun. 2024, 15, 1565.
- 10J. Zhang, C. Zhang, M. Wang, Y. Mao, B. Wu, Q. Yang, B. Wang, Z. Mi, M. Zhang, N. Ling, W. R. Leow, Z. Wang, Y. Lum, Nat. Chem. 2025, 17, 334–343.
- 11F. Bu, Y. Deng, J. Xu, D. Yang, Y. Li, W. Li, A. Lei, Nature 2024, 634, 592–599.
- 12R. M. C. Di Martino, B. D. Maxwell, T. Pirali, Nat. Rev. Drug Discov. 2023, 22, 562–584.
- 13B. Ó. Joensen, Q. Xu, K. Enemark-Rasmussen, V. Frankland, A. Prakash Periasamy, J. R. Varcoe, I. Chorkendorff, B. Seger, ACS Catal. 2025, 15, 1038–1045.
- 14F. Yu, G. Zhang, M. Shu, H. Wang, Angew. Chem. Int. Ed. 2025, 64, e202416467.
- 15Z. Zhu, Y. Zhu, Z. Ren, D. Liu, F. Yue, D. Sheng, P. Shao, X. Huang, X. Feng, A.-X. Yin, J. Xie, B. Wang, J. Am. Chem. Soc. 2024, 146, 1572–1579.
- 16S. Hu, Y. Chen, Z. Zhang, H. Liu, X. Kang, J. Liu, S. Li, Y. Luo, B. Liu, Angew. Chem. Int. Ed. 2025, 64, e202423915.
- 17H. Wu, B. Tian, W. Xu, K. K. Abdalla, Y. Kuang, J. Li, X. Sun, J. Am. Chem. Soc. 2024, 146, 22266–22275.
- 18Y. Zhang, F. Chen, X. Yang, Y. Guo, X. Zhang, H. Dong, W. Wang, F. Lu, Z. Lu, H. Liu, H. Liu, Y. Xiao, Y. Cheng, Nat. Commun. 2025, 16, 1956.
- 19F. Huang, X. Chen, H. Sun, Q. Zeng, J. Ma, D. Wei, J. Zhu, Z. Chen, T. Liang, X. Yin, X. Liu, J. Xu, H. He, Angew. Chem. Int. Ed. 2025, 64, e202415642.
- 20P. Zhao, H. Jiang, H. Shen, S. Yang, R. Gao, Y. Guo, Q. Zhang, H. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202314121.
- 21Y. Cai, J. Fu, Y. Zhou, Y.-C. Chang, Q. Min, J.-J. Zhu, Y. Lin, W. Zhu, Nat. Commun. 2021, 12, 586.
- 22S. Roy, Z. Li, Z. Chen, A. C. Mata, P. Kumar, S. C. Sarma, I. F. Teixeira, I. F. Silva, G. Gao, N. V. Tarakina, M. G. Kibria, C. V. Singh, J. Wu, P. M. Ajayan, Adv. Mater. 2024, 36, 2300713.
- 23J. Zhang, Y. Wang, Y. Li, J. Am. Chem. Soc. 2024, 146, 14954–14958.
- 24A. M. Roth-Zawadzki, A. J. Nielsen, R. E. Tankard, J. Kibsgaard, ACS Catal. 14, 1121–1145.
- 25F. Pan, L. Fang, B. Li, X. Yang, T. O'Carroll, H. Li, T. Li, G. Wang, K.-J. Chen, G. Wu, J. Am. Chem. Soc. 2024, 146, 1423–1434.
- 26A. R. Woldu, A. G. Yohannes, Z. Huang, P. Kennepohl, D. Astruc, L. Hu, X.-C. Huang, Adv. Mater. 2024, 36, 2414169.
- 27P.-P. Yang, M.-R. Gao, Chem. Soc. Rev. 2023, 52, 4343–4380.
- 28W. Pei, S. Zhou, J. Zhao, X. Xu, Y. Du, S. X. Dou, Nano Energy 2020, 76, 105049.
- 29H. Wang, L. Song, X. Lv, H. Wang, F. Zhang, S. Hao, R. Wei, L. Zhang, Q. Han, G. Zheng, Angew. Chem. Int. Ed. 2025, 64, e202500928.
- 30W. Xia, Y. Xie, S. Jia, S. Han, R. Qi, T. Chen, X. Xing, T. Yao, D. Zhou, X. Dong, J. Zhai, J. Li, J. He, D. Jiang, Y. Yamauchi, M. He, H. Wu, B. Han, J. Am. Chem. Soc. 2023, 145, 17253–17264.
- 31Z. Yang, M. Yuan, Z. Cheng, B. Liu, Z. Ma, J. Ma, J. Zhang, X. Ma, P. Ma, J. Lin, Angew. Chem. Int. Ed. 2024, 63, e202401758.
- 32A. Savateev, N. V. Tarakina, V. Strauss, T. Hussain, K. ten Brummelhuis, J. M. Sánchez Vadillo, Y. Markushyna, S. Mazzanti, A. P. Tyutyunnik, R. Walczak, M. Oschatz, D. M. Guldi, A. Karton, M. Antonietti, Angew. Chem. Int. Ed. 2020, 59, 15061–15068.
- 33Z. Chen, A. Savateev, S. Pronkin, V. Papaefthimiou, C. Wolff, M. G. Willinger, E. Willinger, D. Neher, M. Antonietti, D. Dontsova, Adv. Mater. 2017, 29, 1700555.
- 34H. Ou, Y. Qian, L. Yuan, H. Li, L. Zhang, S. Chen, M. Zhou, G. Yang, D. Wang, Y. Wang, Adv. Mater. 2023, 35, 2305077.
- 35H. Shi, Y. Liang, J. Hou, H. Wang, Z. Jia, J. Wu, F. Song, H. Yang, X. Guo, Angew. Chem. Int. Ed. 2024, 63, e202404884.
- 36M. Zheng, P. Wang, X. Zhi, K. Yang, Y. Jiao, J. Duan, Y. Zheng, S.-Z. Qiao, J. Am. Chem. Soc. 2022, 144, 14936–14944.
- 37Y. Wang, B. Li, B. Xue, N. Libretto, Z. Xie, H. Shen, C. Wang, D. Raciti, N. Marinkovic, H. Zong, W. Xie, Z. Li, G. Zhou, J. Vitek, J. G. Chen, J. Miller, G. Wang, C. Wang, Sci. Adv. 2023, 9, eade3557.
- 38N. Wang, W. Jiang, J. Yang, H. Feng, Y. Zheng, S. Wang, B. Li, J. Z. X. Heng, W. C. Ong, H. R. Tan, Y.-W. Zhang, D. Wang, E. Ye, Z. Li, Nat. Commun. 2024, 15, 5913.
- 39Y. Zhu, P. Li, X. Yang, M. Wang, Y. Zhang, P. Gao, Q. Huang, Y. Wei, X. Yang, D. Wang, Y. Shen, M. Wang, Adv. Energy Mater. 2023, 13, 2204143.
- 40S. Mukhopadhyay, M. S. Naeem, G. Shiva Shanker, A. Ghatak, A. R. Kottaichamy, R. Shimoni, L. Avram, I. Liberman, R. Balilty, R. Ifraemov, I. Rozenberg, M. Shalom, N. López, I. Hod, Nat. Commun. 2024, 15, 3397.
- 41S. Zhu, B. Jiang, W.-B. Cai, M. Shao, J. Am. Chem. Soc. 2017, 139, 15664–15667.
- 42Y. Sun, Y. Huang, F. Yao, M. Tian, J. Wang, W. Fan, J. Zhu, J.-M. Lee, Angew. Chem. Int. Ed. 2025, 64, e202418095.
- 43F. Xie, Z. Wang, C.-W. Kao, J. Lan, Y.-R. Lu, Y. Tan, Angew. Chem. Int. Ed. 2024, 63, e202407661.
- 44W. Guo, S. Zhang, J. Zhang, H. Wu, Y. Ma, Y. Song, L. Cheng, L. Chang, G. Li, Y. Liu, G. Wei, L. Gan, M. Zhu, S. Xi, X. Wang, B. I. Yakobson, B. Z. Tang, R. Ye, Nat. Commun. 2023, 14, 7383.
- 45K. Ye, T.-W. Jiang, H. D. Jung, P. Shen, S. M. Jang, Z. Weng, S. Back, W.-B. Cai, K. Jiang, Nat. Commun. 2024, 15, 9781.
- 46H. Zou, G. Zhao, H. Dai, H. Dong, W. Luo, L. Wang, Z. Lu, Y. Luo, G. Zhang, L. Duan, Angew. Chem. Int. Ed. 2023, 62, e202217220.
- 47S. Zhu, T. Li, W.-B. Cai, M. Shao, ACS Energy Lett. 2019, 4, 682–689.
- 48Y.-L. Yang, Y.-R. Wang, L.-Z. Dong, Q. Li, L. Zhang, J. Zhou, S.-N. Sun, H.-M. Ding, Y. Chen, S.-L. Li, Y.-Q. Lan, Adv. Mater. 2022, 34, 2206706.
- 49H.-L. Zhu, J.-R. Huang, X.-W. Zhang, C. Wang, N.-Y. Huang, P.-Q. Liao, X.-M. Chen, ACS Catal. 2021, 11, 11786–11792.
- 50X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu, Y. Xie, Nat. Energy 2019, 4, 690–699.
- 51C. Wang, X. Wang, H. Ren, Y. Zhang, X. Zhou, J. Wang, Q. Guan, Y. Liu, W. Li, Nat. Commun. 2023, 14, 5108.
- 52J. Chen, C. Hu, Y. Liu, Y. Wei, K. Shen, L. Chen, Y. Li, Angew. Chem. Int. Ed. 2025, 64, e202422775.