Trigonal Planar Bis(carbene)Cu(I) Complexes Enable Divergent H2 Activation with H2O for Accelerated Olefin Hydrogenation
In memory of Dr. John C. Linehan for his lasting impact, enthusiasm, and camaraderie within our research program
Graphical Abstract
Abstract
CuH-catalyzed olefin hydrogenation is rare compared to those of carbonyl-derived substrates. Olefin insertion into Cu–H to form Cu-alkyl is ubiquitous; however, subsequent H2 activation remains unknown to our knowledge. Herein, we investigated the transformations of β-H elimination, H2 cleavage, and catalytic olefin hydrogenation in a series of linear and trigonal planar Cu(I)-alkyl complexes supported by monodentate N-heterocyclic carbene and bidentate naphthyridine-bis(carbene) ligands, respectively. Contrary to unreactive linear species, trigonal planar variants promote β-H elimination, hydrogenolysis, and catalytic hydrogenation of unactivated alkenes at mild temperatures and H2 pressure. The rare isolation of a naphthyridine-bis(carbene)CuH monomer further affirms two predominant competing pathways for H2 cleavage of metal–ligand cooperativity at Cu(I)-alkyl or internal electrophilic substitution at Cu(I)-OH. Employing either isolated or in situ generated Cu(I)-OH complex, via protonolysis of alkyl precatalyst by adventitious water, significantly accelerated catalysis compared to that operating primarily by the metal–ligand cooperativity pathway. DFT calculations and energy decomposition analysis on the disparate β-H elimination reactivity between linear and trigonal planar tert-butyl complexes and the mechanism of H2 activation at a hydroxide complex, indicate that coordination geometry at Cu(I) and properties of the naphthyridine-bis(carbene) ligand are integral to the transformations reported here.
Introduction
Catalytic olefin hydrogenation has profoundly impacted a wide range of industrial applications, from commodities and agrochemicals to pharmaceuticals.[1-3] Precious platinum group metal catalysts are a mainstay in industrial olefin hydrogenation; however, the development of Earth-abundant heterogeneous[1, 4] and homogeneous catalysts[5-7] have gained technological interest. In contrast, heterogeneous or homogeneous Cu-catalyzed olefin hydrogenation is scarce[8-10] compared to hydrogenation of carbonyl-derived substrates,[10-17] despite the ability of molecular CuH species to insert olefins under mild conditions to generate nucleophilic Cu alkyl intermediates.[18-20]
The high activation energy for H2 dissociation at Cu nanoparticles and surfaces is widely recognized.[21, 22] Similarly, the dearth of homogeneously catalyzed olefin hydrogenation by Cu complexes can be attributed to the difficult cleavage of H2 at Cu(I) complexes since oxidative addition via a Cu(I/III) redox couple is less readily accessible, especially compared to other low-valent late metal complexes such as those of cobalt.[7] The proclivity of ligand-supported (CuOR)n clusters[23, 24] and monomers toward heterolytic H2 cleavage has been commonly invoked for catalytic hydrogenations of alkynes,[25] ketones,[15, 26] esters,[14] and amides.[13] However, to our knowledge, no mechanistic examination from well-defined complexes has been reported, as studies of these aforementioned catalytic systems are hindered by the presence of transient CuH monomers,[25-27] CuH clusters,[15, 26, 28] ill-defined speciation/coordination environment,[16, 17, 29, 30] high pressure[31] and/or high temperature conditions, and excess alkoxide base that complicate reliable kinetic analysis and identification of definitive on-cycle intermediates.[10, 12]
Straightforward synthetic access to linear Cu alkyl complexes supported by N-heterocyclic carbenes (NHC)[32-34] provides a facile entry for detailed examination. However, theoretical studies postulate that geometric perturbation of linear d10 ML2 by bending of the L-M-L angle, such as with bidentate ligands, leads to orbital mixing to form higher-energy π-symmetry orbitals that facilitate substrate binding and bond activations.[35-37] This increased reactivity of bent versus linear d10 systems has been corroborated by β-alkynyl eliminations of propargylic alkoxide Cu(I) complexes supported by varied NHC and diphosphine ligands,[38] proposed β-H eliminations of (IPr)Cu─CH(Ph)CH2Bpin[39-41] and (DTBM-SEGPHOS)Cu─CH(Me)(C6H4-4-F)[41, 42] (see ref. [41] for abbreviations), and the oxidative addition of C–H bonds,[43] H2,[35] and aryl halides[44] at d10 Pt and Pd species.
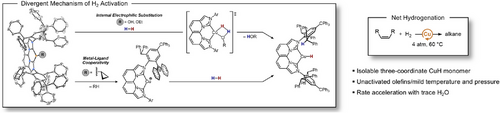
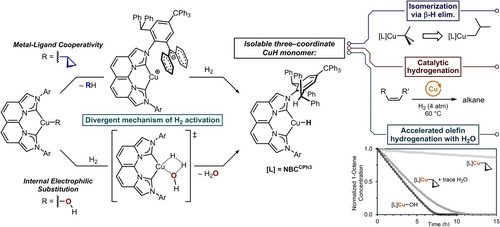
From these insights, we systematically examined the reactivity of linear and trigonal planar alkyl complexes of (IPr*CPh3)CuR[41] and (NBCCPh3)CuR,[41] respectively, towards β-H elimination, hydrogenolysis, and catalytic olefin hydrogenation. Guided by our isolation of monomeric (IPr*CPh3)CuH,[45] our recently designed[46] NBCCPh3 ligand contains proximal and distal steric bulk that can kinetically stabilize the trigonal planar (NBCCPh3)CuH monomer product in these transformations (Figure 1). The successful hydrogenolysis of (NBCCPh3)Cu-alkyl to generate (NBCCPh3)CuH informed a preliminary demonstration of catalytic hydrogenation of terminal and internal unactivated alkenes at mild pressure and temperature. Mechanistic, kinetic, and computational analysis revealed the pivotal role of coordination geometry on reactivity and the influence of adventitious water under varied reaction conditions on the diverse distribution of Cu(I) species and the mechanism of heterolytic H2 cleavage.
Results and Discussion
Investigation of β-H Elimination and Hydrogenolysis of Linear (NHC)Cu-alkyls
Salt-metathesis reactions of (IPr*CPh3)CuCl and 1.3 equiv. of RMgCl for 10 min cleanly produced alkyl complexes 1iPr and 1tBu as determined by 1H NMR spectroscopy. Alternatively, trapping of in situ generated (IPr*CPh3)CuH with 1-hexene or cyclopentene provided complexes 1R (R = hexyl, cPent), respectively (see Supporting Information for details on the isolation of these complexes).[45] To understand the general reactivity pattern of linear (NHC)Cu(I)-alkyl complexes previously reported,[32-34, 39] we evaluated the linear primary, secondary, and tertiary alkyl complexes (IPr*CPh3)CuR (1R, R = hexyl, iPr, cPent, tBu) toward β-H elimination and hydrogenolysis. The adequate solution stability (t1/2 ∼ 12 h, C6D6, 25 °C) and known spectroscopic characterization of the resulting (IPr*CPh3)CuH monomer[45] (1H) in these transformations represent a major advantage for mechanistic studies. 1H NMR spectroscopic studies of isolated primary 1Hex shows no β-H elimination in C6D6 at 25 °C for >4 days or 80 °C for 24 h, nor hydrogenolysis under 4 atm H2 at 25–80 °C (Scheme 1). Additionally, no catalytic hydrogenations of 1-hexene or cyclopentene were observed in the presence of 10 mol% of 1Hex or 1cPent in C6D6 under 4 atm H2 at 25–80 °C over 24 h. The persistent concentration of these linear copper alkyl complexes during attempted catalytic reactions indicates no reactivity other than their gradual decomposition (<5%).
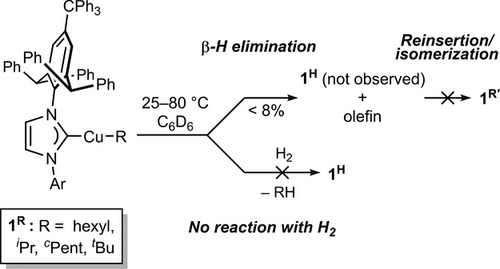
Moreover, the minimal β-H elimination and absence of isomerization with unsubstituted secondary or tertiary alkyl complexes 1iPr or 1tBu contrasts with the proposed tandem β-H elimination/isomerization process in the conversion of (IPr)Cu─CH(Ph)CH2Bpin to (IPr)Cu–CH(BPin)CH2(Ph) at 70 °C for 24 h.[39, 40] The potential role of the boron functionality on β-H elimination in this complex remains unclear. 1H NMR analysis of 1iPr or 1tBu in C6D6 over 7.5 days at 25 °C showed trace formation of propylene and isobutylene, respectively (Figure S11). However, neither 1H nor the primary isomerized 1nPr or 1iBu products were observed. The starting complexes of 1iPr or 1tBu decomposed by 4% and 2%, respectively, with trace detection of free IPr*CPh3 ligand. These observations suggest decomposition follows β-H elimination instead of reinsertion of the eliminated olefin over these long reaction times at low concentrations of 1H and olefin that are produced. Under more forcing conditions (C6D6, 80 °C, 17.5 h), 7.6% decomposition of 1tBu was observed by 1H NMR spectroscopy. Kinetic modeling of this process, assuming an irreversible first-order process (i.e., β-H elimination), yields a barrier of 30.4 kcal mol−1, which is consistent with our DFT investigation (vide infra). The solution stabilities of 1R complexes are consistent with those reported previously for unsubstituted, linear (NHC)Cu-alkyl complexes.[32-34]
Observed β-H Elimination of Trigonal Planar Cu(I)-Alkyls
Salt-metathesis reactions of (NBCCPh3)CuCl and corresponding organomagnesium reagents cleanly generated complexes (NBCCPh3)CuR (2R, R = Me, Et, iPr, cPr, tBu—see Supporting Information for details). Single crystals of 2R were obtained by vapor diffusion of pentane into benzene solution at room temperature. 1H NMR spectroscopic and single crystal X-ray diffraction (SCXRD) analysis of the isolated single crystals revealed stark differences in the stabilities of the alkyl complexes.
Complex 2Me, isolated in 75% yield and devoid of β-hydrogen atoms, is thermally stable as a solid and in solution, yet it is exceedingly sensitive to air and moisture. Single crystals and C6D6 solutions of 2Et are stable under nitrogen at ambient temperature and pressure but decompose to intractable mixtures under vacuum. 1H NMR spectroscopic analysis of 2Et generated in situ at room temperature showed trace ethylene, implying an operative β-H elimination pathway. However, the anticipated hydride product of (NBCCPh3)CuH (2H) was not detected. The independent synthesis and spectroscopic characterization of 2H is discussed in a later section. The minimal β-H elimination is attributed to favorable ethylene reinsertion into (NBCCPh3)CuH in this equilibrium. Moreover, the dissolution of isolated crystalline 2Et in C6D6 reveals no free ethylene over 24 h.
To provide definitive spectroscopic evidence for β-H elimination, we examined the reactions of secondary and tertiary alkyl complexes 2iPr and 2tBu, which can isomerize to the corresponding thermodynamic primary alkyl products (Figure 2).[47-49] Previous kinetic studies indicate that the insertion of unactivated olefins into isolated (IPr*CPh3)CuH monomer proceeds by rate-limiting hydride transfer under pseudo-first-order conditions.[45] Therefore, the expectedly slow second-order reaction between the CuH monomer and reduced olefin concentration should enable the observation of all relevant species in a mechanism of β-H elimination and reinsertion on the 1H NMR timescale.
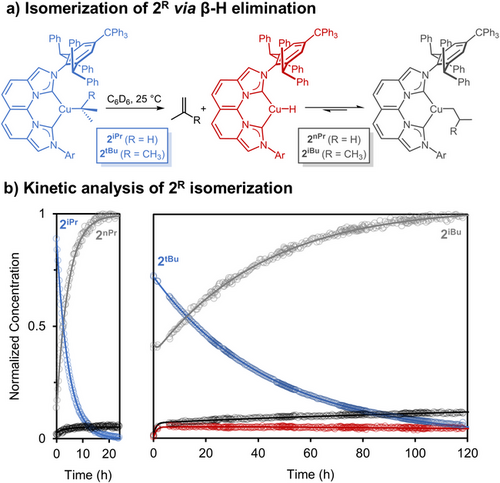
The 1H NMR spectrum obtained immediately after adding iPrMgCl to (NBCCPh3)CuCl indicates clean formation of (NBCCPh3)CuiPr (2iPr). Spectroscopic monitoring of 2iPr generated in situ in C6D6 at 25 °C shows free propylene and isomerization to 2nPr, with no 2iPr remaining after 24 h (Figure 2). After 3 days, 2nPr, 2H (vide infra), and propylene are still detected. The presence of 2H was identified from key NBCCPh3 resonances, as overlapping residual solvent signals obscure the hydride resonance of 2H. Modeling of the reaction kinetics using COPASI[50] provided a free energy barrier of 23.2 kcal mol−1 for the β-H elimination of 2iPr at 25 °C (Supporting Information Pages S34–S39). Additionally, SCXRD of red-brown single crystals obtained from the reaction mixture confirmed the structure of the isomerized product 2nPr (Figure 3a). These observations corroborate the complete isomerization of secondary alkyl complex 2iPr to the thermodynamically favored primary alkyl product 2nPr. Moreover, an equilibrium of β-H elimination/reinsertion from 2nPr is supported by continuous spectroscopic detection of 2H and propylene.
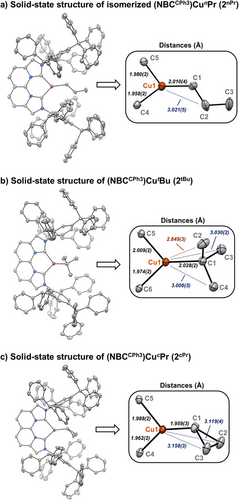
The isomerization of 2tBu is much slower than that of 2iPr (Figure 2b). Initial qualitative 1H NMR spectroscopic analysis of isolated 2tBu in C6D6 over 7 days at 22 °C showed gradual conversion to 2iBu, as determined by the loss of the tBu signal and concomitant growth of other key iBu resonances. In addition to these two species, isobutylene and a third minor set of ligand resonances for 2H (∼5%) are detected. In contrast to 2iPr, the isomerization of isolated 2tBu allowed observation of the corresponding 2H with a CuH singlet at 3.79 ppm (C6D6, 25 °C). After 13 days, only 2iBu, 2H, and isobutylene are observed, consistent with β-H elimination occurring from the isomerized alkyl species. The slow isomerization of 2tBu to 2iBu at 25 °C enabled the collection of time-course concentration data for all observable species (Figure 2b). Kinetic modeling of the reaction determined barriers of β-H elimination for both 2tBu and 2iBu at 24.5 and 24.1 kcal mol−1, respectively, along with an anti-Markovnikov insertion barrier of isobutylene into 2H at 17.6 kcal mol−1 (Supporting Information Pages S28–S33).
The preferential formation of primary alkyl products aligns with the general observation that these complexes are thermodynamically favored based on steric effects, particularly at a congested metal center.[51] Although the current data cannot fully elucidate the origin for the faster β-H elimination rate of 2iPr compared to that of 2tBu (hours vs. days), we propose that 2tBu experiences greater conformational constraints for adopting a syn coplanar rearrangement for β-H elimination, compared to that of 2iPr, leading to a decreased rate of isomerization. The detection of CuH monomer, propylene, and isobutylene in the isomerization of secondary and tertiary alkyl complexes of 2iPr and 2tBu to their corresponding primary alkyl complexes indicates a β-H elimination/reinsertion mechanism rather than a π-allyl complex pathway.[51]
The solid-state structure of 2tBu (Figure 3b), obtained from immediate crystallization after synthesis, shows that one of the three Cu─Cβ distances is significantly shorter (2.849(3) vs. 3.030(2) and 3.006(3) Å) and exhibits a smaller ∠Cu─Cα─Cβ (106.1(1)° vs. 115.8(1)° and 114.5(1)°), suggesting a potential interaction between Cu and this β-CH3 at −173 °C (the temperature of SCXRD data collection). Cooling a toluene-d8 solution of 2tBu to −90 °C revealed no de-coalescence of the β-CH3 resonances by 1H NMR spectroscopy, indicating no observable interactions at this temperature in solution. Moreover, the isomerization of 2tBu occurs at 25 °C, therefore, the structural features of solid-state 2tBu determined at −173 °C by SCXRD are (unsurprisingly) not representative of the relevant reaction conditions. DFT calculations on the geometry optimized structure and complementary occupied-virtual pairs (COVP) analysis of 2tBu do not show significantly shortened β-CH3···Cu distances or significant stabilization/interaction between Cu(I) d-orbitals and β-CH3 groups of the tBu ligand (Supporting Information, Page S115).
We employed a strained alkyl group to disfavor the syn coplanar arrangement for β-H elimination[52] to isolate (NBCCPh3)Cu-cyclopropyl (2cPr) (78% yield) that is stable to β-H elimination between 25–60 °C (vide infra). In addition to strain, the high-energy formation of the cyclopropene product also likely contributes to the stability of this robust alkyl species. Spectroscopic and crystallographic analysis confirmed the formation of 2cPr (Figure 3c), with the strained Cu-cyclopropyl functionality containing no apparent β-CH3 interactions at Cu (Cu–Cβ = 3.119(4) and 3.158(3) Å).
Generation of (NBCCPh3)CuH Monomer by Hydrogenolysis
The generation of metal hydride products by metal alkyl hydrogenolysis is well documented,[7, 53-55] yet hydrogenolysis of Cu-alkyls is uncommon compared to those of Cu-alkoxides.[17, 23, 25, 28, 56, 57] Based on the accepted β-H elimination mechanism of alkyl complexes,[58, 59] the formation of (NBCCPh3)CuH (2H) from (NBCCPh3)Cu-alkyls implies C–H σ-coordination of alkyl ligands before C─H bond cleavage. Dihydrogen might access similar σ-coordination at Cu(I) to facilitate hydrogenolysis;[60, 61] therefore, we investigated the reaction of (NBCCPh3)Cu-alkyl with H2 to generate 2H.
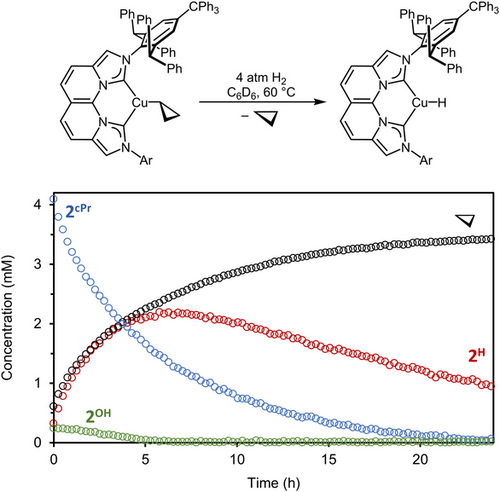
The isolation of stable 2cPr enables direct examination of hydrogenolysis, since competitive β-H elimination of 2Et, 2iPr, and 2tBu and the extreme sensitivity of 2Me to adventitious moisture complicate these studies. Exposure of 2cPr to 4 atm H2(g) in C6D6 at 22 °C produced monomeric 2H as observed by 1H NMR spectroscopy, requiring 11 days to reach 39% conversion of 2cPr. The initial introduction of H2 formed trace (NBCCPh3)CuOH (2OH) by protonolysis of 2cPr by adventitious H2O, in which the 2OH concentration is depleted within the first 5 h of reaction. The identity of 2OH was confirmed by independent synthesis (Supporting Information, Page S101).
Despite incomplete hydrogenolysis of 2cPr over even longer reaction times (22 °C for 22 days), the resulting 1:1.6 2cPr/2H mixture provided clear spectroscopic evidence of monomeric 2H from the integration of the hydride singlet at 3.79 ppm to a single proton relative to the corresponding NBCCPh3 ligand resonances in the 1H NMR spectrum. This hydride signal exhibits a doublet cross-peak (2JC–H = 14.4 Hz) in the 1H–13C HMBC NMR spectrum, with the carbene carbon singlet at 186.8 ppm in the 13C{1H} spectrum. The carbene resonance splits into a doublet (2JC–H = 14.0 Hz) in the proton-coupled 13C spectrum, providing unequivocal evidence of only one hydride in the monomeric 2H (Figure S45–S48). We observed minor decomposition of 2H at room temperature (6% mass-balance loss over 11 days). Monitoring the hydrogenolysis of 2cPr at 60 °C in C6D6 over 4 half-lives in 24 h shows rate acceleration but also increased decomposition to 75%. At this elevated temperature, 2OH is also fully consumed (Figure 4).
Motivated by the persistence of 2H in solution, we generated a copper alkoxide synthon for isolating this monomeric CuH.[19] Because of the steric constraints at the Cu center from the NBCCPh3 ligand, the ethoxide ligand was targeted instead of the commonly employed tert-butoxide analogue. (NBCCPh3)CuOEt (2OEt) was cleanly isolated in 76% by treating (NBCCPh3)CuCl with 10 equiv. of KOEt in THF over 60 h at room temperature. A lack of solubility of KOEt in THF and the sterically hindered pocket at the CuCl center likely both contribute to the sluggish reaction. The crystal structure of 2OEt exhibits orientational disorder of the ethoxide fragment into two separately observed conformations (57:43 ratio)—additional structural analysis is provided in the Supporting Information (Figure S36).
Addition of HSi(OEt)3 to benzene or toluene solutions of 2OEt at 22 °C rapidly and cleanly converts the dark-orange solution to a dark-brown solution containing 2H and Si(OEt)4 as indicated by 1H NMR analysis in C6D6. Immediate precipitation with pentane yields a dark-brown powder, which can be isolated and stored at −30 °C for months without significant deterioration. Extended application of vacuum to remove all residual solvent leads to varying levels of decomposition, but the spectroscopic characterization of this isolated 2H is consistent with all signals observed when generated via 2cPr + H2. Dissolution of 2H in C6D6 engendered more decomposition (∼20% over 50 h, vide infra) when not pressurized with H2. Attempts to synthesize the Cu-D isotopologue using DSi(OEt)3 only led to its partial formation, with deuterium washing into the -CHPh2 arms of the NBCCPh3 ligand while in solution (Figures S42 and S43, vide infra).
The hydride resonance of 2H at 3.79 ppm appears distinctly downfield from those of the three known CuH monomers (1.50–2.14 ppm),[45, 62, 63] all of which are linear complexes supported by monodentate NHC ligands. Notably, (IPr*CPh3)CuH (1H), containing identical flanking aryls to that of 2H, features a hydride resonance at 1.89 ppm (C6D6) in the 1H NMR spectrum. The 2JCH coupling of 14 Hz measured for 2H is smaller than the previously reported 2JC-H coupling (40.0–41.6 Hz) for linear monomeric Cu–H complexes. These spectroscopic differences can be ascribed to the geometry of 2H since a carbene donor trans to the hydride is absent, abating the trans influence that impacts the magnitude of coupling and shielding effects observed in previous reports.[64]
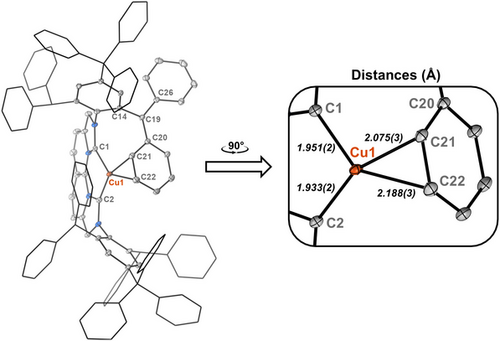
Although 2H is sufficiently stable in solution for spectroscopic characterization at room temperature, attempts at obtaining single crystals were unsuccessful. Rather, a crystal of an intermediate species (Int) was isolated; its structure is shown in Figure 5. Although we were unable to cleanly isolate this complex on a preparative scale, solid-state structural analysis indicates deprotonation of a methine position at one of the -CHPh2 groups of the NBCCPh3 ligand, yielding an anionic fragment, in which the negative charge appears delocalized over the resulting biphenyl unit that coordinates η2 to the now cationic Cu(I) center. Presumably, in the absence of H2 under crystallization conditions, the hydride ligand of 2H undergoes intramolecular deprotonation of a -CHPh2 group with accompanying loss of H2. Although the bound arene formally carries a delocalized negative charge, the Cu─Carene bond lengths observed in Int are similar to those found in [(IPr)Cu(I)(η2arene)]SbF6 complexes.[65, 66]
To provide evidence for the key H/D exchange at the -CHPh2 groups of the NBCCPh3 ligand in this mechanistic proposal, we examined the reaction of 2cPr with D2 (Scheme 2). The reaction of 2cPr at 60 °C with 4 atm of D2 predominantly extruded cyclopropane instead of cyclopropane-d1. A sample of cyclopropane-d1 was independently synthesized for verification (Supporting Information, Page S65). The 1H NMR signal for the four equivalent -CHPh2 groups of the NBCCPh3 ligand, typically observed at 6.00 ppm (C6D6, 60 °C), is absent. A corresponding signal is observed by 2H NMR spectroscopy at the same chemical shift during the reaction. The accompanying loss of a Cu–H signal at 3.65 ppm is also confirmed, and the corresponding Cu–D signal was observed in the 2H NMR spectrum for 2D as well as the detection of HD gas (Figures S54 and S55). These results imply an equilibrium between 2H/D and Int, with the resting state favoring 2H/D under pressurized H2/D2 (Scheme 2). Notably, similar H/D exchange in the -CHPh2 group is observed for the minor quantities of 2OH/OD, occurring rapidly before the consumption of this species during the initial stages of the reaction (vide infra).
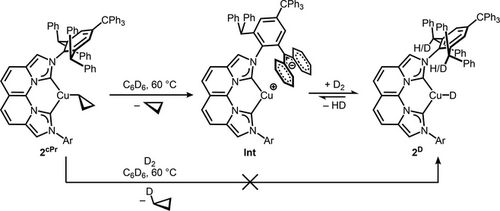
The predominant formation of cyclopropane, instead of cyclopropane-d1, eliminates the conventional σ-bond metathesis mechanism of D2 cleavage across the Cu-cyclopropyl functionality. This evidence supports a mechanism involving intramolecular deprotonation of a -CHPh2 group at NBCCPh3 by Cu-cyclopropyl to release cyclopropane, followed by heterolytic D2 cleavage at Int to generate a Cu-D and the first -CDPh2 group. The complete conversion of all remaining -CHPh2 groups to -CDPh2 groups invokes an iterative process of intramolecular deprotonation of -CHPh2 by the resulting CuD to release HD to generate Int followed by D2 cleavage to reform CuD. This mechanism of H2/D2 cleavage mirrors metal–ligand cooperativity (MLC) pathways in metal-catalyzed hydrogenation,[67-69] and has inspired an emerging strategy for heterolytic H2 cleavage at Cu(I).[14, 26, 56, 70]
Additional trapping experiments were performed to further our mechanistic insight into dihydrogen activation from 2cPr (Figure 6). 1H NMR spectroscopic analysis of a C6D6 solution of 2cPr at 60 °C without H2 showed consumption of 2cPr with concomitant formation of cyclopropane and highly broadened ligand resonances observed for the zwitterionic Int (Figure S52). The formation of cyclopropane corroborates intramolecular deprotonation of 2cPr and eliminates the possibility of competing β-H elimination of 2cPr to generate 2H and cyclopropene. Two sequential intermediates are observed over the course of the reaction kinetics that indicate Int converts to an unidentified species (2Unk). Upon addition of 1 atm H2 to the reaction mixture, the broad resonances attributed to Int rapidly convert to 2H. Moreover, 2Unk undergoes a slow decay due to its ceased production. The consumption of 2cPr is independent of [H2] since the first-order decay kinetic profile of 2cPr remains undeterred with H2 addition. Kinetic modeling of the data yields a 26.5 kcal mol−1 barrier for the intramolecular deprotonation of 2cPr at 60 °C.
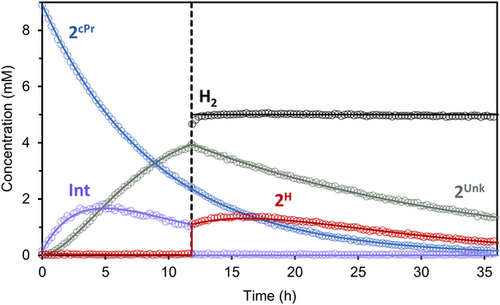
Collectively, the hydrogenolysis of linear and trigonal planar Cu-alkyl complexes supported by IPr*CPh3 and NBCCPh3 ligands, respectively, do not appear to participate in conventional heterolytic H2 cleavage by a σ-bond metathesis mechanism, consisting of a four-center transition state arising from the association of metal-alkyl and H2, that is often invoked for early to middle transition metal alkyl complexes.[53, 55] This lack of reactivity toward H2 at a Cu-alkyl group likely reflects the different polarization of metal-carbon bonds of early and late metals,[71] and contributes to the dearth of hydrogenation reactivity at Cu(I)-alkyl species despite their prevalence. The coordination geometry of Cu-alkyl species supported by the NBCCPh3 ligand overcomes this limitation indirectly by accessing H2 activation via MLC intramolecular deprotonation of the CHPh2 arm by the alkyl ligand. These pathways are not favorable in the linear congener, an effect likely due to both the enforced proximity of these groups as well as increased reactivity of the Cu-alkyl fragment in the three-coordinate system.
Catalytic Hydrogenation of Unactivated Olefins and Mechanistic Study
The successful net hydrogenolysis of 2cPr to 2H motivated the investigation of Cu-catalyzed olefin hydrogenation. Using unoptimized conditions, we examined the hydrogenation of 1-hexene, 1-octene, cyclopentene, cyclohexene, and cis-cyclooctene in C6D6 at 60 °C under 4 atm of H2 for 8–24 h with 5–10 mol% 2cPr. The results are summarized in Table 1. The hydrogenation of cyclopentene and cyclohexene is sluggish and lower yielding compared to that of 1-hexene and 1-octene. The conversion of cyclopentene and cyclohexene to their cycloalkyl products was observed in 51% and 13%, respectively, after 24 h at 60 °C using undried H2. The selected substrates eliminate complicated speciation from β-H elimination/isomerization, simplifying 1H NMR spectroscopic identification of intermediates for mechanistic investigation.
| Olefina) | ΔrH° (kcal mol−1)b) | Yield (time)c) | Resting statec) |
|---|---|---|---|
| 1-hexene | −29.9 | 99% (8.3 h) | 2Hex |
| 1-octene | −29.9 | 99% (11 h) | 2Oct |
| cyclopentene | −26.8 | 51% (24 h) | 2cPent |
| cyclohexene | −28.2 | 13% (24 h) | 2H |
| cis-cyclooctene | −24.6 | 0% (24 h) | 2H |
- a) Reaction conditions: olefin, 10 mol% 2cPr, undried H2 (4 atm), C6D6, 60 °C.
- b) Heat of hydrogenation reported in the NIST database.
- c) Determined by 1H NMR spectroscopy.
Operando 1H NMR kinetic studies show resting states of alkyl complexes of 2Hex, 2Oct, and 2cPent for 1-hexene, 1-octene, and cyclopentene hydrogenation, respectively. In contrast, cyclohexene hydrogenation engenders a resting state of 2H, indicating turnover-limiting olefin insertion. The different insertion barriers for cyclopentene versus cyclohexene are attributed to a kinetic rather than a thermodynamic effect. The heats of hydrogenation, a general descriptor of olefin stability, of cyclopentene and cyclohexene are −26.8 and −28.2 kcal mol−1,[72] respectively, indicating hydrogenation of cyclopentene is more difficult than that of cyclohexene. The sterically congested pocket at the CuH center, combined with the rapid interconversion of the dissymmetric, half-chair conformer of cyclohexene at room temperature, compared to the smaller, less dynamic, and puckered cyclopentene, provides a rationale for disfavored cyclohexene insertion into CuH.[73, 74] Similar results have been reported for iridium-catalyzed hydrogenation of these two cyclic olefins, in which the effect of ring strain and structural conformations (chair vs. boat) are attributed to this observed trend.[75] The hydrogenation of the bulkier cis-cyclooctene provided no conversion, reflecting both the anticipated kinetically unfavored binding to CuH for insertion and the heat of hydrogenation.
To circumvent substrate mass-balance and H2 mass-transport issues observed with 1-hexene hydrogenation (see Supporting Information Pages S68–S74 for details), mechanistic studies were completed with higher boiling 1-octene as a model system, ensuring accurate measurement of substrate decay during catalysis. Kinetic analysis of 1-octene hydrogenation using undried H2 shows rapid olefin insertion into 2H with a resting-state species of 2Oct and trace 2OH throughout the reaction (Figure 8b). Upon complete consumption of 1-octene after 11 h, the resting state of 2Oct converts to 2H, which undergoes gradual decomposition in the absence of substrate. A copper alkyl resting state appears to offer improved stability, whereas a copper hydride resting state increases the rate of catalyst decomposition (Figure 8b, Figures S60 and S62).
To understand the influence of trace H2O on catalysis, we performed a quantitative 1H NMR kinetic analysis of 1-octene hydrogenation with 2cPr using dried H2. Drying the H2 gas stream using a moisture trap before addition to the reaction mixture inhibits the formation of (NBCCPh3)CuOH, but also significantly decreases the catalytic rate (Figure 8a). Using undried H2, the significant acceleration of 2cPr activation and 1-octene decay in the presence of trace 2OH (Figure 8b), generated from adventitious H2O, implied a divergent mechanism of turnover. Control experiments using exclusively 2OH showed an increased rate of catalytic turnover to octane, with 2OH observed as the only resting state. The observed dependence of rate on H2 concentration (Figure S82) indicates that, contrary to 2cPr, which undergoes net hydrogenolysis through preceding intramolecular deprotonation to form Int, 2OH is instead capable of direct H2 activation, likely via an internal electrophilic substitution (IES) mechanism.[76-78] After the formation of 2H, fast olefin insertion into the transient CuH monomer produces 2R, followed by protonolysis to reform 2OH and release the respective alkane to complete turnover. Similar studies to discern the H2 dependence of on-cycle copper alkyl species (2Oct, as compared to 2cPr) using H2 pressure drop experiments and dried H2 show only a minor dependence that can be attributable to trace H2O still entering the system despite these precautions (Supporting Information Page S78).
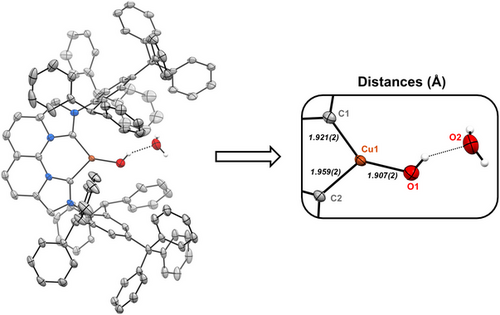
In the presence of additional H2O, the 1H NMR signal for the hydroxide of 2OH is significantly shifted due to hydrogen bonding (Figure S83). Single-crystal X-ray diffraction analysis confirms the presence of a hydrogen-bonding H2O molecule to a monomeric Cu-OH in the solid-state structure of 2OH·H2O (Figure 7) when grown from mixtures containing additional water. In fact, the isolation and characterization of reactive low-coordinate, monomeric late-metal hydroxide complexes is particularly scarce.[79, 80] This presence of excess H2O also significantly influences the rate of catalysis; using 2OH versus 2OH·H2O for 1-octene hydrogenation decreases the initial TOF from 2.8 to 1.6 h−1, respectively (Figure S84).
-
Turnover-limiting hydrogenolysis of 2OEt is significantly impacted based on the depletion of bulk [H2] to a point that is lower than the on-cycle catalyst (an effect not observed spectroscopically with 2OH).
-
With 2OEt, the inflection of 1-octene decay at the onset of the reaction coincides with H2 concentration as it is depleted, slowing to a steady-state during most of the reaction, further supporting the direct H2 activation with this copper alkoxide.
-
Since turnover involves protonolysis of fleeting 2Oct to regenerate 2OR, the different reactivity of 2H/2Oct with EtOH or H2O plays a significant and intricate role in overall catalytic performance.
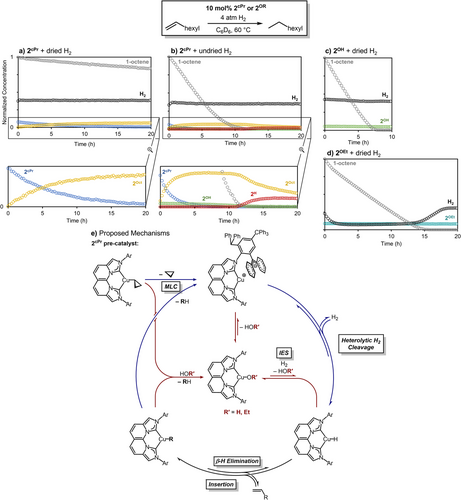
Furthermore, we have demonstrated that 2OEt is robust, as it is recyclable for a second round of 1-octene hydrogenation. After complete hydrogenation of 1-octene in 15.8 h with the initial 10 mol% 2OEt with 4 atm H2 at 60 °C in C6D6, a larger second charge of 1-octene was then added. The reaction mixture, now containing 3 mol% catalyst loading with respect to 1-octene by 1H NMR integration, provided 99% conversion of 1-octene after 16.6 h under 4 atm H2 and 80 °C. Once catalysis was complete, prolonged heating at 80 °C indicates a persistent [2OEt] over 31.5 h, demonstrating its excellent thermal stability (Figure S79).
Based on the combined results of stoichiometric and operando kinetic studies, proposed mechanistic pathways for CuH-catalyzed olefin hydrogenation are summarized in Figure 8e. In catalytic reactions that do not generate 2OH from trace H2O or employ 2OEt, the primary mechanism involves turnover-limiting intramolecular deprotonation of the ligand -CHPh2 of 2R to release alkane and yields the zwitterionic Int (R = cyclopropyl from precatalyst 2cPr, or the resulting alkyl from olefin insertion). Subsequent rapid heterolytic cleavage of H2 by Int produces 2H (Figure 8e, outer blue path) that inserts the respective olefin to regenerate 2R to complete the catalytic cycle. The Cu-cyclopropyl precatalyst 2cPr disfavors β-H elimination to promote exclusive intramolecular deprotonation, whereas the resulting on-cycle alkyl 2R species can participate in competitive β-H elimination/olefin insertion and intramolecular deprotonation. These competing processes result in a sluggish overall turnover and poor catalyst activation (complete 2cPr decay observed after >20 h, Figure 8a).
In contrast, the inclusion of 2OR (R = H, OEt) enables direct H2 activation in conjunction with the slower intramolecular deprotonation pathway (Figure 8e), inner red path). In addition to the more rapid formation of 2H via hydrogenolysis by the IES pathway, the facile reverse reaction between 2H (or 2R) and the respective protic co-catalyst (H2O or EtOH) produced under these conditions further increases the mechanistic complexity. When 2OR is the sole resting-state, the turnover of 1-octene is significantly increased versus the MLC-only pathway (Figure 8a,c,d). It is tenable that eliminating the H2 mass-transport limitations observed with 2OEt would improve the catalytic performance of the ethoxide congener to be at least comparable and plausibly beyond that of 2OH.
In an intermediate scenario, the presence of adventitious H2O forms trace 2OH from 2cPr pre-catalyst, leading to all accessible mechanistic pathways previously described. Under these conditions, acceleration of both pre-catalyst activation and overall turnover is observed due to the reiterative reaction sequence of 2cPr protonolysis to generate 2OH, which undergoes hydrogenolysis to form 2H and regenerate H2O (complete 2cPr decay is observed after ∼5 h, Figure 8b). The magnitude of the accelerated rate of catalysis is dictated by the competitive rates of insertion/β-H elimination (2H + olefin) versus protonolysis of 2H back to 2OH (2H + H2O). Also considering the decreased stability for the catalyst resting state of 2H versus 2R, the barriers of olefin insertion/β-H elimination contribute significantly to turnover efficiency. Importantly, the low-barrier hydrogenolysis of 2OH minimizes the need for exceedingly high H2 pressures that have been previously used for homogeneously CuH-catalyzed hydrogenations.[10, 12] Ultimately, mixtures of 2R/2OR can facilitate faster turnover by trapping of transient HOR by copper-alkyl species if olefin insertion barriers yield a 2R resting state, and in some cases can offer comparable reactivity to exclusively 2OR conditions (Figure 1, bottom right).
Evaluation of this proposed mechanism through kinetic modeling was carried out under specific conditions where excess H2O would not be present to influence the 2OH hydrogenolysis reaction. In an H2 pressure drop experiment, the catalytic hydrogenation of 1-octene at 60 °C was first observed after charging the reaction with 4 atm of undried H2 to yield an observable quantity of 2OH. After monitoring for 6 h, the mixture was flash frozen, and the pressure was decreased to 1 atm. Immediate resumption of the catalytic reaction at 60 °C highlights an inflection in rate due to turnover-dependent hydrogenolysis of 2OH, with slowed substrate consumption until reaction completion (Supporting Information, Page S83). Modeling this experiment provided barriers for 2OH hydrogenolysis (20.7 kcal mol−1), 2OH intramolecular deprotonation (24.0 kcal mol−1), and 1-octene insertion (17.9 kcal mol−1) at 60 °C.
The formation of a basic Cu─OH moiety in 2OH for heterolytic H2 cleavage via an IES pathway circumvents the inability of Cu-alkyl and the less effective MLC pathway to cleave H2. However, the formation of a Cu─OH species does not necessarily result in H2 activation in all cases, as catalytic hydrogenation of 1-hexene with 10 mol% linear 1Hex under 4 atm of undried H2 at 80 °C for 24 h shows no turnover, despite the verified formation of trace 1OH in the reaction mixture by 1H NMR spectroscopy (Figure S13). These observations emphasize the importance of a trigonal planar geometry at a monomeric Cu─OH for effecting H2 activation under these mild reaction conditions.
Computational Analysis of β-H Elimination of Cu(I) Alkyls and Heterolytic H2 Cleavage at a Monomeric Cu─OH
We carried out computational studies to further understand the role of coordination geometry and supporting ligands on the β-H elimination of alkyl complexes and the activation of H2 at a monomeric hydroxide complex 2OH. The computed free energy barriers ΔG‡ for the endergonic β-H eliminations of linear 1tBu and trigonal 2tBu at 25 °C corresponds to 34.1 and 28.9 kcal mol−1 (Figure 9), respectively, based on gas-phase Q-Chem[81] calculation using dispersion-corrected wB97X-D functional,[1, 83] def2-SVP basis set and corresponding def2-ECP effective core potential[84] for Cu. Similar β-H elimination energy barriers of 1tBu and 2tBu were obtained at the wB97X-D/def2-TZVP and wB97X-D4/def2-SVP levels of theory (Supporting Information, Page S112). Though the computed absolute ΔG‡ value for the β-H elimination of 2tBu differs from the modelled experimental kinetic data at 24.5 kcal mol−1, the difference of ΔΔG‡ = 5.2 kcal mol−1 between 1tBu and 2tBu derived computationally supports the lack of β-H elimination for 1tBu at room temperature. Kinetic analysis at 80 °C for 1tBu infers a barrier of 30.4 kcal mol−1, with a corresponding gas-phase calculated barrier of 34.2 kcal mol−1 at 80 °C. In addition, the calculated metrical parameters for the β-H elimination of 1tBu and 2tBu show late transition states with considerable structural similarities at the Cu–tBu fragments (Figures S88 and S89), constituting complete sp3 C─H cleavage to form a Cu(H)(η2-isobutylene) species. The transition state (TS) structure of 1tBu and 2tBu adopts a distorted T shaped and a distorted tetrahedral geometry, respectively, according to the different binding modes of either supporting ligand. A direct structural and molecular orbital comparison of the two TS structures is provided in the Supporting Information (Supporting Information, Pages S113 and S114). These computational results further suggest that the microscopic reverse of Markovnikov isobutylene insertion into the trigonal planar (NBCCPh3)CuH monomer is 1.1 kcal mol−1 more favorable than that linear (IPr*CPh3)CuH monomer.
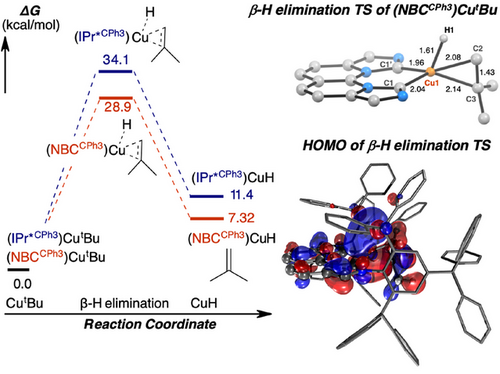
The favorable β-H elimination of 2tBu versus 1tBu was further analyzed by the activation-strain model[85, 86] for the two constituent fragments of carbene ligand and Cu–tBu. This method decomposes ΔE‡ into strain energy (ΔEstr) and interaction energy (ΔEint). ΔEstr captures the deformation-induced energy difference between the transition state and ground state (GS) structure whereas ΔEint reflects the difference in stabilizing interactions between the Cu–tBu fragment and the remaining complex. The results are summarized in Figure 10. Complex 2tBu exhibits a larger stabilization from ΔEint by −10.1 kcal mol−1 and larger strain energy by 4.7 kcal mol−1 compared to those of 1tBu. The remaining difference of ΔEstr is attributed to distortion of carbene ligands/geometry change at Cu.
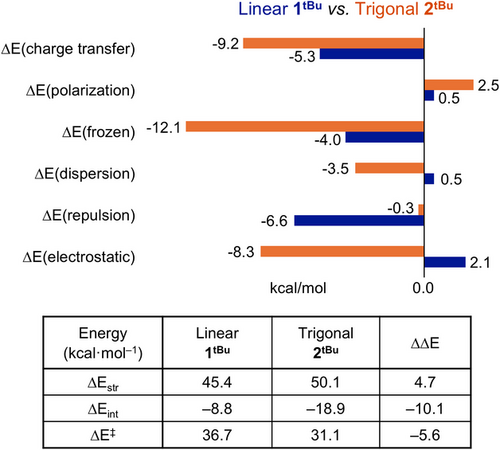
To probe the origin of ΔEint on the energetics of β-H elimination for 1tBu and 2tBu, we performed second generation absolutely localized molecular orbital-energy decomposition analysis (ALMO-EDA).[87-89] This method decomposes the binding energy between the carbene ligand and Cu-alkyl fragment (ΔEint) into components consisting of “frozen” terms (electrostatics, dispersion, and Pauli repulsions) and orbital relaxation terms (polarization and charge transfer). Computational results reveal that, although the strain that is induced upon transition-state formation is slightly higher in 2tBu, the overall lowering of the β-H elimination barrier of 2tBu relative to linear 1tBu is attributed to more stabilizing nonbonding (electrostatics, dispersion) and bonding (metal–ligand charge transfer) interactions that result from their difference in geometry.
The influence of orbital symmetry by substituting IPr*CPh3 for NBCCPh3 ligand is further corroborated by the charge-transfer analysis (ΔECT). The complementary occupied-virtual pairs (COVP)[90, 91] of 2tBu demonstrate increased π-symmetric accepting orbitals on the NBCCPh3 ligand in both the ground and transition states (Figure S92), thus contributing to 3.9 kcal mol−1 greater ΔECT stabilization compared to 1tBu (Figure 10).[92] The combined accepting ability of NBCCPh3 is approximately twice that of IPr*CPh3 in the CutBu complexes, suggesting a summative effect of having two carbene donors in NBCCPh3 (Table S11).[93] Unexpectedly, in this context, there appears to be no synergistic contribution between the two carbene donors and the conjugated naphthyridine linking unit.
We also employed computations to delineate the mechanism of H2 activation by the hydroxide complex 2OH since this reaction is turnover-limiting for accelerated catalytic hydrogenation, based on our kinetic investigation with 1-octene. Computations indicate H2 cleavage at 2OH occurs with a TS energy barrier of 18.6 kcal mol−1 (implicit benzene solvent, 60 °C) (Figure 11), which corroborates the experimental barrier of 20.7 kcal mol−1 at 60 °C by the fitted kinetic model. A discrete intermediate from the coordination of H2 at the Cu(I) center of 2OH prior to the TS was not detected; however, an adduct containing the interaction of H2 through the lone pair of the hydroxide ligand was endergonic by 5.6 kcal mol−1 from the ground state of 2OH and H2. This atypical interaction has been documented in a comprehensive computational investigation for the IES mechanism of varied (pincer)PdOH complexes and H2.[77] This insight hints at yet another adverse effect of excess H2O on the reduced catalytic turnover, since extensive hydrogen-bonding of 2OH/H2O can interfere with 2OH/H2 adduct formation en route to H2 activation.
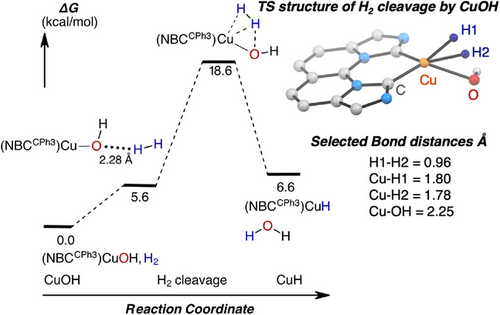
The computed TS structure, summarized in Figure 11, shows a distorted tetrahedral geometry at Cu (τ4′ = 0.67) with a hydrogen atom from H2 oriented directly towards a lone pair of the OH ligand. Bond distances of 1.80 and 1.78 Å of Cu─H1 and Cu─H2, respectively, are significantly longer than expected for a formal Cu─H group based on the Cu─H bond distance of 1.57 Å for geometry-optimized 2H product. Additionally, an H1─H2 bond distance of 0.96 Å in the TS structure indicates a weakly interacting and intact H2 at the Cu(I) center rather than an elongated dihydrogen or a dihydride species.[94] Moreover, notable elongation of the Cu─OH bond by 0.35 Å was observed at the transition state. Taken together, this pathway resembles an early transition state for H2 cleavage and its metrical parameters are consistent with those reported for computational TS structures of IES for Pd-OH and H2.[77]
Conclusion
Comparative studies of two-coordinate linear and three-coordinate trigonal planar Cu-alkyl complexes revealed that only trigonal planar (NBCCPh3)Cu-alkyls promote β-H elimination/isomerization, hydrogenolysis, and catalytic olefin hydrogenation. The spectroscopic observation of monomeric (NBCCPh3)CuH enabled quantitative kinetic analysis for the determination of energy barriers of key elementary steps and mechanistic elucidation of competing pathways. Energy decomposition analysis indicates that the supporting NBCCPh3 ligand in a CutBu complex enhances electrostatics, dispersion, and metal-ligand charge transfer from the increased electron-accepting ability of NBCCPh3 as compared to that of IPr*CPh3, thus contributing to lowering the barrier to β-H elimination by 5.2 kcal mol−1.
The linear and trigonal planar Cu-alkyl species in this study do not appear to mediate heterolytic H2 cleavage by the conventional σ-bond metathesis mechanism. Mechanistic analysis of hydrogenolysis and catalytic olefin hydrogenation from (NBCCPh3)Cu-cyclopropyl by deuterium labeling, trapping, and kinetic studies indicate two predominant competing pathways of metal–ligand cooperativity and internal electrophilic substitution for H2 activation to generate (NBCCPh3)CuH, which has been independently isolated and characterized. The MLC pathway involves intramolecular deprotonation of a -CHPh2 group at the supporting NBCCPh3 by the alkyl ligand to generate a zwitterionic intermediate, composed of a cationic Cu(I) center and an anionic -CPh2 moiety, capable of heterolytic H2 cleavage. The IES pathway indicates direct activation of H2 at the (NBCCPh3)CuOH complex, generated from Cu-alkyl protonolysis, to engender (NBCCPh3)CuH and water, and traverses a four-centered transition state with an energy barrier of 20.7 and 18.6 kcal·mol−1 based on kinetic modeling and computation, respectively.
Importantly, these mechanistic insights motivated the inclusion of isolable on-cycle hydroxide and ethoxide intermediates, (NBCCPh3)CuOR (R = H, Et), for promotion of the IES pathway and led to accelerated catalytic olefin hydrogenation compared to that operating primarily by the MLC pathway. Moreover, kinetic studies involving in situ generation of linear (IPr*CPh3)CuOH for olefin hydrogenation were shown to be catalytically inactive, further highlighting the high reactivity of trigonal planar d10 Cu(I) complexes compared to their linear congeners. Our access to a robust trigonal planar alkoxide catalyst system capable of mediating heterolytic H2 cleavage towards catalytic hydrogenation of unactivated alkenes at mild H2 pressure and temperature provides a promising foray into CuH-catalyzed hydrogenations and is actively being pursued in our lab.
Experimental Section
Details of synthetic procedures, spectroscopic characterizations, kinetic studies, COPASI kinetic modeling, and computational analysis are provided in the Supporting Information. Deposition Numbers 2445645 (for 2nPr), 2445646 (for 2OEt), 2445647 (for 2OH·H2O), 2445648 (for 2tBu), 2445649 (for 2cPr), 2445650 (for 2Et), 2445651 (for Int)) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe AccessStructures service
Acknowledgements
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, Catalysis Science Program, FWP 47319. The authors are grateful to the Collaboratory for Advanced Research Computing at the University of Southern California for computational resources and support. The authors thank Prof. Karen I. Goldberg (Univ. of Pennsylvania) for insightful discussion on H2 activation by late metal hydroxide complexes.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.