Cobalt-Catalyzed Stereodivergent Semihydrogenation of Alkynes: Synthesis of E- and Z-Alkenes
Xiang Ren
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Peng Lu
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
E-mail: [email protected]; [email protected]
Search for more papers by this authorChenggong Zheng
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorProf. Yong Wang
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Zhan Lu
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
State Key Laboratory of Soil Pollution Control and Safety, Zhejiang University, Hangzhou, 310027 China
State Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorXiang Ren
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Peng Lu
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
E-mail: [email protected]; [email protected]
Search for more papers by this authorChenggong Zheng
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorProf. Yong Wang
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Zhan Lu
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
State Key Laboratory of Soil Pollution Control and Safety, Zhejiang University, Hangzhou, 310027 China
State Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
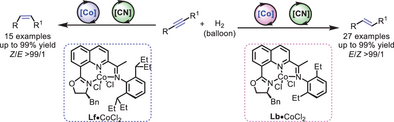
Under the synergistic catalysis of an 8-OIQ cobalt catalyst and CH3CN, a series of E- and Z-alkenes with high stereoselectivity could be synthesized in the semihydrogenation of internal alkynes. Mechanistic studies showed that the acetonitrile plays an important role in controlling isomerization and inhabiting over-hydrogenation during the conversion process.
Abstract
Transition metal-catalyzed semihydrogenation of alkynes is one of the most efficient, sustainable, and environmental-friendly strategies for accessing stereoisomerically pure olefins. Herein, we report a tridentate nitrogen-containing ligand (8-OIQ) promoted cobalt-catalyzed stereodivergent semihydrogenation of internal alkynes; a series of Z- and E-alkenes could be synthesized with high stereoselectivity. Besides, this protocol exhibits excellent functional group tolerance and operates under mild reaction conditions (1 bar H2, room temperature). A preliminary mechanistic study revealed that acetonitrile plays an important role in suppressing over-reduction and controlling the stereoselectivity in this transformation.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.
Supporting Information
| Filename | Description |
|---|---|
| anie202511269-sup-0001-SuppMat.pdf15.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Oger, L. Balas, T. Durand, J.-M. Galano, Chem. Rev. 2013, 113, 1313–1350.
- 2R. C. Larock, in Comprehensive Organic Transformations: A Guide to Functional Group Preparations, 2nd ed., Wiley-VCH, New York 1999.
- 3G. C. Tron, T. Pirali, G. Sorba, F. Pagliai, S. Busacca, J. Med. Chem. 2006, 49, 3033–3044.
- 4G. Wittig, U. Schöllkopf, Chem. Ber. 1954, 87, 1318–1330.
- 5D. J. Peterson, J. Org. Chem. 1968, 33, 780–784.
- 6W. S. Wadsworth, W. D. Emmons, J. Am. Chem. Soc. 1961, 83, 1733–1738.
- 7P. R. Blakemore, J. Chem. Soc., Perkin Trans. 1 2002, 2563–2585.
- 8B. K. Keitz, K. Endo, P. R. Patel, M. B. Herbert, R. H. Grubbs, J. Am. Chem. Soc. 2012, 134, 693–699.
- 9 Metal-catalyzed cross-coupling reactions (Eds: A. De Meijere, F. Diederich), Wiley-VCH, Weinheim 2004.
10.1002/9783527619535 Google Scholar
- 10K. Rafał, G. Karol, Chem. Rev. 2025, 125, 4397–4527.
10.1021/acs.chemrev.4c00001 Google Scholar
- 11W.-Y. Siau, Y. Zhang, Y. Zhao, in Stereoselective Alkene Synthesis (Ed.: J. Wang), Springer, Berlin, 2012, pp. 33–58.
10.1007/128_2012_315 Google Scholar
- 12B. J. Gregori, M. W. S. Schmotz, A. J. von Wangelin, ChemCatChem 2022, 14, e202200886.
- 13D. M. Sharma, B. Punji, Chem. Asian. J. 2020, 15, 690–708.
- 14H. Lindlar, R. Dubuis, Org. Synth. 1966, 46, 89–92.
- 15S. P. Desai, J. Ye, J. Zheng, M. S. Ferrandon, T. E. Webber, A. E. Platero-Prats, J. Duan, P. Garcia-Holley, D. M. Camaioni, K. W. Chapman, J. Am. Chem. Soc. 2018, 140, 15309–15318.
- 16Z. Huang, Y. Wang, X. Leng, Z. Huang, J. Am. Chem. Soc. 2021, 143, 4824–4836.
- 17V. Chugh, J. Wu, M. Leutzsch, H Randel, T.W., A. A. Auer, C. Farès, C. Werlé, Chem Catalysis 2024, 4, 101078.
- 18Z. Wang, Q. Luo, S. Mao, C. Wang, J. Xiong, Z. Chen, Y. Wang, Nano Res. 2022, 15, 10044–10062.
- 19G. J. Baker, A. J. P. White, I. J. Casely, D. Grainger, M. R. Crimmin, J. Am. Chem. Soc. 2023, 145, 7667–7674.
- 20Y. Liang, U. K. Das, J. Luo, Y. Diskin-Posner, L. Avram, D. Milstein, J. Am. Chem. Soc. 2022, 144, 19115–19126.
- 21L. Ling, C. Hu, L. Long, X. Zhang, L. Zhao, L. L. Liu, H. Chen, M. Luo, X. Zeng, Nat. Commun. 2023, 14, 990.
- 22D. J. Hale, M. J. Ferguson, L. Turculet, ACS Catal. 2022, 12, 146–155.
- 23S. Fu, N. Y. Chen, X. Liu, Z. Shao, S. P. Luo, Q. Liu, J. Am. Chem. Soc. 2016, 138, 8588–8594.
- 24K. Tokmic, A. R. Fout, J. Am. Chem. Soc. 2016, 138, 13700–13705.
- 25C. Chen, Y. Huang, Z. Zhang, X. Q. Dong, X. Zhang, Chem. Commun. 2017, 53, 4612–4615.
- 26V. G. Landge, J. Pitchaimani, S. P. Midya, M. Subaramanian, V. Madhu, E. Balaraman, Catal. Sci. Technol. 2018, 8, 428–433.
- 27K. Li, R. Khan, X. Zhang, Y. Gao, Y. Zhou, H. Tan, J. Chen, B. Fan, Chem. Commun. 2019, 55, 5663–5666.
- 28X. Liu, B. Liu, Q. Liu, Angew. Chem. Int. Ed. 2020, 59, 6750–6755.
- 29H. Alawisi, H. D. Arman, Z. J. Tonzetich, Organometallics 2021, 40, 1062–1070.
- 30D. K. Pandey, J. R. Khusnutdinova, ChemCatChem 2025, 17, e202500041.
- 31M. T. Sheikh, P. Sakharov, S. Raje, G. de Ruiter, ACS Catal. 2025, 15, 5370–5377.
10.1021/acscatal.5c00792 Google Scholar
- 32K. Chen, H. Zhu, Y. Li, Q. Peng, Y. Guo, X. Wang, ACS Catal. 2021, 11, 13696–13705.
- 33W.-F. Tian, Y.-Q. He, X.-R. Song, H.-X. Ding, J. Ye, W.-J. Guo, Q. Xiao, Adv. Synth. Catal. 2020, 362, 1032–1038.
- 34P. Lu, X. Ren, H. Xu, D. Lu, Y. Sun, Z. Lu, J. Am. Chem. Soc. 2021, 143, 12433–12438.
- 35J. Zhao, G. Xu, X. Wang, J. Liu, X. Ren, X. Hong, Z. Lu, Org. Lett. 2022, 24, 4592–4597.
- 36X. Ren, Z. Lu, Org. Lett. 2021, 23, 8370–8374.
- 37I. A. Mkhalid, R. B. Coapes, S. N. Edes, D. N. CovEntry, F. E. Souza, R. L. Thomas, J. J. Hall, S. W. Bi, Z. Lin, T. B. Marder, Dalton Trans. 2008, 8, 1055–1064.
- 38A. P. Singh, R. Singh, S. S. Verma, V. Rai, C. H. Kaschula, P. Maiti, S. C. Gupta, Med. Res. Rev. 2019, 39, 1851–1891.
- 39A. Nowicki, P. Skupin-Mrugalska, M. Jozkowiak, M. Wierzchowski, M. Rucinski, P. Ramlau, V. Krajka-Kuzniak, J. Jodynis-Liebert, H. Piotrowska-Kempisty, Int. J. Mol. Sci. 2020, 21, 1100.
- 40Y. Jiang, Y. Y. Liu, X. Liu, H. Lin, K. Gao, W. Y. Lai, W. Huang, Chem. Soc. Rev. 2020, 49, 5885–5944.
- 41J. E. Anthony, Angew. Chem. Int. Ed. 2008, 47, 452–483.
- 42S. F. Rach, F. E. Kühn, Chem. Rev. 2009, 109, 2061–2080.
- 43P. Lu, H. Wang, Y. Mao, X. Hong, Z. Lu, J. Am. Chem. Soc. 2022, 144, 17359–17364.
- 44M. R. Friedfeld, M. Shevlin, G. W. Margulieux, L.-C. Campeau, P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3314–3324.
- 45T. Ouyang, C. Hou, J.-W. Wang, W.-J. Liu, D.-C. Zhong, Z.-F. Ke, T.-B. Lu, Inorg. Chem. 2017, 56, 7307–7311.
- 46W.-Y. Chu, R. Gilbert-Wilson, T. B. Rauchfuss, M. van Gastel, F. Neese, Organometallics 2016, 35, 2900–2914.
- 47J. Lee, J. Lee, J. Seo, Nat. Commun. 2024, 15, 8688.
- 48V. Aryal, S. Inaththappulige, A. Acharya, R. Giri, J. Am. Chem. Soc. 2025, 147, 1667–1676.