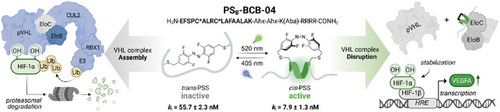
Conditional Stabilization of the Hypoxia-Inducible Factor HIF1α— Photoswitchable Stapled Peptides Prevent Elongin BC–Mediated Degradation
Graphical Abstract
Visible-light photoswitchable peptides enable the stabilization of the hydroxylated hypoxia transcription factor 1α (HIF1α—OH) by targeting the pVHL-EloBC interaction. Significant differences in conformation, binding, and transcription of HIF-targeted genes between the photostationary states (PSS) were demonstrated.
Abstract
Oxygen levels and its distribution are tightly regulated due to their critical impact on health. Hypoxia-inducible factors (HIFs) are traditionally recognized as key regulators of the transcriptional response to low oxygen (hypoxia). Recent research expanded their functions, highlighting their potential as therapeutic targets. Despite these advances, there is still a need for chemical biology tools that offer precise spatiotemporal control within the HIF network and output. Here, we introduce an optochemical approach that enables significant differences in expression of HIF1α-target genes depending on the photostationary state (PSS). Our photoswitchable stapled peptide PSB-BCB-04 stabilized HIF1α under normoxic conditions by targeting EloBC (ki = 7.9 ± 1.3 nM) and preventing VHL-mediated degradation. Visible light allowed reversible regulation of peptide conformation, which entailed a sevenfold difference in its EloBC-binding capacity. In a proof-of-concept study, we demonstrated that inhibition of HIF1α degradation enabled isomer-specific expression of the vascular endothelial growth factor (VEGFA) in prostate cancer cells. Our results validated the potential of photopharmacological stabilization of HIF1α and provided a new toolbox for on-demand photocontrol over the HIF-signaling pathway as well as VHL-mediated degradation.
Oxygen homeostasis is essential for all aerobic organisms.[1] In addition to its fundamental role in respiration, molecular oxygen also influences multiple biological processes, including metabolism, erythropoiesis, angiogenesis, proliferation, immune response, and survival.[2, 3] Therefore, cells must rapidly detect and adapt to fluctuations in oxygen levels by changing gene expression. In this context, the highly conserved transcription factors (TFs) hypoxia-inducible factors (HIFs) regulate the expression of over a hundred genes with the purpose of adaptation to low oxygen concentrations (hypoxia). Although there are other TFs affected by hypoxic conditions,[4-6] HIFs are positioned in the central pathway to respond to oxygen deprivation—a discovery recognized with the Nobel Prize in 2019.[7] Under normoxic conditions (21% O2), the predominant isoform HIF1α undergoes hydroxylation in Pro402 and Pro564; this post-translational modification is responsible for its recognition by the Elongin BC (EloBC)-containing ubiquitin ligase Von Hippel–Lindau (VHL) and, subsequently, proteasomal degradation of HIF1α (Figure 1).[8] On the contrary, hydroxylation does not occur in hypoxia, which means that HIF1α remains unmodified, escaping VHL recognition. As a result, the O2-sensor α-unit (HIF1α) is stabilized and can dimerize with the DNA-binding β-unit (HIF1β). This transcriptionally active heterodimer[9] translocates into the nucleus and, in turn, binds the hypoxia-response elements (HREs, 5′-RCGTG-3′ R, purine) (Figure 1) to initiate the downstream expression of numerous genes, such as those encoding erythropoietin (EPO) and vascular endothelial growth factor (VEGFA).[10, 11] This behavior clearly illustrates the critical role of HIFs in many signaling pathways, affecting both cellular and systemic functions beyond hypoxic adaptation. Hence, control over the canonical responses to hypoxia has medical potential.[12-14] Thus, on the one hand, inhibition of HIF is beneficial for treating cancer, pulmonary hypertension, and retinal neovascularization; small molecules[15, 16] together with the recently reported cyclic peptides[17] have succeeded in preventing HIF activity. On the other hand, the stabilization of HIF in normoxia has been therapeutically applied in renal anemia, ischemia, inflammation, restoration, and skin healing.[18] For instance, HIF1α activation promoted regeneration and functional recovery in axon injury by inducing VEGFA expression.[19, 20] HIF1α levels have been stabilized by targeting either the prolyl hydroxylase domain protein 2 (PHD2)[21] or the pVHL substate pocket as elegantly demonstrated the VHL ligand VH298.[22] Despite the remarkable pharmacological advances during the past decade, which culminated with many ongoing clinical trials and even approval drugs for HIF1α (molidustat, vadadustat, daprodustat, and desidustat),[18] challenges still remain. The complex involvement of HIF1α in multiple pathways, along with the spatiotemporal variation of oxygen levels in tissues, context dependency, and isoforms, require programmable manipulation to trigger on-demand HIF responses. Photoreactive chemical probes with cellular activity should offer a promising approach for this goal. To our knowledge, existing examples are limited to two, which indeed use the same general approach, i.e., irreversible photocontrol of PHD2, either with photocaged inhibitors[23] or photocaged delivery systems of siRNA.[24] Along these lines, reversible photocontrol of HIF-downstream genes has not been addressed yet. Here, we report a novel optochemical strategy to control HIF stabilization with downstream effects in normoxia by directly targeting the pVHL-EloBC interaction. Our photoswitchable peptide PSB-BCB-04 is a potent inhibitor (ki = 7.9 ± 1.3 nM) with up to 91% cis-ratio in aqueous solution, which was translated into a sevenfold affinity difference between isomers. Importantly, our compound enabled isomer-dependent upregulation of VEGFA levels in LNCaP prostate cancer cells. Therefore, PSB-BCB-04 represents the first light-controllable HIF1α activator functioning by disrupting EloBC-pVHL interaction. Besides HIF1α control at the cellular level, we believe that our compound would open new avenues in the emerging field of conditional proteasomal degradation.[25, 26]

Given the limited examples of visible-light photo-crosslinkers, some even recently released during our investigation,[27-33] we first aimed to develop an analogue of Woolley's bis-haloacetamide-based azobenzenes,[34-37] specifically, the 4,4′-bis(bromoacetamido)-tetra-ortho-fluoroazobenzene (PSA) (Figure 2a). Regarding the synthesis, we followed Hecht's reported procedure[38] to obtain the tetra-ortho-fluoroazobenzene core, which was then functionalized at the para position to yield the desired photo-crosslinker PSA (see Supporting Information). Tetra-ortho-fluorination was selected to increase the separation of the n→π* bands between isomers.[38, 39] However, we anticipated that the electron-donating effect of the para-acetamides in PSA could partially counteract this separation.[38] Therefore, we also evaluated the previously synthesized 4,4′-bis(bromomethyl)-tetra-ortho-fluoroazobenzene (PSB),[40] but never used in the context. Its bromomethyl functionalization should be suitable for peptide stapling via cysteine alkylation. Besides, the direct alkylation should reduce the electron-donating effect too.[32, 41] Indeed, as expected, spectroscopic analysis in DMSO (Figure S1) confirmed a more pronounced band separation for PSB (Figure S2) than for PSA (Figure S3) with an isomer ratio of 88% for the trans-isomer at the photostationary state under 405 nm irradiation (trans-PSS405nm) and 87% for the cis-isomer at the photostationary state under 520 nm irradiation (cis-PSS520nm) via NMR (Figures S4 and S5).
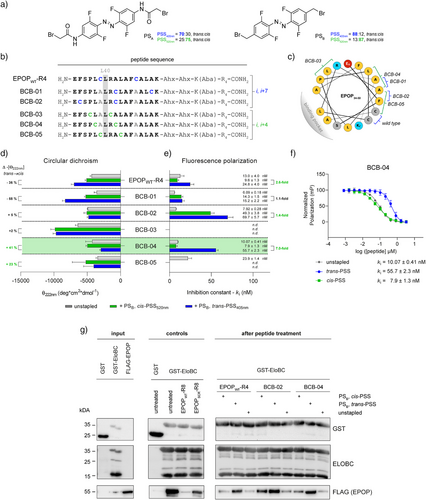
Once the superiority of PSB was demonstrated, we designed a peptide library based on our recently published EPOP (34–50) BC-box derivative that binds EloBC with nM affinity.[42] In addition to stapling the parental cysteines, which are conveniently positioned in i, i + 7 and could bridge two helical turns, we aimed at identifying alternative positions to exert the highest functional impact. For this purpose, we obtained an EloBC-affinity profile by a systematic alanine scan using competitive fluorescence polarization (Table 1; Figures S6 and S7). Parental alanines were conserved. To prevent solubility issues, we introduced four C-terminal arginines that were spaced by two 6-aminohexanoic acids (Ahx) and a lysine conjugated with the p-aminobenzoic acid (Aba) chromophore. Having a total of five arginines, counting the BC-box sequence (BCB) was expected to yield cellular uptake[43, 44] without compromising both the dynamic range and binding affinity, as occurred for the octaarginine conjugates targeting the EPOP-EloBC interaction.[42] Almost all peptides conserved the nM affinity. In fact, the variants in which the parental cysteines were exchanged, i.e., EPOP-A6 (C39A) and EPOP-A11 (C46A), even increased binding, confirming the possibility of additional stapling positions for PSB incorporation. The replacement of leucine, i.e., EPOP-A5 (L38A), -A7 (L40A) -A9 (L43A), and -A12 (L48A), decreased the affinity, which is in agreement with the validated binding of the EPOP-derivative peptides in the hydrophobic H3-H4 groove of EloC.[42, 45] As expected, the interaction was impaired (ki = ∼109 ± 12 nM) for the variant missing the known key L40 residue[42] (EPOP-A7). No binding was observed for EPOP-A4 (P37A) and EPOP-A8 (R41A); interestingly, EPOP-A4 (P37A) displayed unexpected high values of polarization when the concentration increases as EPOP-A13 (K50A). Dynamic light scattering (DLS) measurements suggested that this effect may be due to precipitation (Figure S8). Moreover, four of the thirteen variants improved binding: EPOP-A1 (E34A), EPOP-A6 (C39A), EPOP-A10 (F45A), and EPOP-A11 (C46A).
| Peptide | Variant | Sequence | Inhibition constant (ki, nM)b) |
|---|---|---|---|
| EPOPWT-R4 | wild-type | H2N–E F S P L C L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 13 ± 4 |
| EPOP-A1 | E34A | H2N–A F S P L C L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 12.2 ± 1.7 |
| EPOP-A2 | F35A | H2N–E A S P L C L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 14.2 ± 2.8 |
| EPOP-A3 | S36A | H2N–E F A P L C L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 19.5 ± 1.6 |
| EPOP-A4 | P37A | H2N–E F S A L C L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | n.d. |
| EPOP-A5 | L38A | H2N–E F S P A C L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 17.5 ± 1.9 |
| EPOP-A6 | C39A | H2N–E F S P L A L R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 9.0 ± 1.9 |
| EPOP-A7 | L40A | H2N–E F S P L C A R A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | ∼109 ± 12 |
| EPOP-A8 | R41A | H2N–E F S P L C L A A L A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | n.d. |
| EPOP-A9 | L43A | H2N–E F S P L C L R A A A F C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 36 ± 9 |
| EPOP-A10 | F45A | H2N–E F S P L C L R A L A A C A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 8 ± 4 |
| EPOP-A11 | C46A | H2N–E F S P L C L R A L A F A A L A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 1.7 ± 1.1 |
| EPOP-A12 | L48A | H2N–E F S P L C L R A L A F C A A A K–Ahx–Ahx–K(Aba)–RRRR–CONH2 | 21.6 ± 3.5 |
| EPOP-A13 | K50A | H2N–E F S P L C L R A L A F C A L A A–Ahx–Ahx–K(Aba)–RRRR–CONH2 | ∼10 ± 4 |
- Note: The peptide binding affinities for EloBC were determined in competitive binding experiments against EPOPWT-FAM[a] in 50 mM NaH2PO4/Na2HPO4, 150 mM NaCl, 0.1 mM TCEP, and 0.02% Triton X-100 (Figures S6 and S7).
- a) This peptide was previously published;[42] under the here applied conditions, kD = 0.28 ± 0.02 nM was determined and used to calculate the inhibitory constants (Figure S7).
- b) The ki values represent mean ± standard deviation and are derived from three independent measurements with technical triplicates per data point each. Peptides for which ki could not be calculated in the applied concentration range due to no binding, insufficient data points, or precipitation/aggregation are indicated with n.d., i.e., not determined. For EPOP-A7 and EPOP-A13, estimated ki values were provided and indicated with “∼”.
Based on these results, we designed five new BC-box sequences (BCB) in addition to EPOPWT-R4 that were stapled with PSB to study their conformational and inhibitory behavior under irradiation (Figure 2b). Our library included peptides stapled in the i, i + 7 position (PSB-EPOPWT-R4, PSB-BCB-01, and PSB-BCB-02) and in the i, i + 4 position (PSB-BCB-03 to PSB-BCB-05), ensuring that these did not interfere with the EloBC-binding interface (Figure 2c). To assess conformational changes of the PSB-containing peptides upon visible-light irradiation, we employed circular dichroism (CD) spectroscopy (Figures 2d and S9). Although the accurate determination of helicity from mean residue ellipticity (θ) is complicated, differences in θ at 222 nm generally indicate changes in fractional helicity (FH). Unlike the precedents of the bis-haloacetamide-based azobenzenes,[46, 47] the cis-PSS520nm for i, i + 7 stapled peptides displayed a decrease in the negative θ222nm up to −58%, which means reduced fractional helicities from the trans-to-cis (Figure 2d and Table S5). This trend would usually occur for i, i + 11 stapled peptides.[31, 34, 46] In contrast, the i, i + 4 stapled peptides exhibited an increase in the negative θ222 nm up to +48% for PSB-BCB-04, suggesting a rise in the fractional helicity content.
Next, we evaluated the photomodulation of the inhibitory binding affinities (ki) of our photoswitchable peptides (Figures 2e,f and S10). The highest affinity difference between PSS was obtained for PSB-BCB-04 that exhibited a sevenfold change in inhibitory binding (ki,cis-PSS = 7.9 ± 1.3 nM versus ki,trans-PSS = 55.7 ± 2.3 nM). Moreover, a nearly threefold difference was obtained for the stapled wild-type peptide PSB-EPOPWT-R4 (ki,cis-PSS = 9.6 ± 1.3 nM versus ki,trans-PSS = 24.8 ± 4.0 nM). The remaining peptides either did not exceed the affinity of their unstapled variants or showed no interaction, as observed for PSB-BCB-03, possibly because of both affinity-compromising modifications, i.e., R41A and P37A. Interestingly, our results highlight that CD screenings alone must be interpreted carefully. Thus, it is widely accepted that an increase in the negative θ222nm in peptides binding in an α-helical conformation should enhance affinity by reducing conformational entropy.[48] However, in our study, only BCB-04 follows this trend (Figure 2d,e and Table S5), which suggests that FH alone cannot explain the sevenfold improvement of the cis-PSS520nm PSB-BCB-04, highlighting the importance of optimal positioning of the interacting residues. Gratifying, we also demonstrated dynamic modulation in both directions by in situ irradiation under the biologically relevant glutathione (GSH)-reducing conditions (Figure S11), which avoided minor hydrazobenzene formation observed with the stronger reducing agent TCEP (Figure S12).[49]
Afterward, we performed pull-down experiments using the three peptides with the highest difference in ki between their respective cis-PSS and trans-PSS (Figure 2e) to corroborate that the PSB-containing inhibitors retained the disruptive properties of the parental EPOP peptide[42] to affect the cellular interactors of EloBC (Figure 2g). Using expressed GST-EloBC as bait, the lysate of FLAG-EPOP-transfected HEK293T cells was treated with 25 µM of either unstapled or PSB-modified peptides in cis-PSS520nm or trans-PSS405nm. Of note, EPOPWT-R8 and its scramble version, EPOPSCR-R8, were used as controls.[42] Gratifyingly, there was a clear correlation between the inhibitory constants and the pull-down experiments. The peptides that had detectable isomer differences in the affinity (>1.4-fold), which were PSB-EPOPWT-R4 and PSB-BCB-04, affected the intensity of the detected FLAG-EPOP bands. Consistently, in their cis-PSS520nm always showed higher disruption of the EloBC-EPOP interaction. Among the tested peptides, PSB-BCB-04, whose ki was the lowest (ki = 7.9 ± 1.3 nM), was the most effective peptide, indicating the potential to control HIF1α turnover rates to induce distinct downstream effects depending on the PSS. Therefore, taken together these results led us to select PSB-BCB-04 for further studies. According to our HPLC measurements, our best candidate PSB-BCB-04 reached the highest isomerization ratio up to 91% of cis-PSS520nm (180 s) and 81% trans-PSS405nm (5 s) under physiological conditions with 10 mM GSH (Figures 3a,b and S13–15). To our knowledge, our outcome represents the highest isomerization ratios for a stapled peptide under these conditions using visible light so far.[32, 33, 46, 47, 50] The ratios remained remarkably stable within 20 h, with only slight changes after 44 h (Figure 3a,b). Under cell culture conditions (37 °C and FBS), PSB-BCB-04 displayed a half-life time of t½ = 1.5 days—far beyond the reported 4 h window needed for HIF stabilization (Figure S15).[22] Changes in pure water were even less than 1% for both PSS after 44 h (Figure S16). Of note, continuous switching showed no detectable fatigue (Figure 3c).
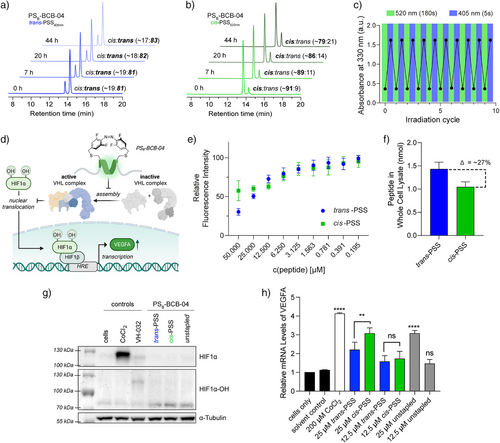
Finally, we decided to test PSB-BCB-04 in a realistic biological context. Thus, in a proof-of-concept setup, we aimed at employing PSB-BCB-04 as a photoswitchable HIF1α activator by targeting pVHL-EloBC interaction and, in turn, inhibiting proteasomal degradation, which subsequently should enable PSS-selective induction of the hypoxic response elements (HREs) (Figure 3d). First, we characterized cell permeabilization. Independent methodologies using either the chloroalkane penetration assay (CAPA)[51] or HPLC revealed a preferential uptake of the trans-PSS405nm above 12.5 µM. (Figures 3e,f and S17–19A; Table S6). LNCaP cells treated with the cis-PSS520nm showed ∼27% less peptide in the whole-cell lysate compared to those with trans-PSS405nm. Despite these differences, Western blot experiments demonstrated that both PSS of PSB-BCB-04 can mainly induce the hydroxylated HIF1α-OH (Figure 3g) more efficiently than the controls, even with higher concentrations. To detect statistically significant differences between PSS, we increased concentration and employed the more sensitive RT-qPCR method. For this purpose, LNCaP prostate cancer cells were treated with 25 µM of BCB-04 or PSB-BCB-04 in trans-PSS405nm or cis-PSS520nm for 6 h (Figure 3e) to quantify the expression levels of the HIF-downstream gene VEGFA. Of note, this concentration is below their respective IC50 values (Figure S19B). The treatment with either unstapled BCB-04 or the PSB-BCB-04 in its cis-PSS520nm demonstrated a remarkable threefold increase in VEGFA expression levels (p = <0.0001, ****), which rivalled the well-known hypoxia mimetic CoCl2, even at 8-times higher concentration (200 µM); CoCl2 stabilizes HIF1α under normoxic conditions.[52] More importantly, even though the uptake of cis-PSS520nm at 25 µM is lower than the trans analogue, this photoswitchable peptide induced a threefold increase in VEGFA expression with a statistically significant difference (p = 0.0097, **) between PSSs. This result reinforces the validity of our approach and verified the feasibility of achieving selective HIF1α activation under normoxic conditions using light-responsive peptidomimetics. Interestingly, these experiments also shed some light on our previous findings: EPOP-derivative peptides activated apoptotic pathways in cancer cells by targeting EloBC.[42] Thus, it may be possible that the underlying mechanism is via induction of HREs, as described for CoCl2-treated cancer cells.[53, 54]
In summary, we present a new optochemical strategy for HIF1α stabilization based on visible-light photoswitchable peptides targeting pVHL-EloBC interaction. We also introduced a novel photo-crosslinker for peptide stapling; PSB is a promising reagent due to its straightforward synthesis and peptide incorporation together with its excellent photochemical properties. PSB-BCB-04 reached photoisomerization rates as high as 91% for the cis-PSS520nm at physiological conditions, which surpasses the current precedents of photoswitchable stapling peptides.[46, 47] Our photoswitchable peptide did not only control both conformation and binding affinity (ki = 7.9 ± 1.3 nM) in vitro but also its trans-PSS and cis-PSS affected the transcription of HIF-targeted genes, such as VEGFA, differently. Therefore, we firmly believe that our photoreactive chemical probe will provide the first steps toward achieving spatiotemporal resolution within the complex HIF network. Importantly, beyond our first demonstration of photocontrol of HIF-downstream genes, we anticipate that both PSB and PSB-BCB-04 can be easily implemented in other contexts, thereby expanding the repertoire of light-controllable tools available in chemical biology. Finally, considering the crucial role of proteolysis targeting chimeras (PROTACs) in drug discovery, we expect that this novel methodology via EloBC-targeting will encourage further developments.
Acknowledgements
This work was financially supported by Marburg University and the DFG programs: “Control of epigenetic states through light-triggered protein-protein interaction mediators” VA1002/5-1 (AOBJ666911) to O.V; TRR81: “Chromatin changes in differentiation and malignancies” TRR81/3, A17, Z04 (109546710) to R.L. and O.V. The authors thank the students of the chemical biology master practical course (CB-MPR) for their contribution to the early steps of this project; Dr. Clara Simon for her input on initial RT-qPCR experiments; Tim Beunings for assistance in the alanine scan library; Prof. Alessio Ciulli, Dr. Alessandra Salerno, Prof. Ali Tavassoli, and Prof. Tilman Borggrefe for their advice on Western blots experiments; Prof. Joshua Kritzer for his input on the CAPA assays; and Valeska Viereckt for providing the linker for the CAPA assays. Figures 1 and 2d and the TOC were created in BioRender. Trinh, V. (2025) https://BioRender.com/f66p087.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.